Abstract
Gene transfer to the central nervous system provides powerful methodology for the study of gene function and gene-environment interactions in vivo, in addition to a vehicle for the delivery of therapeutic transgenes for gene therapy. The aim of the present study was to determine patterns of tropism exhibited by pseudotyped lentiviral vectors in the rat substantia nigra, in order to evaluate their utility for gene transfer in experimental models of Parkinson’s disease. Isogenic lentiviral vector particles encoding a GFP reporter were pseudotyped with envelope glycoproteins derived from vesicular stomatitis virus (VSV), Mokola virus (MV), lymphocytic choriomeningitis virus (LCMV), or Moloney murine leukemia virus (MuLV). Adult male Lewis rats received unilateral stereotactic infusions of vector into the substantia nigra; three weeks later, patterns of viral transduction were determined by immunohistological detection of GFP. Different pseudotypes gave rise to transgene expression in restricted and distinct cellular populations. VSV and MV pseudotypes transduced midbrain neurons, including a subset of nigral dopaminergic neurons. In contrast, LCMV and MuLV-pseudotyped lentivirus produced transgene expression exclusively in astrocytes; the restricted transduction of astroglial cells was not explained by the cellular distribution of receptors previously shown to mediate entry of LCMV or MuLV. These data suggest that pseudotyped lentiviral vectors will be useful for experimental gene transfer to the rat substantia nigra. In particular, the availability of neuronal and astrocytic-targeting vectors will allow dissociation of cell-autonomous and cell non-autonomous functions of key gene products in vivo.
Keywords: lentivirus, pseudotype, vesicular stomatitis virus, mokola virus, lymphocytic choriomeningitis virus, moloney murine leukemia virus, tropism, substantia nigra, Parkinson’s disease
1. Introduction
Parkinson’s disease (PD) is a common, sporadic, late-onset neurodegenerative disease, characterized pathologically by loss of discrete populations of cerebral neurons, including dopaminergic neurons of the substantia nigra (Forno, 1996). The resulting cardinal motor signs of bradykinesia, rigidity and tremor can be ameliorated by using drugs that augment striatal dopamine production or directly stimulate dopamine receptors. However, currently available treatments do not prevent or slow disease progression, and there is an urgent, and presently unmet, need for effective neuroprotective agents. There has been significant recent progress in determining the molecular basis of neurodegeneration in PD. Rare Parkinsonism phenocopies are caused by mutations in genes encoding α-synuclein, leucine-rich receptor kinase 2, Parkin, pten-induced kinase 1 (PINK1) and DJ1(Bonifati, et al., 2003, Kitada, et al., 1998, Paisan-Ruiz, et al., 2004, Polymeropoulos, et al., 1997, Valente, et al., 2004, Zimprich, et al., 2004). Although mutations in these genes account for a minority of cases of PD, it is possible that elucidation of the abnormal biochemical processes underlying these familial forms of Parkinsonism will identify pathogenic mechanisms that are shared with the common sporadic form of PD. There is consequently considerable interest in understanding the roles of these genes in vivo, particularly with regard to how they may inform on the mechanisms underlying selective vulnerability of dopaminergic neurons in PD, but also in relation to how the relevant gene products interact with environmental influences thought important in the pathogenesis of sporadic PD, for example pesticide exposure (Betarbet, et al., 2000, Costello, et al., 2009, Ritz, et al., 2009). Methods for reliable experimental manipulation of gene expression in the substantia nigra may therefore yield valuable insights into the mechanisms underlying neurodegeneration in PD, in addition to providing a means for testing putative therapeutic approaches by effecting the ectopic expression of molecules such as growth factors or antioxidants.
Direct gene transfer to the brain in vivo offers some advantages for these experimental applications. First, genetic manipulation is not restricted to organisms in which germline transgenic and gene inactivation technologies are applicable, broadening the range of animal models that can be subjected to these analyses. Second, gene transfer in adult animals circumvents the possibility of compensatory developmental changes altering observed phenotypes. Third, targeted alterations in gene expression allow elucidation of the neuroanatomical basis of behavioral phenotypes without confounding effects of widespread alterations in gene expression. Finally, gene expression changes that appear neuroprotective in models of sporadic PD could be developed as possible gene therapy approaches. The most effective gene delivery vehicles in the CNS in vivo are recombinant virus vectors, in which viral genes mediating pathogenic functions are replaced with transgene sequences of interest (Burton, et al., 2005). A variety of viral vectors has been used to deliver transgenes to the substantia nigra in vivo. Of these, vectors based on adeno-associated virus (AAV) have received particular attention, because they show impressive tropism for nigral dopamine neurons, and the parent virus does not cause disease in humans. AAV vectors have been used recently in Parkinson’s disease clinical gene therapy trials (Feigin, et al., 2007, Kaplitt, et al., 2007, Marks, et al., 2008). However, the limited capacity for the insertion of heterologous genetic sequences into the AAV genome precludes the use of AAV-based vectors for applications demanding delivery of large transgene sequences. Lentiviral-based vectors can accommodate larger transgene sequences (up to ≈8kb). The utility of the HIV-1 based lentivirus vectors commonly used experimentally has been greatly enhanced by pseudotyping, in which the endogenous viral envelope proteins are replaced by envelope proteins from other viruses, altering the tropic range of the vectors. For example, the native envelope glycoproteins of HIV-1 bind to cell surface receptors present on lymphocytes, and impart specific viral tropism for these cells in the pathogenesis of AIDS; replacement of these envelope proteins with the vesicular stomatitis virus G-protein allows the resulting vectors to transduce many different types of cells. A range of lentiviral pseudotypes has been described, including envelope proteins derived from natively neurotropic viruses. A systematic evaluation of the relative utility of these pseudotyped vectors for gene delivery to the substantia nigra has not yet been reported. Here we show that the type of cell, neuronal or astrocytic, transduced by lentiviral vectors in the substantia nigra in vivo can be altered by pseudotyping the particles with envelope proteins derived from different viruses.
