Abstract
The lack of appropriate animal models has hampered efforts to develop therapies for Duchenne muscular dystrophy (DMD). A new mouse model lacking both dystrophin and telomerase (Sacco et al., 2010) closely mimics the pathological progression of human DMD and shows that muscle stem cell activity is a key determinant of disease severity.
Duchenne muscular dystrophy (DMD) is among the most common inherited disorders and is characterized by progressive muscle wasting leading to loss of ambulation and premature death. DMD and other forms of muscular dystrophy are the focus of intensive efforts to develop treatments, as current interventions have only a minor impact on disease progression. Testing of experimental therapies has centered on various strains of dystrophin mutant (mdx) mice. Despite sharing the same genetic defect as DMD patients (mutations in the dystrophin gene) mdx mice have a slowly progressive muscle pathology which, except for the diaphragm muscle, does not lead to the widespread formation of fibrous connective tissue and loss of muscle mass that characterize the human disease. Unlike DMD patients, mdx mutant mice do not lose the ability to walk and display only a 20% reduction in lifespan (Chamberlain et al., 2007). Although studies of mdx mutant mice have led to important advances in our understanding of the pathophysiology of dystrophy and have allowed development of several promising approaches to therapy (Muir and Chamberlain, 2009), the slowly progressive phenotype has prompted efforts to create an mdx model that more closely resembles the human disorder. Sacco et al. (2010) report in this issue a new mdx model that lacks telomerase (mTR) activity, and therefore shows a reduction in the regenerative capacity of myogenic stem cells. These mdx/mTR double knockout mice display a more severe, rapidly progressing phenotype and a greatly reduced lifespan. This new strain could enhance our understanding of the mechanisms of disease progression in DMD, while providing a new platform for testing genetic, stem cell and pharmacologic approaches for treating DMD.
Dystrophin, the protein product encoded by the DMD locus, links the internal muscle cytoskeleton to the extracellular matrix, enabling lateral transmission of the forces of contraction from within muscle cells to the surrounding matrix. Loss of dystrophin renders multinucleated skeletal muscle cells, known as myofibers, susceptible to exercise-induced injury. Injury results in necrosis of segments of the myofiber, triggering a cycle of degeneration and regeneration, the latter of which is supported by myogenic stem cells (Figure 1). Earlier efforts to generate a mouse DMD model with a more severe phenotype led to the creation of a dystrophin:utrophin double knockout mouse. The severe pathology in these mice results from the loss of expression of the dystrophin paralog utrophin, which can compensate for the lack of dystrophin in muscle (Deconinck et al., 1997). However, DMD patient muscles do express utrophin and as such this mouse model does not fully mimic the human disorder. In the past year an mdx double mutant mouse involving cytidine monophosphate–sialic acid hydroxylase (Cmah) was generated that appears to more closely resemble the human DMD pathophysiology at both a phenotypic and molecular level. The Cmah/mdx double knockout mouse carries a human-specific mutation that prevents protein glycosylation with N-glycolylneuraminic acid (Neu5Gc), leading to a more severe phenotype and shortened lifespan (Chandrasekharan et al., 2010). It is unclear how the inability of cells to incorporate the sialic acid Neu5Gc onto glycoproteins and glycolipids leads to a more severe dystrophy, but this change presumably alters the integrity of myofiber plasma membranes (the sarcolemma; Figure 1). Sacco et al. now create an mdx/mTR double mutant to test the hypothesis that murine muscles have increased regenerative capacity relative to human muscles due to the longer telomeres found in mouse relative to human myogenic stem cells (Figure 1). Thus a greater regenerative capacity in mouse muscles may contribute to the relatively mild phenotype of mdx mutant mice.
