Abstract
Metabotropic glutamate receptor 5 (mGlu5) has been implicated in a variety of learning processes and is important for inhibitory avoidance and conditioned taste aversion learning. MGlu5 receptors are physically connected with NMDA receptors and they interact with, and modulate, the function of one another in several brain regions. The present studies used systemic co-administration of an mGlu5 receptor positive allosteric modulator, 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) and an NMDA receptor antagonist dizocilpine maleate (MK-801) to characterize the interactions of these receptors in two aversive learning tasks. Male Sprague-Dawley rats were trained in a single-trial step-down inhibitory avoidance or conditioned taste aversion task. CDPPB (3 or 10 mg/kg, s.c.), delivered by itself prior to the conditioning trial, did not have any effect on performance in either task 48 hours after training. However, CDPPB (at 3 mg/kg) attenuated the MK-801 (0.2 mg/kg, i.p.) induced learning deficit in both tasks. CDPPB also reduced MK-801-induced hyperactivity. These results underlie the importance of mGlu5 and NMDA receptor interactions in modulating memory processing, and are consistent with findings showing the efficacy of positive allosteric modulators of mGlu5 receptors in reversing the negative effects of NMDA receptor antagonists on other behaviors such as stereotypy, sensorimotor gating, or working, spatial and recognition memory.
Keywords: inhibitory avoidance, conditioned taste aversion, open-field, metabotropic glutamate receptor 5, NMDA receptor
1. Introduction
Glutamate, the major excitatory neurotransmitter in the adult central nervous system, acts through ionotropic (NMDA, AMPA, kainate) and metabotropic glutamate receptors (mGlus: group I, mGlu1 and mGlu5; group II, mGlu2 and mGlu3; group III, mGlu4, mGlu6, mGlu7 and mGlu8) (Niswender & Conn, 2010). Recently, the interaction between group I mGlu and NMDA receptors on synaptic plasticity has received a great deal of attention. The functional interaction between mGlu5 and NMDA receptors has been studied at multiple levels from the molecular to the whole animal. However, although major progress has been made at the molecular and cellular levels, assessment of the effects of these interactions on cognitive functioning remains relatively unexplored.
Stimulation of mGlu5 receptor positively modulates the NMDA receptor through PKC phosphorylation and/or tyrosine kinase phosphorylation depending on the brain regions and specific conditions involved (Collett & Collingridge, 2004; Kotecha, Jackson, Al-Mahrouki, Roder, Orser, & MacDonald, 2003; Lu, Xiong, Lei, Orser, Dudek, Browning, & MacDonald, 1999). NMDA enhances mGlu5 receptor responses via calcineurin activation, which dephosphorylates the mGlu5 receptor at a PKC phosphorylation site (Alagarsamy, Rouse, Gereau, Heinemann, Smith, & Conn, 1999). The two receptors interact in a positive reciprocal manner, whereby stimulation of one receptor potentiates the function of the other. As individual synapses have specific signaling components, and different mGlu5 and NMDA receptor subtype/splice variants may be expressed, several mechanisms have been implicated in the upregulation of NMDA receptor functions by mGlu5 receptor and vice versa (Bruno, Battaglia, Copani, D’Onofrio, Di Lorio, De Blasi, Melchiorri, Flor, & Nicoletti, 2001; Hermans & Challiss, 2001). The functional interactions between the two receptors are of widespread significance as these have been reported in the hippocampus, prefrontal cortex, striatum, subthalamic nucleus, nucleus accumbens and spinal cord (Attucci, Carla, Mannaioni, & Moroni, 2001; Awad, Hubert, Smith, Levey, & Conn, 2000; Fitzjohn, Irving, Palmer, Harvey, Lodge, & Collingridge, 1996; Kotecha et al., 2003; Mannaioni, Marino, Valenti, Traynelis, & Conn, 2001; Martin, Nie, & Siggins, 1997; Pisani, Gubellini, Bonsi, Conquet, Picconi, Centonze, Bernardi, & Calabresi, 2001; Ugolini, Corsi, & Bordi, 1997). The two receptors physically link through anchoring proteins: mGlu5 receptor binds Homer proteins (Fagni, Ango, Perroy, & Bockaert, 2004), NMDA receptor interacts with PSD-95, and Homer and PSD-95 can be clustered by Shank – a postsynaptic density protein (Naisbitt, Kim, Tu, Xiao, Sala, Valtschanoff, Weinberg, Worley, & Sheng, 1999; Tu, Xiao, Naisbitt, Yuan, Petralia, Brakeman, Doan, Aakalu, Lanahan, Sheng, & Worley, 1999). NMDA and mGlu5 receptors can act synergistically to activate a number of proteins such as MAPKs, CaMKII, and CREB (Mao & Wang, 2002; Yang, Mao, Tang, Samdani, Liu, & Wang, 2004). Accordingly, coactivation of the receptors is required for distinct forms of LTP (Fujii, Sasaki, Mikoshiba, Kuroda, Yamazaki, Mostafa Taufiq, & Kato, 2004). Other electrophysiological evidence for the interaction has been recently reviewed (Homayoun & Moghaddam, 2010).