2. Materials and Methods
Unless otherwise noted, all chemical supplies were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.1 Viral vectors
Lentiviral vectors expressing green fluorescent protein (GFP) were prepared by Penn Vector Core (University of Pennsylvania, Philadelphia, PA). The gene transfer plasmid pHR-EGFP contains HIV-1 LTRs/packaging signals and a cassette in which the immediate early promoter from cytomegalovirus drives GFP expression (Kobinger, et al., 2001). Vector particles were prepared as described (Kobinger, et al., 2001), by transient transfection of HEK293T cells with: (i) pHR-EGFP; (ii) packaging plasmid pCMVΔR8.2 encoding viral genes, except for envelope glycoproteins (Naldini, et al., 1996); and (iii) a plasmid encoding envelope glycoproteins derived from vesicular stomatitis virus (VSV; pMD.G (Naldini, et al., 1996)), Mokola virus (MV; pLTRMVG (Mochizuki, et al., 1998)), lymphocytic choriomeningitis virus (LCMV; pHCMV-LCMV-GP(WE-HPI) (Beyer, et al., 2002)) or Moloney murine leukemia virus (MuLV; pHIT456 (Kobinger, et al., 2001)). Vector particles were harvested from culture supernatants, filtered and concentrated by ultracentrifugation, as described (Watson, et al., 2002). For infectious titer determination, HEK293T cells were transduced with limiting dilutions of the concentrated vector suspension and the number of proviral DNA genomes determined by qPCR using a primer and probe set corresponding to the 5’ non-coding region downstream of the 5’ LTR. Titers were: VSV, 1.1 × 1011 IU/mL; MV, 7.57 × 109 IU/mL; LCMV, 1.03 × 109 IU/mL; MuLV, 2.50 × 109 IU/mL.
2.2 Surgery
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and in accordance with National Institutes of Health guidelines. Adult male Lewis rats (4 per pseudotype; Hilltop Lab Animals, Inc., Scottdale, PA, USA) were anesthetized with isofluorane and placed in a stereotactic frame. The scalp was shaved and a longitudinal incision was made. The dorsal surface of the skull was then exposed and a burr hole was drilled above the infusion site. Using a specialized infusion pump (UMP2; Sarasota, FL, USA) 2 μL of vector was infused slightly dorsal to the pars compacta region of the substantia nigra (-5.2 A/P, 2.0 R/L, -7.5 mm V to the bregma) according to the Paxinos and Watson rat brain atlas (Paxinos and Watson, 1998). A 10 μL Hamilton syringe with a 30 gauge needle was used for the infusion (Hamilton Co., Reno, NV, USA). The infusion rate was 0.2 μL/min and the needle remained in place for 5 minutes after the infusion for vector absorption. Bone wax was then applied to the burr hole and the site was stitched closed. 21 days after vector infusion, animals were anesthetized with pentobarbital (50 mg/kg; Hospira Inc., Deerfield, IL, USA) and perfusion fixed with ~50 mL ice-cold 0.1 M PBS, followed by ~250 mL 4% paraformaldehyde. The brain was removed, post-fixed overnight and cryoprotected in 30% sucrose. Brains were then sectioned coronally on a freezing microtome at thickness of 35 μM.
2.3 Immunohistology
The following primary antibodies were used: mouse anti-green fluorescent protein (GFP) (1:4,000, MAB3580, Millipore, Billerica, MA, USA); sheep anti-tyrosine hydroxylase (TH) (1:2,000, AB1542, Millipore); rabbit anti-ionized calcium binding adaptor molecule 1 (Iba-1) (1:25,000, 019-19741, Wako Chemicals USA, Inc., Richmond, VA, USA); rabbit anti-glial fibrillary acidic protein (GFAP) (1:500, Z0334, DakoCytomation, Denmark), rabbit anti-S100β (1:20,000, #37, Swant, Bellinzona Switzerland), mouse anti-α-dystroglycan (1:1,000, 05-593, Millipore); mouse anti- α-dystroglycan (1:100, HM5010, Hycult Biotech, distributed by Cell Sciences, Canton, MA, USA), mouse anti-acetylglucosaminyl-transferase-like protein (LARGE) (1:500, sc-33435, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), protein O-mannosyltransferase 1 (POMT-1) (1:2,000, sc-98908, Santa Cruz Biotechnology, Inc.), rabbit anti-cationic amino acid transporter-1 (CAT-1) (1:1,000, sc-66825, Santa Cruz Biotechnology, Inc.), rabbit anti- CAT-1 (1:2,000, 14195-1-AP, Protein Tech. Group, Inc., Chicago, IL, USA). For chromagenic development, sections were treated with 3% hydrogen peroxide, blocked in 10% normal donkey serum, 0.3% triton x100, PBS, then incubated in the primary antibody solution, containing 1% normal donkey serum and 0.3% triton x100 at 4°C. Biotinylated secondary antibodies (1:200; 715-065-151 or 711-065-152; Jackson ImmunoResearch, West Grove, PA, USA) and Advidin-Biotin Complex solution (Vectastain, Vector Labs; Burligame, CA, USA) were used to detect bound primary antibodies using a DAB substrate reaction (sk-4100, Vector Labs, Burligame, CA, USA). Indirect immunofluorescence was carried out similarly, except that hydrogen peroxide treatment was omitted, and alexa488 donkey anti-mouse (1:500; A21208; Invitrogen Corp., Carlsbad, CA, USA), Cy3 donkey anti-sheep, Cy3 donkey anti-rabbit, or Cy5 donkey anti-mouse (1:500; 713-165-003, 711-165-152, or 711-175-152; Jackson ImmunoResearch) secondary antibodies were used.
2.4 Image acquisition
Images of chromagenically stained sections were acquired by light microscopy. Immunofluorescence images were acquired using a confocal microscope; the acquisition settings were optimized over several sections and the settings at each magnification were maintained throughout acquisition. Any adjustments to intensity, brightness or contrast were made identically to every image in a series at a given magnification, except for GFP labeling in Figure 4, where intensity varied dramatically between pseudotypes and the goal was to simply show that microglial labeling was present in a region that had been transduced by the vector.
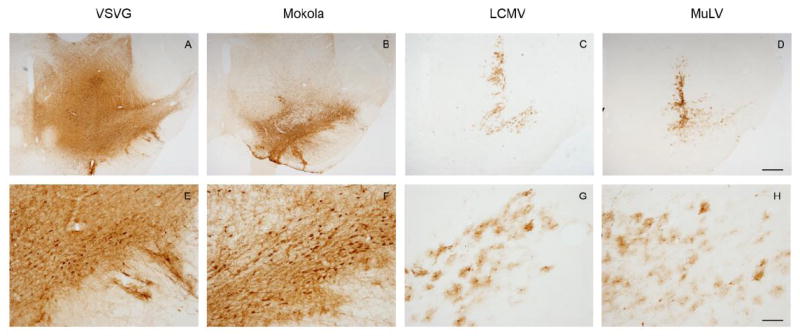
Figure 4. GFP transgene expression in the striatum following infusion of pseudotyped lentiviral vectors into the substantia nigra.