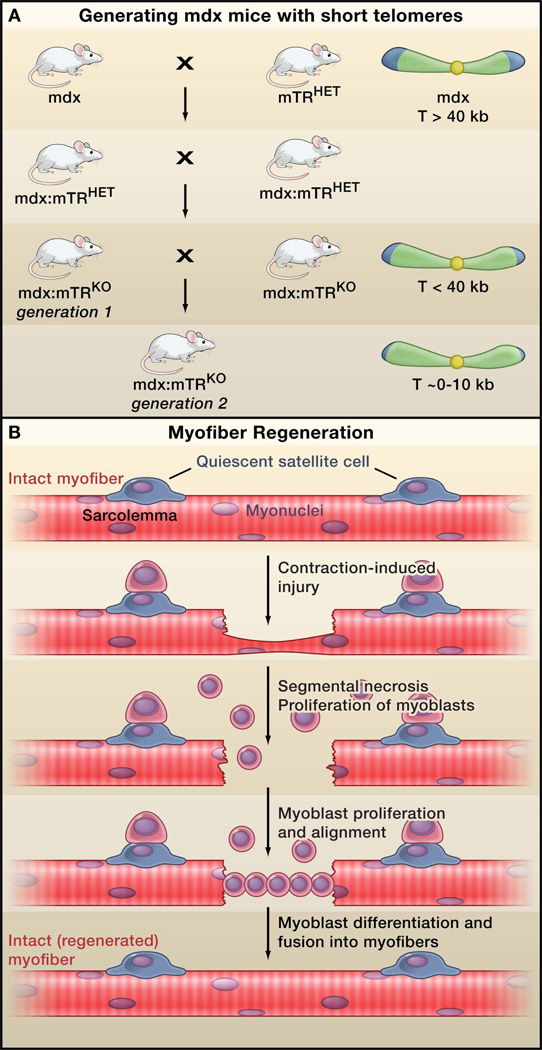
Figure.
New model for Duchenne muscular dystrophy (DMD). (A) Sacco et al. (2010) generate a new mouse model for DMD by crossing dystrophin-deficient mdx mice (the current DMD model) with mice carrying a mutation in the telomerase gene (mTRHET mice). Inbreeding of the resulting heterozygotes (mdx/mTRHET) produces double knockout mice (mdx/mTRKO) that show decreasing telomere length (T) in the first and second generations, and a corresponding decrease in the capacity of myogenic stem cells (satellite cells) to proliferate. These mice display a rapidly progressing muscular dystrophy phenotype that more closely mimics the human disease compared to the current model. Note that mdx is an X-linked gene. For simplicity, the complete mdx genotype is not depicted in the figure. (B) Dystrophic muscles are highly susceptible to contraction-induced injury, which impairs the protective sheath around myofibers (the sarcolemma), leading to myofiber necrosis and activation of nearby satellite cells. Activated satellite cells divide asymmetrically, giving rise to one satellite cell, and a proliferating daughter cell that generates myoblasts. The myoblasts migrate to the site of injury, differentiate into myocytes, align and fuse into the damaged myofiber, repairing the lesion.
The longer telomeres in murine somatic cells relative to humans greatly reduce their propensity to undergo replicative senescence, i.e. lose the ability to enter mitosis (Blasco et al., 1997; Kipling and Cooke, 1990). Previous studies have also shown that loss of telomerase, the enzyme that extends telomeres, can accelerate a number of pathophyiological changes associated with aging in mice (Rudolph et al., 1999). However telomerase deficient mice do not show abnormalities of muscle, probably due to the stable post-mitotic nature of this tissue. By crossing a telomerase mutation onto the dystrophic mdx background Sacco et al. create a situation where myogenic stem cells carrying shortened telomeres (due to loss of telomerase) are forced into active and continuous proliferation to counter the ongoing cycles of myofiber necrosis and regeneration (due to loss of mdx) (Fig. 1). These mutations may therefore create conditions in mice that mimic the human DMD phenotype.