In contrast to in vitro studies, in vivo data examining this interaction in learning are very limited. Studies have used co-administration of mGlu5 and NMDA receptor antagonists or NMDA receptor antagonists and mGlu5 receptor positive allosteric modulators (PAMs). Homayoun, Stefani, Adams, Tamagan, and Moghaddam (2004) showed that co-application of behaviorally inactive doses of MK-801 (dizocilpine maleate, an NMDA receptor antagonist) and MPEP (2-methyl-6-(phenylethynyl)-pyridine, an mGlu5 receptor antagonist) impaired working memory in a four-arm maze and instrumental, appetitive light-nosepoke association learning task. MPEP also enhanced the effects of MK-801 on locomotion and stereotypy (Homayoun et al., 2004). In addition, phencyclidine (NMDA receptor antagonist) and MPEP impaired spatial learning in a radial arm maze task (Campbell, Lalwani, Hernandez, Kinney, Conn, & Bristow, 2004). In passive avoidance learning, co-administration of MK-801 and MTEP (3-[2-methyl-1,3-thiazol-4yl)ethynyl]pyridine, an mGlu5 receptor antagonist) impaired retention when given before training (Gravius, Pietraszek, Schmidt, & Danysz, 2006). Recently, DFB (3,3′-difluorobenzaldazine), an mGlu5 receptor PAM, was shown to increase memory in a Y-maze spatial alternation task (Balschun, Zuschratter, & Wetzel, 2006) and to attenuate ketamine-induced impairment in object recognition (Chan, Chiu, Sou, & Chen, 2008). CDPPB (3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide), another mGlu5 receptor PAM, reduced MK-801-induced impairment in an operant-based set-shifting task (Darrah, Stefani, & Moghaddam, 2008). Uslaner, Parmentier-Batteur, Flick, Surles, Lam, McNaughton, Jacobson and Hutson (2009) investigated the effect of CDPPB in an object recognition task, and found that CDPPB can increase novel object recognition and also can attenuate the MK-801-induced deficit in this task.
Previous data from our laboratory as well as other laboratories have indicated the unique importance of NMDA and mGlu5 receptors in aversive learning tasks, especially in inhibitory avoidance learning and conditioned taste aversion (Gravius, Pietraszek, Schafer, Schmidt, & Danysz, 2005; Izquierdo, Bevilaqua, Rossato, Bonini, Medina, & Cammarota, 2006; Nunez-Jaramillo, Ramirez-Lugo, Herrera-Morales, & Miranda, 2010; Schachtman, Bills, Ghinescu, Murch, Serfozo, & Simonyi, 2003; Simonyi, Serfozo, Parker, Ramsey, & Schachtman, 2009; Simonyi, Serfozo, Shelat, Dopheide, Coulibaly, & Schachtman, 2007). It was shown that these glutamate receptors play a critical role in acquisition and memory consolidation of these tasks. Inhibitory avoidance learning is a hippocampus-dependent associative learning task in which a response during training produces an aversive outcome. In the step-down inhibitory avoidance, the animal is placed on a platform and receives a shock when it steps off the platform. Memory for the shock is measured as an increased latency to step off the platform on subsequent trials (Gold, 1986). Conditioned taste aversion is a form of aversive classical conditioning in which a taste or flavored substance (the conditioned stimulus, CS) is paired with a drug or experience that produces internal malaise (the unconditioned stimulus, US), and this pairing results in the conditioned response — the subjects avoid the substance on a test trial (Bures, Bermudez-Rattoni, & Yamamoto, 1998).
The present study aimed to investigate whether the previously documented interaction between NMDA and mGlu5 receptors is necessary for learning in aversively motivated tasks, specifically inhibitory avoidance and conditioned taste aversion. The effects of CDPPB, an mGlu5 receptor PAM, on an MK-801-induced impairment in learning was examined in order to characterize mGlu5 and NMDA receptor interaction on hippocampal-dependent and - independent memory formation. In addition, an open-field test was used to examine possible changes in exploratory behavior after drug injection.
2. Materials and methods
2.1. Animals
2.1.1. Inhibitory avoidance and open-field tasks
Male, Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200-230 g were housed in groups of 2-3 with a 12 hr light/12 hr dark cycle (time on at 6:00 AM). Food and water were available ad libitum. Experiments were initiated near the beginning of the light period between 10:00 AM and 12:00 PM. In all experiments, animals were randomly assigned to groups. All experiments were conducted blind to the treatment condition of the rat. The work was carried out in accordance with National Institutes of Health guidelines and with permission from the University of Missouri-Columbia Animal Care and Use Committee (Protocol #6494).
2.1.2. Conditioned taste aversion
Male, Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 220-240 g were housed individually with a 16 hr light/8 hr dark cycle (time on at 6:00 AM). Food and water were available ad libitum until the beginning of the behavioral procedures. In all experiments, animals were randomly assigned to groups except for counterbalancing of water consumption prior to the start of the experiment (Protocol #6474).
2.2. Behavioral procedures
2.2.1. Inhibitory avoidance
Procedures were similar to those described earlier (Simonyi et al., 2007). Animals were handled daily for one week before the experiment. On the conditioning day, animals were injected with MK-801 (0 or 0.2 mg/kg, i.p.) and CDPPB (0, 3 or 10 mg/kg, s.c.) (n=10-12) or CDPPB alone (n=8-9) 20 min before being placed on the 2.5-cm high, 8-cm wide platform in one side of a shuttle-box (Med Associates, St. Albans, VT). The grid floor was connected to a scrambled shock generator. The detection system consisted of six pairs of photobeams, located 3.5 cm above the floor. The system was remotely controlled through an interface connected to an IBM-PC operating Med Associates software (version SOF-700RA-11). Latency to step down onto the grid was measured. Upon stepping down, the animals received a 0.4 mA, 0.5 sec footshock and were removed from the shuttle box. The retention test occurred 48 hours after training. The test session was identical to the training session except that the footshock was omitted. Step-down latency was recorded with a maximum latency of 180 sec.
2.2.2. Open-field
Med Associates Open Field Test Environments (ENV-515) were used to conduct open-field tests. Each activity box resided in a sound-resistant cubicle (ENV-017M) containing a clear, acrylic cage (43.2 × 43.2 × 30.5 cm). Twenty minutes after injection with MK-801 (0 or 0.2 mg/kg, i.p.) and CDPPB (0, 3 or 10 mg/kg, s.c.) (n=7) or CDPPB alone (n=6-7), rats were placed into the center of the square arena of an unlit activity box for 30 min. Locomotor activity data was collected in 5-min intervals using Med Associates’ Open-Field Activity Software (SOF-811), and measured as distance traveled (cm).