Light micrographs of coronal forebrain sections are shown, 21 days after intranigral infusion of lentiviral vectors pseudotyped with envelope proteins derived from VSV (A, E), MV (B, F), LCMV (C, G), or MuLV (D, H). Low magnification images (A-D; bar = 500 μm) are shown for each pseudotype, in order to illustrate restricted expression of the GFP transgene within the striatum. High magnification images (E-H; bar = 50 μm) of the striatum illustrate the striking differences in striatal GFP expression between the different pseudotypes.
2.5. Estimation of maximal percent transduction of dopamine neurons after viral infusion
Immunofluorescence staining for dopaminergic (TH+) and transgene expressing (GFP+) cells was conducted as above. Confocal images obtained near the geometric center of the substantia nigra were acquired at 20x magnification from animals injected with either VSVG or MV. To estimate the maximal transduction efficiency, the number of TH+ neurons expressing GFP and the total number of TH+ neurons for each image were counted and used to determine the percentage of nigral dopamine neurons transduced.
2.6 Striatal dopamine terminal integrity
The integrity of striatal dopaminergic terminals on the virally infused side was assessed by fluorescence intensity of tyrosine hydroxylase positive terminal density. We have successfully used this method to quantify the loss of striatal dopamine terminals after toxin administration (Tapias, et al., 2010). For this experiment, 1 in 12 striatal sections were stained for fluorescence detection as described above, except donkey anti-mouse IR 800 (1:500, LI-COR Biosciences; Lincoln, NE, USA) was used as the secondary antibody. To quantify tyrosine hydroxylase immunoreactivity, sections from each animal were scanned at 800 nm on an Odyssey Infrared Imaging System (Licor Inc., Lincoln Nebraska, U.S.A.) at a resolution of 21 μm. Fluorescence intensity was adjusted below saturation. Using Odyssey Software, V 3.0, the dorsal striatum was selected (i.e., nucleus accumbens was excluded) and the average pixel intensity was obtained. For each animal, the average fluorescence intensity was obtained over 4-6 coronal sections on both the side receiving virus infusion and the uninfused side.
2.7. Statistical analysis
Transduction efficiency for VSV and MV was compared using a Student’s t-test. For striatal fluorescence intensity, the viral infused side was compared to the uninfused side by a paired Student’s t-test. For all experiments, p<0.05 was deemed significant.
3. Results
3.1 Transduced cells in the midbrain
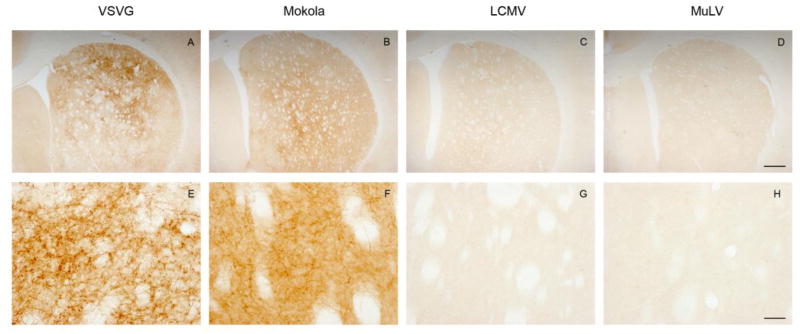
Twenty-one days after infusion of viral vectors into the midbrain, expression of the virally-encoded GFP reporter gene was examined by immunohistochemistry. GFP expression was evident in the midbrain of samples transduced by each of the vectors (Figure 1A-D and see below). Importantly, however, the morphology of transduced cells differed between pseudotypes. GFP-expressing cells from animals that received VSV-G or Mokola pseudotypes primarily showed neuronal morphology (figure 1E, F), whereas LCMV and MuLV pseudotypes produced transgene expression in cells with relatively small cell bodies and extensive processes, morphological characteristics highly suggestive of glial cells.
Figure 1. GFP transgene expression in the midbrain following infusion of pseudotyped lentiviral vectors into the substantia nigra.
Light micrographs of coronal midbrain sections are shown, 21 days after intranigral infusion of lentiviral vectors pseudotyped with envelope proteins derived from VSV (A, E), MV (B, F), LCMV (C, G), or MuLV (D, H). GFP expression was localized immunohistochemically, with a chromogenic substrate yielding a brown product. Low magnification images (A-D; bar = 500 μm) of sections are shown for each pseudotype, in order to illustrate the topographical distribution of transgene expression. High magnification images (E-H; bar = 100 μm) of the substantia nigra show the morphology of transduced cells.
Interestingly, the distribution of transduced cells also differed between pseudotypes. VSV and Mokola pseudotyped lentiviral vectors gave rise to widespread expression of GFP throughout the midbrain, including the pars compacta tier of the substantia nigra (figure 1A,B) and extending through the rostro-caudal extent of the nigra (figure 2), whereas GFP expression was more localized within the substantia nigra in animals that received LCMV or MuLV pseudotyped vectors (figure 1C, D) and dramatic decreases in GFP expression were observed anterior and posterior to the needle track. These differences in topological distribution of GFP expression may have been attributable to the differential tropism of the vectors in addition to variation of the in vitro titers between pseudotypes (see discussion).
Figure 2. GFP transgene expression extends throughout the midbrain after infusion of VSV or MV.
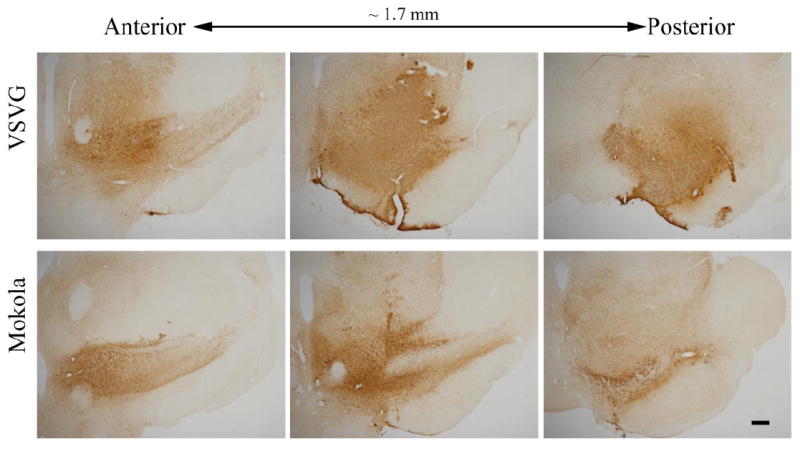
Light micrographs of serial coronal midbrain sections (1 in 24 sections) are shown, 21 days after intranigral infusion of lentiviral vectors. GFP expression was localized immunohistochemically, with a chromogenic substrate yielding a brown product. Low magnification images (bar = 500 μm) of sections are shown for each pseudotype, in order to illustrate the topographical distribution of transgene expression.