Muscle regeneration requires the activation and proliferation of resident stem cells known as satellite cells (Collins et al., 2005). Sacco et al. demonstrate a marked decrease in the ability of activated satellite cells from mdx/mTR mice to proliferate and support regeneration in vitro, in vivo in dystrophic mdx/mTR muscles or when transplanted into wild type or dystrophic muscles. With their reduced regenerative capacity, these double mutant mouse satellite cells more closely resemble human DMD satellite cells, and as a result the mdx/mTR mice display an accelerated onset and progression of the dystrophic phenotype, more akin to the human disease. The disease model created by Sacco et al. thus suggests that reduced regenerative capacity in humans (relative to mice) may explain at least some of the differences in the severity of disease caused by dystrophin mutations. Replicative senescence is usually observed in mitotically active cells derived from elderly individuals, and as such DMD has characteristics of a premature aging syndrome given that the myogenic stem cells become prematurely senescent. The mdx/mTR double mutant muscle phenotype underscores the tremendous requirement for continuous myogenic stem cell proliferation needed to support dystrophic muscle regeneration. This ongoing, lifelong myogenic stem cell proliferation may also contribute to the acquisition of mutations that lead to a high frequency of rhabdomyosarcoma tumors in muscles of old mdx mice (Chamberlain et al., 2007).
What is the immediate utility of the new mouse model for DMD? One is the potential to gain new insights into mechanisms of dystrophic progression that take into account limitations on muscle regenerative capacity. A second is to reinforce the concept that DMD arises not just from intrinsic defects in muscle function but also from a progressive loss of regenerative capacity due to the continuous proliferation requirements placed on myogenic stem cells in a dystrophic environment. Thus an effective therapy for DMD will need to not only restore function to mature myofibers but also to halt or greatly slow ongoing myofiber necrosis to minimize the recruitment of satellite cells in order to avoid acquisition of replicative senescence. This study may also spur creative approaches to overcome limitations inherent to stem cell transplantation therapies due to the limited proliferative capacity of adult somatic stem cells. New stem cell therapies for muscular dystrophies will need to take into account the replicative lifespan of donor cells, and may benefit from the use of myogenic progenitors derived from induced pluripotent stem cells, which maintain normal telomere lengths (Takahashi and Yamanaka, 2006).
Even if the new variants of mdx mutant mice do not spur the development of new therapies for muscular dystrophies, they are likely to prove valuable. The mdx/mTR double mutant mice increase the power of therapeutic testing by providing greater resolution between normal and dystrophic muscle function (Sacco et al., 2010; Chandrasekharan et al., 2010). For example, a variety of structurally distinct microdystrophins (miniaturized versions of dystrophin that can be efficiently delivered to dystrophic muscles using adeno-associated viral vectors) are being tested for use in gene therapy for DMD (Muir and Chamberlain, 2009). However it can be challenging to identify the constructs that work best in ameliorating the relatively mild pathophysiology of mouse mdx mutant muscles. The stronger phenotype of the mdx/mTR double mutant mice provides a better background for testing therapies. This issue is significant because even subtle functional changes in mdx muscle function could translate into significant quality of life issues for patients. Similar arguments can be made for testing almost all types of experimental DMD therapies.
One limitation of the new mdx/mTR mouse model is the somewhat elaborate breeding scheme required to achieve the desired genotype. Given the significant therapeutic advances achieved using the current mdx models, it should be possible to perform initial or routine testing in the standard mdx strains and use the more severely affected double mutant strains for secondary and follow-up testing. Nevertheless, the increased power of analysis afforded by the newer mutations may reduce the number of large animal model studies needed for preclinical testing of DMD therapeutics and thereby accelerate the development of a treatment for DMD.
ACKNOWLEDGMENTS
J.S.C. was supported by NIH grant AG033610
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan K, Yoon JH, Xu Y, deVries S, Camboni M, Janssen PM, Varki A, Martin PT. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000692. 42ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Kipling D, Cooke HJ. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Muir LA, Chamberlain JS. Expert Rev. Mol. Med. 2009;11:e18. doi: 10.1017/S1462399409001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Cell. 1999;96:701–702. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. Cell. 2010 doi: 10.1016/j.cell.2010.11.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]