2.2.3. Conditioned taste aversion
The procedures have been described previously (Schachtman et al., 2003; Simonyi et al., 2009). Rats were water deprived for 24 hours. Then, animals were acclimated to drinking from the drinking tubes for four days to obtain their daily water within 15 minutes in their home cages and water consumption was measured. Fluids were provided at the same time every day in the experiments. Animals were handled on these days. On the conditioning day, animals were injected with MK-801 (0 or 0.2 mg/kg, i.p.) and CDPPB (0, 3 or 10 mg/kg, s.c.) (n=10-14) or CDPPB alone (n=7-8) and were presented with access to 8 ml of the 0.1% saccharin solution (the CS) 20 minutes later. Immediately after consumption of the saccharin, LiCl (US) was injected i.p. (0.15M, 1.33% body weight). The animals were observed for behaviors that indicate internal malaise (e.g., “lying on belly”). In the 48-hour period between conditioning and testing, the rats received no treatment except for exposure to 15 minutes of water access. One or three-four test trials were administered in each experiment (one in every 24 hours) in which saccharin was presented in drinking tubes for 15 minutes.
2.3. Drugs
3-cyano-N-(1,2,-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) was synthesized by IQsynthesis (St. Louis, MO; according to (Lindsley, Wisnoski, Leister, O’Brien, Lemaire, Williams, Burno, Sur, Kinney, Pettibone, Tiller, Smith, Duggan, Hartman, Conn, & Huff, 2004), was suspended in phosphate-buffered saline (PBS with 10% Tween-80), and dosed at 1 ml/kg (s.c.). (+)–MK-801 maleate (Ascent Scientific, Princeton, NJ) was administered intraperitoneally at 1 ml/kg in PBS. Animals were administered CDPPB/Vehicle immediately after being given MK-801/PBS.
2.4. Statistical analysis
Because step-down latencies are not normally distributed, inhibitory avoidance data were analyzed using the Kruskal-Wallis test followed by pairwise comparisons using the Mann-Whitney U test. Results from the open-field and conditioned taste aversion tasks are presented as mean ± SEM, and data were analyzed either by one-way analysis of variance (ANOVA) or by two-way repeated measures ANOVA followed by pairwise comparisons using Bonferroni’s test. P values of <0.05 were considered statistically significant.
3. Results
3.1. CDPPB does not influence inhibitory avoidance learning but reduces MK-801-induced impairment in learning
Step-down latencies of animals given CDPPB (0, 3, or 10 mg/kg, s.c.) prior to training are shown in Fig. 1A. There were no significant differences in training or test latencies among CDPPB treatment groups. Fig. 1B shows step-down latencies of animals co-administered MK-801 (0 or 0.2 mg/kg, i.p.) and CDPPB (0, 3, 10 mg/kg, s.c.). Animals given 0.2 MK-801/0 CDPPB had significantly shorter training latencies than 0 MK-801/0 CDPPB controls (p <0.001). Kruskal-Wallis test revealed a significant difference in test latencies among groups (p<0.001). Pairwise comparisons revealed test latencies of rats given 0.2 MK-801/0 CDPPB and 0.2 MK-801/10 CDPPB were significantly shorter than those of 0 MK-801/0 CDPPB controls (p<0.001). Administration of 3 mg/kg CDPPB attenuated this MK-801 effect, restoring performance to the control level (p<0.05, Fig. 1B).
Fig. 1.
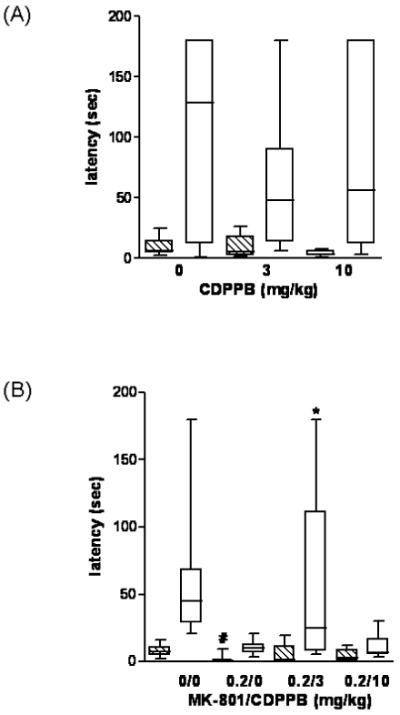
CDPPB does not influence inhibitory avoidance learning but reduces MK-801-induced impairment in learning. (A) Vehicle (0), 3 or 10 mg/kg CDPPB was injected s.c. 20 minutes before training. (B) PBS/Vehicle (0/0), 0.2 mg/kg MK-801/Vehicle (0.2/0), 0.2 mg/kg MK-801/3 mg/kg CDPPB (0.2/3) or 0.2 mg/kg MK-801/10 mg/kg CDPPB (0.2/10) were injected s.c/i.p. simultaneously 20 minutes before training. Retention was tested 48 hours after training. The figure depicts the latencies during training (shaded bars) and test (open bars). Results represent the median, interquartile range, and the lowest and highest values (n=8-12). *p<0.05 vs. 0.2 mg/kg MK-801/Vehicle group, #p<0.001 vs. PBS/Vehicle group (Kruskal-Wallis test, Mann-Whitney U test).
3.2. CDPPB does not influence spontaneous locomotor activity but attenuates MK-801-induced hyperlocomotion
Fig. 2 shows the total distance traveled in an open field for animals given CDPPB alone (Fig. 2A) or MK-801 and CDPPB (Fig. 2B). There were no significant differences among treatment groups given CDPPB alone. One-way ANOVA revealed a significant effect of treatment among animals co-administered MK-801 and CDPPB (F3, 22 = 10.69, p = 0.0002). Animals given 0.2 MK-801/0 CDPPB were significantly more active than 0 MK-801/0 CDPPB controls (p<0.001, Bonferroni’s posttest). Administration of 3 mg/kg CDPPB attenuated MK-801-induced hyperactivity (p<0.01, Bonferroni’s posttest).