3.2 Immune response
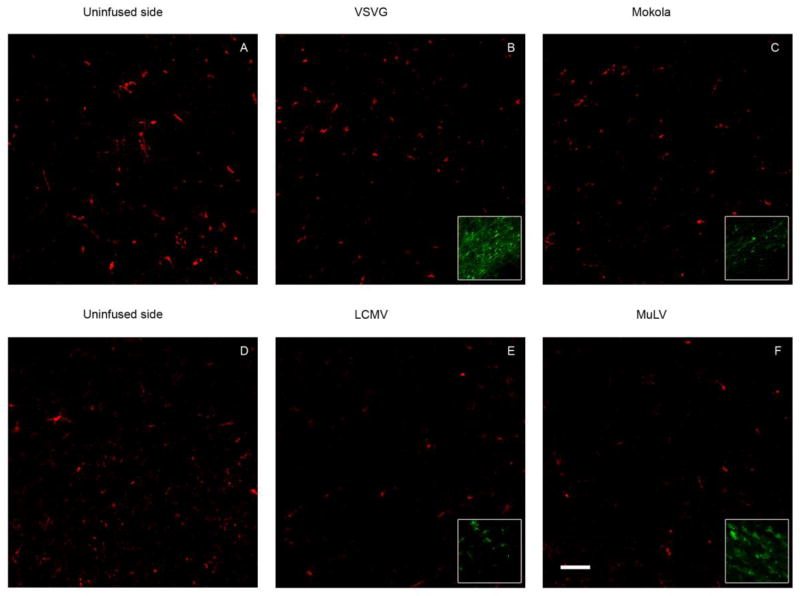
No evidence of tissue necrosis or acute inflammation was seen in any of the sections examined, with the exception of some microglial activation near the needle track. Immunohistochemistry for IBA-1, a marker for microglial cells, was used to assess samples for evidence of microglial proliferation, migration or activation. Microglial numbers and morphology were similar in both vector transduced regions and the uninfused control side (Figure 3). These data indicate that none of the pseudotyped lentiviral vectors elicited an observable immune response at the titers used and with the infusion parameters tested. Consequently the observed differences in patterns of transduction between pseudotypes were not attributable to major differences in host response to the different envelope proteins.
Figure 3. Pseudotyped lentiviral infusion does not induce a demonstrable immune response in the midbrain.
The images show confocal micrographs of the substantia nigra from animals that received intranigral infusions of VSV (B), MV (C), LCMV (E) or MuLV (F) pseudotyped lentiviral vectors 21 days previously (bar = 50 μm). Examples of images from the substantia nigra on the control (uninfused) side are shown for comparison (A, D). Sections were labeled by indirect immunofluorescence to detect IBA-1, a marker for microglial cells (red; A-F) and GFP in order to confirm that the IBA-1 images were obtained from regions that had been transduced by the vectors (green; inset in panels B, C, E, F). Images were acquired using identical confocal microscope settings for IBA-1 in order to allow direct comparison of the distribution and intensity of immunoreactivity. GFP images were individually adjusted in order to optimize illustration of vector transduction.
3.3 Transduction of dopaminergic neurons of the substantia nigra by VSV and MV pseudotyped vectors
Immunohistochemistry was employed in order to detect virally-encoded GFP in the striatum, which is densely innervated by the axons of ipsilateral nigral dopaminergic neurons. Following nigral infusion, both VSV and MV pseudotyped vectors gave rise to GFP expression in the striatum, indicating transduction of dopaminergic neurons forming the ascending nigrostriatal system (Figure 4A, B, E, F). LCMV and MuLV pseudotypes, however, produced no observable striatal transgene expression (Figure 4C, D, G, H), indicating that these vectors did not transduce dopaminergic neurons after infusion into the substantia nigra.
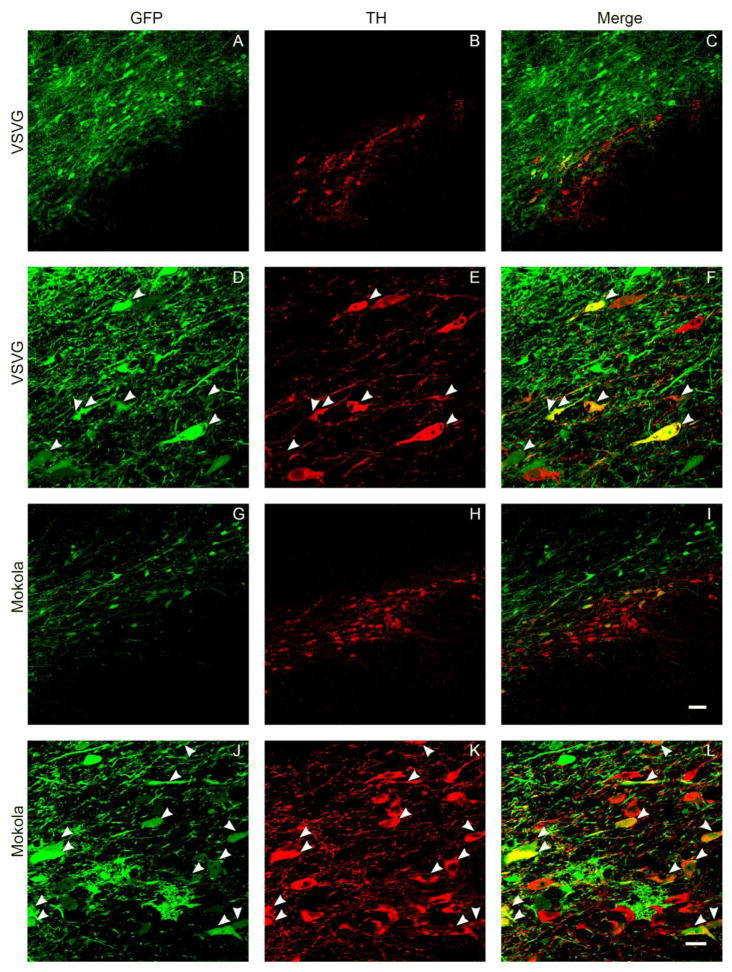
Double label indirect immunofluorescence was used in order to confirm co-localization of GFP and tyrosine hydroxylase (TH) in dopaminergic neurons of the substantia nigra after exposure to VSV and MV pseudotyped lentiviral vectors. At low magnification, GFP transgene expression was seen throughout the substantia nigra (Figure 5A-C, G-I). High power confocal images showed co-localization of GFP and TH in a substantial minority of TH-expressing cells (<50% TH+ cells in sections showing maximal transgene expression, see below), in addition to GFP expression in numerous other cells that did not express TH but showed neuronal morphology (Figure 5D-F, J-L). The maximal transduction efficiency, calculated as % of TH+ cells that were also GFP+ in sections showing maximal transduction, was similar between pseudotypes (47.5 ± 7.5 vs. 44.0 ± 13.0; p = 0.8; mean % dopamine neuron transduction ± S.E.M.; VSV vs. MV). These data indicate that VSV and MV pseudotyped lentiviral vectors transduced a subset of nigral dopaminergic neurons, in addition to other midbrain neuronal populations.
Figure 5. Neuronal expression of GFP following intranigral delivery of VSV or MV-pseudotyped lentivirus vectors.
The images show confocal micrographs of the substantia nigra from animals that received intranifral infusions of VSV (A-F) or MV (G-L) pseudotyped lentiviral vectors 21 days previously. Sections were labeled by indirect immunofluorescence in order to detect GFP (green; A, D, G, J) and tyrosine hydroxylase (red; B, E, H, K). Low magnification images (A-C, G-I; bar = 50 μm) illustrate the relative topographical distributions of transduced neurons and dopaminergic neurons. High magnification images (D-F, J-L bar = 20 μm) demonstrate examples of vector-transduced dopamine neurons, identified by co-expression of GFP and TH (arrowheads).
To determine whether viral transduction by VSV-G or MV pseudotyped vectors impaired the integrity of the nigrostriatal dopamine system, we determined the density of striatal dopaminergic terminals by quantitative infrared immunofluorescence to measure striatal tyrosine hydroxylase expression. Mean striatal TH immunofluorescence intensity (figure 6) on the side that received viral infusion was similar to the uninfused side for both VSV (703.3 ± 47.3 vs.749.9 ± 41.5; p = 0.3, paired Student’s t-test; average pixels ± S.E.M.; virus side vs. uninfused side) and MV pseudotypes (736.6 ± 133.5 vs. 778.0 ± 119.5; p = 0.2). These data show that there was no significant reduction in striatal TH expression following vector transduction of the nigra, and consequently exclude a significant depletion of dopaminergic terminals or loss of neurochemical phenotype resulting from transgene delivery using these vectors.
Figure 6. Viral transduction of dopaminergic neurons does not affect striatal tyrosine hydroxylase positive terminal density.
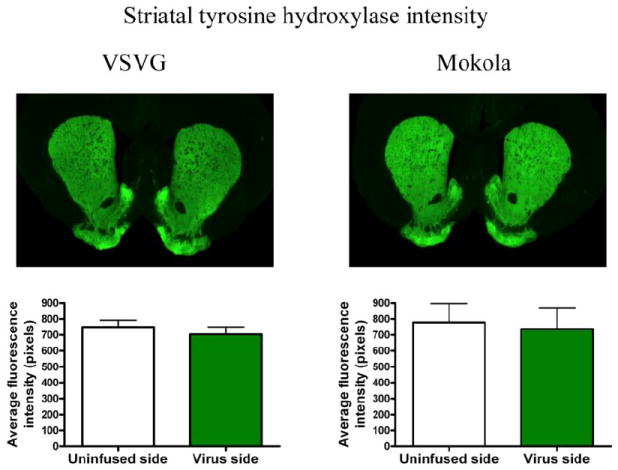
The intensity of TH immunoreactivity was determined in the striatum, ipsilateral and contralateral to nigral transduction, in the same sections by quantitative infrared immunofluorescence. Five sections were quantified from each animal that received VSV (n = 5) or MV (n = 4); the graphs show the mean striatal TH intensity (± SE) for control and transduced striatum. There were no statistically significant differences between the virally infused side and unifused side for either pseudotype.
3.4 Transduction of astrocytes by LCMV and MuLV pseudotyped vectors
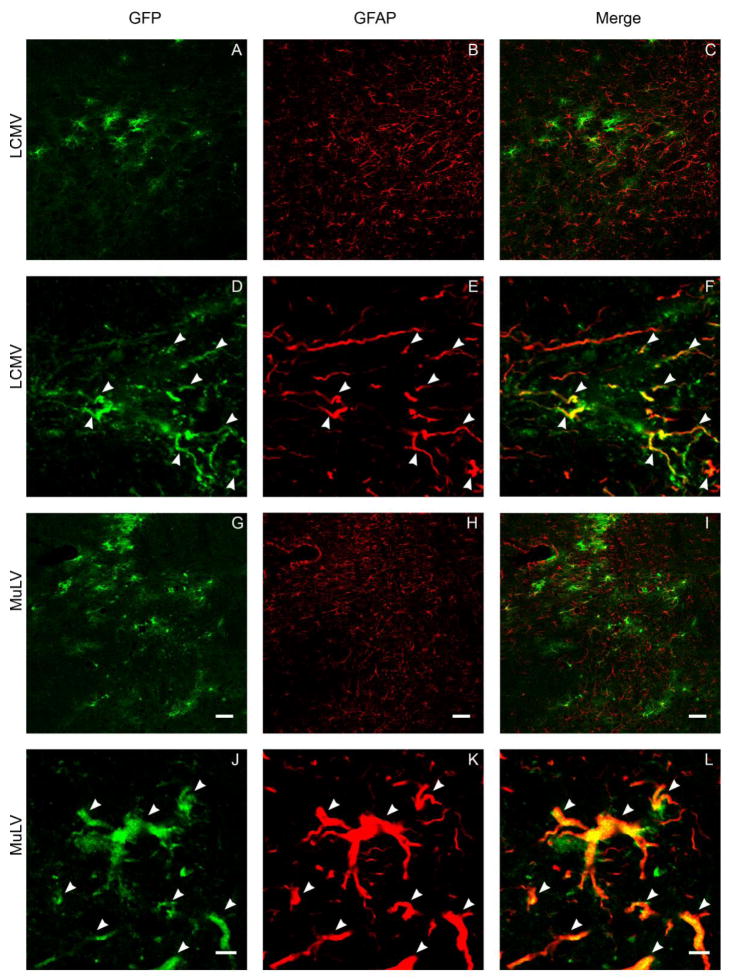
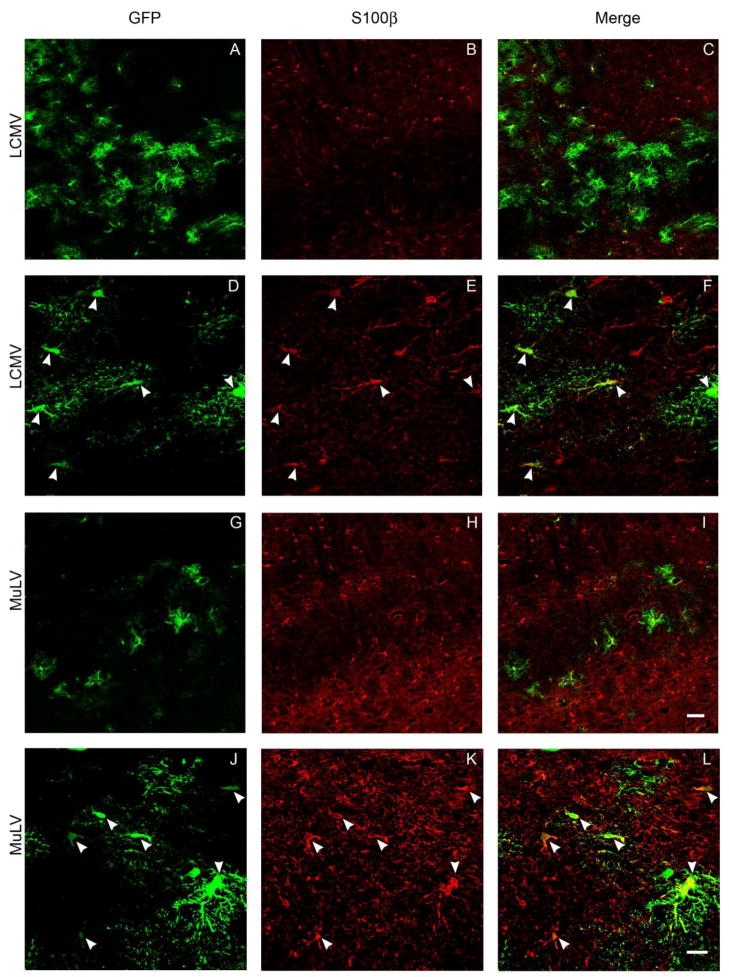
Following nigral infusion, LCMV and MuLV pseudotyped vectors gave rise to GFP transgene expression in cells with glial morphology. Double label indirect immunofluoresence was employed to detect the vector-encoded GFP transgene and markers of specific glial populations simultaneously, in order to clarify the identity of the transduced glial cells. After nigral infusion of either LCMV or MuLV pseudotyped lentivirus, GFP transgene expression was seen to colocalize with glial fibrillary acidic protein (GFAP) within the proximal processes of astrocytes (Figure 7) and S100β within the cell bodies of astrocytes (Figure 8). These data show that LCMV and MuLV pseudotyped lentiviral vectors specifically transduced astrocytes after nigral infusion. We next asked whether this striking pattern of glial tropism was explained by the distribution of receptors known to mediate entry of the viruses