Fig. 2.
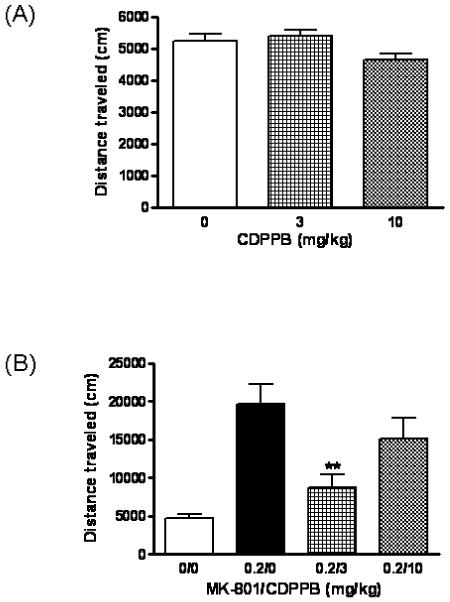
CDPPB does not influence spontaneous locomotor activity but attenuates MK-801-induced hyperlocomotion. (A) Animals were injected s.c. with Vehicle (0), 3 or 10 mg/kg CDPPB 20 minutes before being placed in the activity chamber. (B) Animals were injected (s.c./i.p.) with PBS/Vehicle (0/0), 0.2 mg/kg MK-801/Vehicle (0.2/0), 0.2 mg/kg MK-801/3 mg/kg CDPPB (0.2/3) or 0.2 mg/kg MK-801/10 mg/kg CDPPB (0.2/10) 20 minutes before being placed in the activity chamber. Activity was recorded for 30 minutes. Data are expressed as mean ± SEM of distance traveled (n=6-7). **p<0.01 vs. 0.2 mg/kg MK-801/Vehicle group (One-way ANOVA, Bonferroni’s posttest).
3.3. CDPPB does not influence conditioned taste aversion but attenuates MK-801-induced disruption in conditioned taste aversion
Saccharin consumption of animals on the test trials is shown in Fig. 3. There were no statistical differences among groups in saccharin consumption during conditioning (data not shown). There were no statistical differences among CDPPB treatment groups at test (Fig. 3A). However, one-way ANOVA revealed a significant effect of treatment among animals co-administered MK-801 and CDPPB (F3, 49 = 6.21, p = 0.012) (Fig. 3B). Animals given 0.2 MK-801/0 CDPPB consumed more saccharin on test day than 0 MK-801/0 CDPPB controls; MK-801 attenuated conditioned taste aversion (p<0.001, Bonferroni’s posttest). Additionally, there was a significant difference between 0.2 MK-801/0 CDPPB and 0.2 MK-801/3 CDPPB groups (p<0.05), indicating that CDPPB partially reversed the learning deficit induced by MK-801.
Fig. 3.
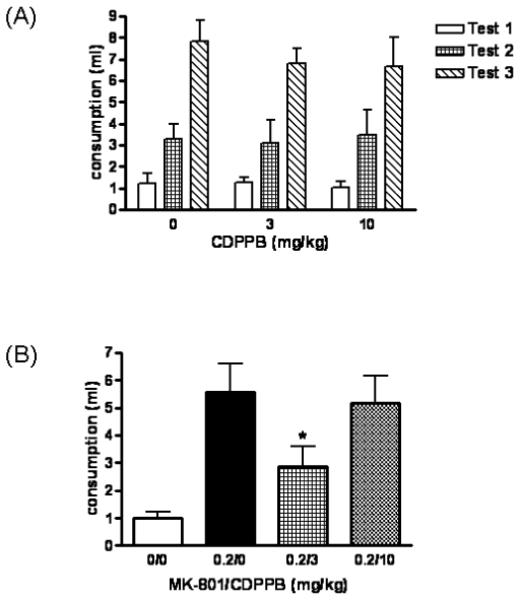
CDPPB does not influence conditioned taste aversion but attenuates MK-801-induced disruption in classical conditioning. (A) Vehicle (0), 3 or 10 mg/kg CDPPB was injected s.c. 20 minutes before saccharin intake during conditioned taste aversion conditioning. Testing was performed for three successive days, once per day, beginning 2 days after conditioning. (B) PBS/Vehicle (0/0), 0.2 mg/kg MK-801/Vehicle (0.2/0), 0.2 mg/kg MK-801/3 mg/kg CDPPB (0.2/3) or 0.2 mg/kg MK-801/10 mg/kg CDPPB (0.2/10) were injected s.c/i.p. simultaneously 20 minutes before saccharin intake during conditioned taste aversion conditioning. Testing was performed 48 hours later. Results are expressed as mean ± SEM (n=7-14). *p<0.05 vs. 0.2 mg/kg MK-801/Vehicle group (One-way ANOVA, Bonferroni’s posttest).
Cognitive enhancing drugs are often only effective in a narrow range of doses, and frequently follow a U-shaped dose response curve. Optimally effective drug doses can depend on the experimental conditions and parameters used (McGaugh & Roozendaal, 2008). To investigate the potential cognitive enhancing effects of CDPPB, we conducted a conditioned taste aversion experiment using a conditioning procedure aimed at producing a weaker conditioned taste aversion by using 0.075M LiCl, in 1.33% b.w. injected 30 minutes after saccharin consumption (Miranda, Quirarte, Rodriguez-Garcia, McGaugh, & Roozendaal, 2008). There were no significant differences in saccharin consumption between groups on test days 1-4 using this procedure, confirming the above result and indicating that CDPPB did not significantly enhance the learning of a conditioned taste aversion (data not shown).