from which the envelope glycoproteins of the pseudotyped vectors were derived. LCMV cell entry is mediated by binding to α-dystroglycan, which requires a specific series of post-translational modifications in order to function as the LCMV receptor. MuLV enters cells following an initial binding event to the cationic amino acid transporter CAT-1. We hypothesized that CAT-1 and either α-dystroglycan or the enzymes involved in its post-translational modification, LARGE and POMT1, would exhibit restricted expression patterns on astroglial cells. However, immunohistochemistry using a panel of different primary antibodies raised against distinct epitopes of these proteins indicated that α-dystroglycan immunoreactivity was most prominently detected on blood vessels in the substantia nigra (figure 9A, D), and expression of CAT-1 (figure 9C, F), LARGE (figure 9B) and POMT1 (figure 9E) was most easily visualized on cells with neuronal morphology. Together, these data show that LCMV and MuLV pseudotyped lentiviral vectors show unexpectedly selective transduction of astrocytes in the substantia nigra, but this is not explained by the tissue distribution of receptors for the viruses from which the envelope proteins were derived.
Figure 7. GFP expression in the proximal processes of astrocytes following intranigral infusion of LCMV and MuLV-pseudotyped lentiviral vectors.
The images show confocal micrographs of the substantia nigra from animals that received intranigral infusions of LCMV (A-F) or MuLV (G-L) pseudotyped lentiviral vectors 21 days previously. Sections were labeled by indirect immunofluorescence in order to detect GFP (green; A, D, G, J) and glial fibrillary acidic protein (GFAP; red; B, E, H, K). Low magnification images (A-C, G-I; bar = 50 μm) illustrate the relative topographical distributions of transduced cells and astrocytes. High magnification images (D-F, J-L bar = 5 μm) demonstrate co-localization of GFP and GFAP in the proximal processes of individual astrocytes (arrowheads).
Figure 8. GFP expression in the cell bodies of astrocytes following intranigral infusion of LCMV and MuLV-pseudotyped lentiviral vectors.
The images show confocal micrographs of the substantia nigra from animals that received intranigral infusions of LCMV (A-F) or MuLV (G-L) pseudotyped lentiviral vectors 21 days previously. Sections were labeled by indirect immunofluorescence in order to detect GFP (green; A, D, G, J) and S100β (red; B, E, H, K). Low magnification images (A-C, G-I; bar = 50 μm) illustrate the relative topographical distributions of transduced cells and astrocytes. High magnification images (D-F, J-L bar = 20 µm) demonstrate co-localization of GFP and S100β in the cell bodies of individual astrocytes (arrowheads).
Figure 9. Expression of proteins involved in cellular entry of LCMV and MuLV in the rat substantia nigra.
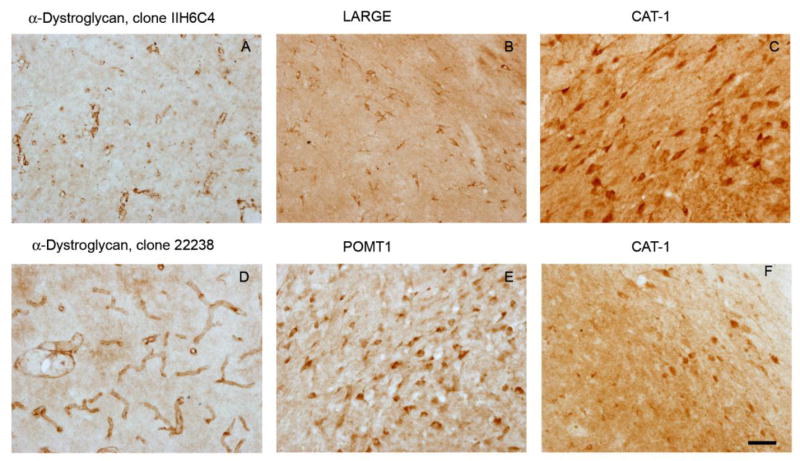
The images show light micrographs of rat substantia nigra (bar = 50 μm) labeled by immunohistochemistry with a chromogenic reaction yielding a brown product in oder to localize proteins implicated in cellular entry of LCMV (α-dystroglycan, A, D; LARGE, B; POMT1, E) or MuLV (CAT-1, C, F). Two independent antibodies for each antigen confirmed the observed expression patterns of α-dystoglycan (clones IIH6C4 A and 2238 D) and CAT-1 (Santa Cruz, C; Protein Tech, F).