4. Discussion
The present study demonstrated that CDPPB, while by itself is devoid of any effect, attenuates an MK-801-induced disruption of inhibitory avoidance and conditioned taste aversion learning. Furthermore, CDPPB was found to attenuate the hyperactivity induced by MK-801. The mGlu5 receptor PAM, CDPPB, was administered prior to conditioning since mGlu5 receptors are implicated in both acquisition and consolidation of learning and memory processes. The drug has a brain half-life of approximately 4.4 hours (Kinney, O’Brien, Lemaire, Burno, Bickel, Clements, Chen, Wisnoski, Lindsley, Tiller, Smith, Jacobson, Sur, Duggan, Pettibone, Conn, & Williams, 2005), thus it was still active in the brain during consolidation. The behavioral measures employed in the present study involve a relatively short conditioning trial (duration of the CS-US pairing) so it is likely that both acquisition and consolidation were affected by CDPPB administration.
The NMDA receptor antagonist, MK-801, has been found to impair learning in a variety of tasks including inhibitory avoidance and conditioned taste aversion when administered systemically (Golden & Houpt, 2007; Ishiyama, Tokuda, Ishibashi, Ito, Toma, & Ohno, 2007; Roesler, Vianna, De-Paris, & Quevedo, 1999; Saute, da Silveira, Soares, Martini, Souza, & Ganzella, 2006). Dosages commonly used range from 0.1 mg/kg to 0.5 mg/kg, but have been found to induce hyperactivity at these doses (e.g. Stefani & Moghaddam, 2010; Uslaner et al., 2009; Vales, Svoboda, Benkovicova, Bubenikova-Valesova, & Stuchlik, 2010). A dose of 0.2 mg/kg MK-801 was used in the present study because a higher dose of 0.3 mg/kg was found to produce taste aversion on its own (Jackson & Sanger, 1989) and a lower dose of 0.1 mg/kg was found to be behaviorally ineffective in inhibitory avoidance (Gravius et al., 2006). No pre-exposure to the inhibitory avoidance apparatus was used in the present study because habituation to a context has been shown to facilitate learning. That is, pre-exposure to the inhibitory avoidance box or exposure to a low intensity footshock prior to training prevented an NMDA receptor antagonist-induced memory impairment (Roesler, Vianna, Sant’Anna, Kuyven, Kruel, Quevedo, & Ferreira, 1998). Context pre-exposure has also been found to facilitate learning and memory in fear conditioning (e.g. Biedenkapp & Rudy, 2007; Huff, Wright-Hardesty, Higgins, Matus-Amat, & Rudy, 2005).
In the present study, systemic administration of MK-801 significantly impaired performance in both the inhibitory avoidance and conditioned taste aversion procedures, which is consistent with findings that indicate the importance of NMDA receptor function in learning (see Riedel, Platt, & Micheau, 2003 for a review). Local administration of NMDA receptor antagonists demonstrated that both hippocampal and amygdalar NMDA receptors are necessary for memory processing in inhibitory avoidance learning (Jerusalinsky, Ferreira, Walz, Da Silva, Bianchin, Ruschel, Zanatta, Medina, & Izquierdo, 1992; Szapiro, Vianna, McGaugh, Medina, & Izquierdo, 2003). Additionally, cortical and amygdalar NMDA receptors are required for learning of a conditioned taste aversion (Berman, Hazvi, Neduva, & Dudai, 2000; Escobar, Alcocer, & Bermudez-Rattoni, 2002; Ferreira, Gutierrez, De La Cruz, & Bermudez-Rattoni, 2002; Gutierrez, Hernandez-Echeagaray, Ramirez-Amaya, & Bermudez-Rattoni, 1999; Yasoshima, Morimoto, & Yamamoto, 2000). Similarly, involvement of mGlu5 receptors in aversive learning has been well documented for a variety of learning tasks (see Simonyi, Schachtman, & Christoffersen, 2010 for a review). Studies using systemic administration of mGlu5 receptor antagonists prior to training have demonstrated that activation of mGlu5 receptors is required for inhibitory avoidance learning (Genkova-Papazova et al., 2007; Gravius et al., 2005; Jacob, Gravius, Pietraszek, Nagel, Belozertseva, Shekunova, Malyshkin, Greco, Barberi, & Danysz, 2009; Simonyi, Schachtman, & Christoffersen, 2005). Another study showed that hippocampal mGlu5 receptors contribute to consolidation, but not extinction in this task (Simonyi et al., 2007). Moreover, earlier studies from our laboratory revealed that mGlu5 receptor antagonism attenuates conditioned taste aversion and latent inhibition (Bills, Schachtman, Serfozo, Spooren, Gasparini, & Simonyi, 2005; Schachtman et al., 2003). In a recent study, MTEP, an mGlu5 receptor antagonist, when administered to the basolateral amygdala, resulted in normal conditioned taste aversion on the initial test trial, but slowed extinction (Simonyi et al., 2009).
At the molecular and cellular level, multiple studies have confirmed the functional interactions of mGlu5 and NMDA receptors (see details in the Introduction). At the behavioral level, antagonists of mGlu5 receptors potentiate the effects of NMDA receptor antagonists on locomotor activity, working memory, spatial memory, prepulse inhibition and stereotypy (Campbell et al., 2004; Henry, Lehmann-Masten, Gasparini, Geyer, & Markou, 2002; Homayoun et al, 2004; Kinney, Burno, Campbell, Hernandez, Rodriguez, Bristow, & Conn, 2003; Pietraszek, Gravius, Schafer, Weil, Trifanova, & Danysz, 2005). Similarly, MK-801 and MTEP when co-administered at doses which were behaviorally inactive when administered alone, were found to impair performance in a step-through inhibitory avoidance task (Gravius et al., 2006). Finally, mGlu5 receptor PAMs are able to reverse the cognitive deficits induced by NMDA receptor antagonism. For example, DFB attenuated hyperactivity and impairment in object recognition induced by ketamine (Chan et al., 2008). CDPPB reversed the MK-801-induced deficit in cognitive flexibility, object recognition, and working and spatial memory (Darrah et al., 2008; Stefani & Moghaddam, 2010; Vales et al., 2010). The effects of mGlu5 receptor PAMs in these learning tasks are likely due to cognitive effects and not motivational, sensory or motoric effects of the drug. These studies, along with the present study suggest that mGlu5 and NMDA receptors interact to affect learning and memory although results obtained using systemic injection cannot support any anatomical hypothesis about the sites at which these receptors interact on memory processing. Some non-associative (e.g., motoric or sensory) effects of MK-801 occur, such as the differences between groups on the conditioning trial of the avoidance experiment reported here, but such effects of the drug cannot readily explain differences in test performance (poor learning produced by this drug and reversal by CDPPB) when drug is absent.
Several recent studies have found that mGlu5 receptor PAMs enhance performance in a variety of tasks. For example, both CDPPB and ADX-47273 enhanced performance in the Morris water maze (Ayala, Chen, Banko, Sheffler, Williams, Telk, Watson, Xiang, Zhang, Jones, Lindsley, Olive, & Conn, 2009) and novel object recognition (Liu, Grauer, Kelley, Navarra, Graf, Zhang, Atkinson, Popiolek, Wantuch, Khawaja, Smith, Olsen, Kouranova, Lai, Pruthi, Pulicicchio, Day, Gilbert, Pausch, Brandon, Beyer, Comery, Logue, Rosenzweig-Lipson, & Marquis, 2008; Uslaner et al., 2009), and DFB increased retention of a spatial alternation task (Balschun et al., 2006). In the present study, CDPPB alone did not appear to enhance performance in either task. However, a higher dose of CDPPB, along with weaker conditioning procedures was not investigated, so we cannot adequately comment on the potential memory enhancing effects of CDPPB. Nonetheless, our results in the open-field test support and complement previous findings (Gass and Olive, 2009; Liu et al., 2008; Rosenbrock, Kramer, Hobson, Koros, Grundl, Grauert, Reymann, & Schroder, 2010; Stefani & Moghaddam, 2010; Vales et al., 2010). In addition, similar to the study by Uslaner and his colleagues (2009), our study found that 3 mg/kg CDPPB attenuated the MK-801-induced learning impairment but 10 mg/kg CDPPB was ineffective. The Uslaner study reported that 3 mg/kg CDPPB reversed an MK-801-induced deficit in novel object recognition; but higher doses of 10 and 30 mg/kg CDPPB did not, suggesting an inverted-U-shaped dose response curve. This type of dose-response curve is not uncommon among cognitive enhancers, and for a specific drug can vary depending on which behavioral task is being used. Uslaner et al. (2009) note that mGlu5 receptor activation produces multiple downstream effects which are not always straightforward, such as influences on LTP and LTD. Interestingly, different mGlu5 receptor PAMs can influence mGlu5 receptor-related signaling pathways differentially (Zhang, Rodriguez, & Conn, 2005). The fact that mGlu5 receptor activation leads to different downstream effects, depending on which behavioral task is being used and the amount of drug administered, may account for the present dose-response relationship.
NMDA and mGlu5 receptors are highly co-localized in regions associated with learning and memory, such as the cortex, hippocampus and amygdala (Laurie & Seeburg, 1994; Romano, Sesma, McDonald, O’Malley, Van den Pol, & Olney, 1995), and are physically linked through anchoring proteins, which allow the synergistic activation of many signaling proteins such as MAPKs and CREB underlying synaptic plasticity (Mao & Wang, 2002; Yang et al., 2004). CDPPB has also been shown to increase CREB phosphorylation as well as activation of CaMKII in the hippocampus and the prefrontal cortex (Uslaner et al., 2009). However, further studies are needed to test whether these effects are more universal or region-specific. A recent study found that administering CDPPB (10 mg/kg, i.p.) prior to MK-801 administration prevented an NMDA receptor antagonist-induced increase in prefrontal cortex neuron firing rate and also normalized disruptions in burst activity (Lecourtier, Homayoun, Tamagnan, & Moghaddam, 2007). Researchers analyzed prefrontal cortex neuron firing rate for two hours after drug administration and found the firing rate remained elevated for over an hour, suggesting that the mGlu5 receptor PAM, CDPPB, does not induce rapid mGlu5 receptor desensitization (Lecourtier et al., 2007).
These studies have important implications for translational research seeking to identify potential drug treatments for a variety of diseases involving glutamate receptor dysfunction such as schizophrenia. The negative symptoms and cognitive deficits of schizophrenia may be the result of NMDA receptor hypofunction (for a review see Conn, Tamminga, Schoepp, & Lindsley, 2008). Current antipsychotic drugs treat the positive symptoms of schizophrenia, but are largely ineffective in treating the negative symptoms. NMDA receptor agonists are not a viable treatment option because they pose a high risk of excitotoxicity, which is why selective mGlu5 PAMs are receiving so much empirical attention (Gravius, Pietraszek, Dekundy, & Danysz, 2010). PAMs, such as CDPPB, potentiate mGlu5 receptor activity, which provides a way to increase NMDA receptor activity without the risk of excitotoxicity (Gass & Olive, 2009). Recent research using different animal models further supports the potential antipsychotic efficacy of mGlu5 receptor PAMs (Kinney et al., 2005; Liu et al., 2008; Vardigan, Huszar, McNaughton, Hutson, & Uslaner, 2010).
The results of the present study are consistent with the notion that receptor interaction is involved in the regulation of induction and persistence of LTP and LTD, and more generally with the concept of metaplasticity (for a review see Abraham, 2008). Our study demonstrates the functional interaction of mGlu5 and NMDA receptors in conditioned taste aversion and inhibitory avoidance learning. This is an important basic feature of synaptic plasticity underlying learning and memory and supports previous studies, as well as adds to their results describing the interaction between mGlu5 and NMDA receptors.