4. Discussion
The original report of VSV pseudotyped lentiviral vectors showed transduction of both neurons and glial cells in the hippocampus and striatum of adult mice (Naldini, et al., 1996). VSV pseudotyped lentiviral vectors have also been shown to transduce cells in the murine substantia nigra, although previous studies have reached differing conclusions regarding transduction of dopaminergic neurons. Transgene expression was found in both neurons and astrocytes in the mouse substantia nigra, with transduction of approximately 20% of nigral dopaminergic neurons (Bensadoun, et al., 2000). In the rat, transduction of up to 40% substantia nigra dopaminergic neurons was reported in one study (Deglon, et al., 2000). However, another study showed significant inefficiency in transducing neurons of the pars compacta region of the rat substantia nigra using VSV-pseudotyped vectors (Rosenblad, et al., 2000); lentiviral infusion into the striatum resulted in predominantly neuronal transduction, whereas infusion into the substantia nigra resulted in little transduction in the pars compacta, but transgene expression in both astrocytes and neurons in surrounding areas. The reasons for these apparently discordant findings are unclear, although inter-species differences and variation in techniques for preparation and infusion of vector may have contributed. Our data are broadly compatible with previous reports, and show that intranigral infusion of VSV-pseudotyped lentivirus results in transgene expression in neurons, including a subset (< 50%) of nigral dopamine neurons.
Other lentiviral pseudotypes have not been studied in the rat substantia nigra previously, and the primary aim of this work was to characterize their patterns of cellular transduction and to determine whether they present any specific advantages over VSV-pseudotyped vectors for gene delivery to dopaminergic neurons. Mokola-pseudotyped lentiviral particles have been reported to be highly neurotropic in the mouse striatum, olfactory tubercles (Desmaris, et al., 2001), and hippocampus (Watson, et al., 2002). However, non-neuronal cell types have been transduced by Mokola-pseudotyped vectors, including cells of the corpus callosum (Watson, et al., 2002), retinal pigment epithelium (Auricchio, et al., 2001) and myocytes (MacKenzie, et al., 2002). The present study is the first to directly assess the tropism of Mokola-pseudotyped lentiviruses following intranigral infusion in the rat. The pattern of reporter gene expression that we observed was very similar to that seen after infusion of VSV-pseudotyped lentivirus; most transduced cells showed neuronal morphology, but there was transduction of only a fraction of nigral dopaminergic neurons. Since it is straightforward to prepare high-titer vector stocks pseudotyped with the G envelope protein of VSV, we conclude that Mokola-pseudotyped vectors may not confer any advantage for gene transfer to the rat substantia nigra.
In the mouse brain in vivo, LCMV pseudotyped lentivirus vectors transduced cells within the white matter after striatal infusion, and showed limited neuronal transduction after infusion near the hippocampus, whereas MuLV-pseudotyped lentivirus transduced striatal cells (cell type undetermined) and granule neurons of the hippocampus after hippocampal infusion (Watson, et al., 2002). Neither pseudotype has been previously studied in the rat substantia nigra. Unexpectedly, we found that both LCMV- and MuLV-pseudotyped vectors showed selective transduction of astrocytes in the rat substantia nigra. The parent LCM virus from which the envelope glycoprotein was derived enters cells by binding to a modified form of α-dystroglycan (Cao, et al., 1998, Spiropoulou, et al., 2002); glycosylation by the enzyme LARGE and O-mannosylation mediated by the enzyme POMT1 have been shown to be critical for viral entry in vitro (Imperiali, et al., 2005, Kunz, et al., 2005), although the dependence of viral entry on O-mannosylation is weaker in vivo (Imperiali, et al., 2008). MuLV cell entry occurs after initial binding of envelope protein to the cell surface cationic amino acid transporter CAT-1 (Albritton, et al., 1989, Kim, et al., 1991). We hypothesized that the selective transduction of astrocytes by LCMV- and MuLV-pseudotyped lentivirus vectors was attributable to cell-specific expression of one or more of these proteins. However, an immunohistochemical study, carried out using multiple different antibodies to localize these antigens in the substantia nigra, did not provide any evidence to support this hypothesis, leading us to conclude that the pattern of transgene expression after intranigral infusion of either LCMV- or MuLV-pseudotyped lentivirus vectors is not explained by the distribution of cellular receptors for LCMV or MuLV. This suggests that the processes mediating cell entry of LCMV- or MuLV-pseudotyped lentiviral vectors may differ from the mechanisms by which LCMV and MuLV enter cells, or that the cellular receptors for the relevant envelope glycoproteins vary between species. An alternative explanation involving cell-type specific modulation of post-entry mechanisms, for example reverse transcription or nuclear import, is not favored by the striking differences in tropism shown by vectors that were identical except for their envelope glycoproteins.
The observed differences in topographical distribution of the transgene product were most likely attributable to the morphology of the neuronal and glia cells for which the vectors showed differential tropism. VSV-G and MV vectors gave rise to widespread GFP expression by transduction of neurons and subsequent diffusion of GFP within their axonal projections and dendritic arborizations. In contrast, the relatively compact morphology of astrocytes caused localized GFP expression after transduction by MuLV and LCMV pesudotyped vectors. In keeping with this explanation, GFP expression in regions remote from the vector infusion site, such as the striatum, occurred only after VSV or MV transduction, and was exclusively seen in axonal terminals rather than cell bodies at these locations. Factors that might have affected vector distribution after delivery, such as infusion parameters and volumes, were identical between different groups, although the vectors were administered at a wide range of titers, which may have contributed to the topographical transduction patterns noted. We did not attempt to match titers between groups of several reasons. First, the purpose of this study was to determine whether pseudotyped vector stocks manufactured using available preparation techniques conferred any specific advantage with respect to one another for gene delivery to the rat nigra. Since vector preparation can be a limiting factor for these studies, the differences in titer obtained by standard techniques constituted an important part of the comparison we carried out. Second, there is no unequivocally valid way to match the titers of these vectors for in vivo experiments, because no technique gives a measurement of the number of transduction-competent particles that is universally applicable in different experimental settings. The titers of the vector stocks were determined in vitro by proviral genome counts after transduction of human 293T cells. Since the vectors have different envelope glycoproteins, which confer different entry kinetics into different cell types (confirmed by our in vivo data), it is uncertain that the in vitro titers are an appropriate index by which to compare in vivo doses. Other measures, such as particle count and ELISA for virion proteins do not distinguish defective particles, the proportion of which likely also differs between pseudotypes. Consequently, we elected to use constant volumes from preparations that were made by a standard technique. In order to ensure that the differences in titer did not affect the cellular tropism, we carried out dilution experiments in mixed primary neuronal/astrocytic cultures. These experiments (not shown) confirmed that neuronal-specific transduction by VSV-psuedotyped vector persisted at a 100-fold dilution that matched the in vitro titer of the LCMV stock. We conclude that neuronal tropism is a function of the envelope glycoprotein rather than the titer.