Acknowledgement
This study was partially supported by 1R21 AT 003859 and 1R01 DA024355 from NIH. We thank Drs. Dennis K. Miller and Kelli R. Rodvelt for generously providing the opportunity and training to use the Med Associates Open Field Test apparatus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews Neuroscience. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Rouse ST, Gereau RWT, Heinemann SF, Smith Y, Conn PJ. Activation of N-methyl-D-aspartate receptors reverses desensitization of metabotropic glutamate receptor, mGluR5, in native and recombinant systems. Annals of the New York Academy of Sciences. 1999;868:526–530. doi: 10.1111/j.1749-6632.1999.tb11321.x. [DOI] [PubMed] [Google Scholar]
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. British Journal of Pharmacology. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. Journal of Neuroscience. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. Journal of Neuroscience. 2000;20:7017–7023. doi: 10.1523/JNEUROSCI.20-18-07017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context preexposure prevents forgetting of a contextual fear memory: implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learning and Memory. 2007;14:200–203. doi: 10.1101/lm.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills C, Schachtman TR, Serfozo P, Spooren WP, Gasparini F, Simonyi A. Effects of metabotropic glutamate receptor 5 on latent inhibition in conditioned taste aversion. Behavioural Brain Research. 2005;157:71–78. doi: 10.1016/j.bbr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D’Onofrio M, Di Lorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptors subtypes as targets for neuroprotective drugs. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Bures J, Bermudez-Rattoni F, Yamamoto T. Conditioned taste aversion: memory of a special kind. Oxford University Press; Oxford: 1998. [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology. 2004;175:310–318. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- Chan MH, Chiu PH, Sou JH, Chen HH. Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology. 2008;198:141–148. doi: 10.1007/s00213-008-1103-1. [DOI] [PubMed] [Google Scholar]
- Collett VJ, Collingridge GL. Interactions between NMDA receptors and mGlu5 receptors expressed in HEK293 cells. British Journal of Pharmacology. 2004;142:991–1001. doi: 10.1038/sj.bjp.0705861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Molecular Interventions. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behavioral Pharmacology. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar ML, Alcocer I, Bermudez-Rattoni F. In vivo effects of intracortical administration of NMDA and metabotropic glutamate receptors antagonists on neocortical long-term potentiation and conditioned taste aversion. Behavioural Brain Research. 2002;129:101–106. doi: 10.1016/s0166-4328(01)00329-1. [DOI] [PubMed] [Google Scholar]
- Fagni L, Ango F, Perroy J, Bockaert J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Seminars in Cell and Developmental Biology. 2004;15:289–298. doi: 10.1016/j.semcdb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Ferreira G, Gutierrez R, De La Cruz V, Bermudez-Rattoni F. Differential involvement of cortical muscarinic and NMDA receptors in short- and long-term taste aversion memory. European Journal of Neuroscience. 2002;16:1139–1145. doi: 10.1046/j.1460-9568.2002.02174.x. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, Collingridge GL. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neuroscience Letters. 1996;203:211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- Fujii S, Sasaki H, Mikoshiba K, Kuroda Y, Yamazaki Y, Mostafa Taufiq A, Kato H. A chemical LTP induced by co-activation of metabotropic and N-methyl-D-aspartate glutamate receptors in hippocampal CA1 neurons. Brain Research. 2004;999:20–28. doi: 10.1016/j.brainres.2003.11.058. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biological Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genkova-Papazova M, Petkova B, Stankova I, Ossowska K, Lazarova-Bakarova M. Effects of MPEP on avoidance learning in rats. Methods and Findings in Experimental & Clinical Pharmacology. 2007;29:205–209. doi: 10.1358/mf.2007.29.3.1075356. [DOI] [PubMed] [Google Scholar]
- Gold PE. The use of avoidance training in studies of modulation of memory storage. Behavioral and Neural Biology. 1986;46:87–98. doi: 10.1016/s0163-1047(86)90927-1. [DOI] [PubMed] [Google Scholar]
- Golden GJ, Houpt TA. NMDA receptor in conditioned flavor-taste preference learning: blockade by MK-801 and enhancement by D-cycloserine. Pharmacology Biochemistry & Behavior. 2007;86:587–596. doi: 10.1016/j.pbb.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravius A, Pietraszek M, Dekundy A, Danysz W. Metabotropic glutamate receptors as therapeutic targets for cognitive disorders. Current Topics in Medicinal Chemistry. 2010;10:187–206. doi: 10.2174/156802610790411018. [DOI] [PubMed] [Google Scholar]
- Gravius A, Pietraszek M, Schafer D, Schmidt WJ, Danysz W. Effects of mGlu1 and mGlu5 receptor antagonists on negatively reinforced learning. Behavioral Pharmacology. 2005;16:113–121. doi: 10.1097/00008877-200503000-00007. [DOI] [PubMed] [Google Scholar]
- Gravius A, Pietraszek M, Schmidt WJ, Danysz W. Functional interaction of NMDA and group I metabotropic glutamate receptors in negatively reinforced learning in rats. Psychopharmacology. 2006;185:58–65. doi: 10.1007/s00213-005-0249-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, Hernandez-Echeagaray E, Ramirez-Amaya V, Bermudez-Rattoni F. Blockade of N-methyl-D-aspartate receptors in the insular cortex disrupts taste aversion and spatial memory formation. Neuroscience. 1999;89:751–758. doi: 10.1016/s0306-4522(98)00360-1. [DOI] [PubMed] [Google Scholar]
- Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–1209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochemical Journal. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: Role in modulating cortical activity and relevance to cognition. European Journal of Neuroscience. 2010;639:33–39. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Huff NC, Wright-Hardesty KJ, Higgins EA, Matus-Amat P, Rudy JW. Context pre-exposure obscures amygdala modulation of contextual-fear conditioning. Learning and Memory. 2005;12:456–460. doi: 10.1101/lm.6705. [DOI] [PubMed] [Google Scholar]
- Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. European Journal of Neuroscience. 2007;572:160–170. doi: 10.1016/j.ejphar.2007.06.058. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends in Neurosciences. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Jackson A, Sanger DJ. Conditioned taste aversions induced by phencyclidine and other antagonists of N-methyl-D-aspartate. Neuropharmacology. 1989;28:459–464. doi: 10.1016/0028-3908(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Jerusalinsky D, Ferreira MB, Walz R, Da Silva RC, Bianchin M, Ruschel AC, Zanatta MS, Medina JH, Izquierdo I. Amnesia by post-training infusion of glutamate receptor antagonists into the amygdala, hippocampus, and entorhinal cortex. Behavioral and Neural Biology. 1992;58:76–80. doi: 10.1016/0163-1047(92)90982-a. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. Journal of Pharmacology and Experimental Therapeutics. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. Journal of Pharmacology and Experimental Therapeutics. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. Journal of Biological Chemistry. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. Journal of Neuroscience. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biological Psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O’Brien JA, Lemaire W, Williams DL, Jr., Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo. Journal of Medicinal Chemistry. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piper idin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. Journal of Pharmacology and Experimental Therapeutics. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nature Neuroscience. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. Journal of Neuroscience. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Interactions between ionotropic and metabotropic glutamate receptors regulate cAMP response element-binding protein phosphorylation in cultured striatal neurons. Neuroscience. 2002;115:395–402. doi: 10.1016/s0306-4522(02)00400-1. [DOI] [PubMed] [Google Scholar]
- Martin G, Nie Z, Siggins GR. Metabotropic glutamate receptors regulate N-methyl-D-aspartate-mediated synaptic transmission in nucleus accumbens. Journal of Neurophysiology. 1997;78:3028–3038. doi: 10.1152/jn.1997.78.6.3028. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology. 2008;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Quirarte GL, Rodriguez-Garcia G, McGaugh JL, Roozendaal B. Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learning and Memory. 2008;15:468–476. doi: 10.1101/lm.964708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual Review of Pharmacology and Toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Jaramillo L, Ramirez-Lugo L, Herrera-Morales W, Miranda MI. Taste memory formation: Latest advances and challenges. Behavioural Brain Research. 2010;207:232–248. doi: 10.1016/j.bbr.2009.10.040. [DOI] [PubMed] [Google Scholar]
- Pietraszek M, Gravius A, Schafer D, Weil T, Trifanova D, Danysz W. mGluR5, but not mGluR1, antagonist modifies MK-801-induced locomotor activity and deficit in prepulse inhibition. Neuropharmacology. 2005;49:73–85. doi: 10.1016/j.neuropharm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behavioural Brain Research. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Roesler R, Vianna M, Sant’Anna MK, Kuyven CR, Kruel AV, Quevedo J, Ferreira MB. Intrahippocampal infusion of the NMDA receptor antagonist AP5 impairs retention of an inhibitory avoidance task: protection from impairment by pretraining or preexposure to the task apparatus. Neurobiology of Learning and Memory. 1998;69:87–91. doi: 10.1006/nlme.1997.3810. [DOI] [PubMed] [Google Scholar]
- Roesler R, Vianna MR, De-Paris F, Quevedo J. Memory-enhancing treatments do not reverse the impairment of inhibitory avoidance retention induced by NMDA receptor blockade. Neurobiology of Learning and Memory. 1999;72:252–258. doi: 10.1006/nlme.1999.3910. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van Den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. Journal of Comparative Neurology. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, Reymann KG, Schroder UH. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. European Journal of Neuroscience. 2010;639:40–46. doi: 10.1016/j.ejphar.2010.02.057. [DOI] [PubMed] [Google Scholar]
- Saute JAM, da Silveira LE, Soares FA, Martini LH, Souza DO, Ganzella M. Amnesic effect of GMP depends on its conversion to guanosine. Neurobiology of Learning and Memor. 2006;85:206–212. doi: 10.1016/j.nlm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Schachtman TR, Bills C, Ghinescu R, Murch K, Serfozo P, Simonyi A. MPEP, a selective metabotropic glutamate receptor 5 antagonist, attenuates conditioned taste aversion in rats. Behavioural Brain Research. 2003;141:177–182. doi: 10.1016/s0166-4328(02)00378-9. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News & Perspective. 2005;18:353–361. doi: 10.1358/dnp.2005.18.6.927927. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. European Journal of Pharmacology. 2010;639:17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Parker KE, Ramsey AK, Schachtman TR. Metabotropic glutamate receptor 5 in conditioned taste aversion learning. Neurobiology of Learning and Memory. 2009;92:460–463. doi: 10.1016/j.nlm.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Shelat PB, Dopheide MM, Coulibaly AP, Schachtman TR. Differential roles of hippocampal metabotropic glutamate receptors 1 and 5 in inhibitory avoidance learning. Neurobiology of Learning and Memory. 2007;88:305–311. doi: 10.1016/j.nlm.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. European Journal of Pharmacology. 2010;639:23–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by group I mGluR in spinal cord motoneurons. Neuropharmacology. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vales K, Svoboda J, Benkovicova K, Bubenikova-Valesova V, Stuchlik A. The difference in effect of mGlu2/3 and mGlu5 receptor agonists on cognitive impairment induced by MK-801. European Journal of Pharmacology. 2010;639:91–98. doi: 10.1016/j.ejphar.2009.11.067. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacology, Biochemistry and Behavior. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. Journal of Neuroscience. 2004;24:10846–10857. doi: 10.1523/JNEUROSCI.2496-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Research. 2000;869:15–24. doi: 10.1016/s0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. Journal of Pharmacology and Experimental Therapeutics. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]