In this study, we did not find evidence of overt toxicity from vector infusion. Microglial morphology was unaltered, dopamine neuron morphology and striatal dopamine terminals were preserved, and no behavioral symptoms indicative of dopamine neuron loss were observed up to 21 days after viral infusion. It is possible that minor neuroinflammation occurred at earlier time-points and was not apparent by the time point at which we examined the samples. It will be important to address this issue in future experiments utilizing transgene delivery as a potential neuroprotective intervention in experimental models, since neuroinflammation could act as a preconditioning stimulus that could confound results. This potential issue should be addressed for future experiments evaluating neuroprotective interventions, by use of a paradigm in which bilateral infusion allows comparison of test and control (isogenic except for the test transgene) vectors in the same animal.
The distinct patterns of tropism exhibited by these pseudotyped vectors expands the range of molecular tools available for inducing cell-type specific changes in gene expression in experimental rat models of Parkinson’s disease. It has become apparent that critical functions of proteins involved in PD pathogenesis may take place in non-neuronal cell populations. For example, recent work has shown that DJ-1 is upregulated in astrocytes during the pathogenesis of neurodegeneration in vivo, and that astrocyte-mediated neuroprotective effects in vitro are abrogated by DJ-1 knockdown and augmented by over-expression of DJ-1 (Mullett, et al., 2009, Mullett and Hinkle, 2009). Delivery of a DJ-1 expression cassette or DJ-1 gene-targeting shRNA in vivo could be directed to astrocytes or neurons using these vector pseudotypes, in order to elucidate the respective roles of astrocytic or neuronal functions of DJ-1 in the pathogenic mechanisms underlying experimental rat models of PD. Since recent findings highlight a critical role for the internal environment of the brain in the pathogenesis of PD (Kordower, et al., 2008, Mendez, et al., 2008), the characterization of readily available tools enabling the dissociation of cell autonomous and non-autonomous functions of proteins in the pathogenesis neurodegeneration will be of significant utility.
Acknowledgments
We thank Paras Minhas at the University of Pittsburgh for help with the histology. We also thank Dr. Arbans Sandhu and Dr. Julie Johnston at the Penn Vector core for assistance with the viral vectors. This work was supported by the American Parkinson Disease Association, NINDS grant 1P01NS059806, and VA grant 1I01BX000548.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albritton LM, Tseng L, Scadden D, Cunningham JM. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM, Wilson JM, Bennett J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 3.Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P. Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson’s disease using GDNF. Exp Neurol. 2000;164:15–24. doi: 10.1006/exnr.2000.7409. [DOI] [PubMed] [Google Scholar]
- 4.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 5.Beyer WR, Westphal M, Ostertag W, von Laer D. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J Virol. 2002;76:1488–1495. doi: 10.1128/JVI.76.3.1488-1495.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 7.Burton EA, Fink DJ, Glorioso JC. Vectors and Gene Therapy. In: Meyers RA, editor. Encyclopedia of Molecular and Cell Biology and Molecular Medicine. Wiley, Winheim; 2005. pp. 233–286. [Google Scholar]
- 8.Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 9.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L, Zufferey R, Trono D, Aebischer P. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson’s disease. Hum Gene Ther. 2000;11:179–190. doi: 10.1089/10430340050016256. [DOI] [PubMed] [Google Scholar]
- 11.Desmaris N, Bosch A, Salaun C, Petit C, Prevost MC, Tordo N, Perrin P, Schwartz O, de Rocquigny H, Heard JM. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther. 2001;4:149–156. doi: 10.1006/mthe.2001.0431. [DOI] [PubMed] [Google Scholar]
- 12.Feigin A, Kaplitt MG, Tang C, Lin T, Mattis P, Dhawan V, During MJ, Eidelberg D. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:19559–19564. doi: 10.1073/pnas.0706006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Imperiali M, Sporri R, Hewitt J, Oxenius A. Post-translational modification of {alpha}-dystroglycan is not critical for lymphocytic choriomeningitis virus receptor function in vivo. J Gen Virol. 2008;89:2713–2722. doi: 10.1099/vir.0.2008/004721-0. [DOI] [PubMed] [Google Scholar]
- 15.Imperiali M, Thoma C, Pavoni E, Brancaccio A, Callewaert N, Oxenius A. O Mannosylation of alpha-dystroglycan is essential for lymphocytic choriomeningitis virus receptor function. J Virol. 2005;79:14297–14308. doi: 10.1128/JVI.79.22.14297-14308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW, Closs EI, Albritton LM, Cunningham JM. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- 18.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 19.Kobinger GP, Weiner DJ, Yu QC, Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- 20.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 21.Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MB. Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 2005;79:14282–14296. doi: 10.1128/JVI.79.22.14282-14296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S, Wilson JM, Verma IM, Flake AW. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther. 2002;6:349–358. doi: 10.1006/mthe.2002.0681. [DOI] [PubMed] [Google Scholar]
- 23.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 24.Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki H, Schwartz JP, Tanaka K, Brady RO, Reiser J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol. 1998;72:8873–8883. doi: 10.1128/jvi.72.11.8873-8883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullett SJ, Hamilton RL, Hinkle DA. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology. 2009;29:125–131. doi: 10.1111/j.1440-1789.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 27.Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 29.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 31.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 32.Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenblad C, Gronborg M, Hansen C, Blom N, Meyer M, Johansen J, Dago L, Kirik D, Patel UA, Lundberg C, Trono D, Bjorklund A, Johansen TE. In vivo protection of nigral dopamine neurons by lentiviral gene transfer of the novel GDNF-family member neublastin/artemin. Mol Cell Neurosci. 2000;15:199–214. doi: 10.1006/mcne.1999.0817. [DOI] [PubMed] [Google Scholar]
- 34.Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapias V, Cannon JR, Greenamyre JT. Melatonin treatment potentiates neurodegeneration in a rat rotenone Parkinson’s disease model. J Neurosci Res. 2010;88:420–427. doi: 10.1002/jnr.22201. [DOI] [PubMed] [Google Scholar]
- 36.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 37.Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther. 2002;5:528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- 38.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]