1. Introduction
Despite years of wide-spread drug treatment efforts, hookworm infection remains endemic in many regions of the world, with the highest infection prevalence in Latin and South America, Sub-Saharan Africa, South-East Asia, and China.[1] The global burden is substantial, with over a half-billion individuals worldwide infected, including 44 million pregnant women.[2–4] While mortality due to infection is rare, morbidities such as anemia, malnutrition, stunted growth, and cognitive impairment are common and are particularly associated with heavy intensity and long term infections.[5–7] Although infection is often asymptomatic, young children and women of reproductive age are at particularly high risk for anemia.[8] Infection-induced anemia can hamper productivity, contributing substantially to the overall societal burden of disease.[9–12] Latin America and the Caribbean bear approximately 8.7% of the world’s disability-adjusted life year (DALY) burden of hookworm sequaele. Of the countries in this region, Brazil has an infection burden ten times that of any other country in Latin America, with several studies reporting an infection prevalence of 68–70%.[2, 13–14]
Hookworm vaccines are currently under development. Regular mass antihelminthic drug chemotherapy remains the primary method for treating hookworm and preventing further transmission.[15] Treatment interventions in Latin America and other regions are often school-based, as this is a low cost method of reaching a large proportion of the population at risk.[2, 15–17] Community-based initiatives often target other high risk groups such as women of reproductive age.[18] Drug treatment is usually administered to all individuals in the target group at least once annually regardless of infection status in regions where prevalence is estimated to be ≥40% due to the substantial asymptomatic rate and high cost of diagnosis.[19] Reported decreases in drug efficacy over the past two decades raises concerns about emerging drug resistance and further emphasizes the need to develop alternative hookworm control methods.[20–24] A candidate hookworm vaccine completed a phase I clinical trial in the United States in 2006.[25–27] Formation of the Human Hookworm Vaccine Initiative highlights the push to bring a hookworm vaccine to market.[28–29] Rather than guarantee sterilizing (total) immunity, such a vaccine would probably primarily prevent the incidence of heavy intensity infections and associated morbidities.[25, 27] Additionally, other vaccine candidates currently in pre-clinical development function mainly by inhibiting hemoglobin digestion and decreasing adult worm fecundity, thereby reducing the risk of anemia as well as infection duration.[25, 30]
Understanding the potential economic value of a hookworm vaccine can assist scientists, manufacturers, public health officials, and other decision makers and help guide clinical development, investment, marketplace positioning, and eventual implementation. Constructing economic models early in a vaccine's development when vaccine characteristics and market strategy can still be adjusted may improve a vaccine’s chances of success.[31] A computer simulation model was constructed to evaluate the potential economic value of a hookworm vaccine and how this value may change with varying vaccine characteristics (prevention, probability of egg reduction, and cost) and different environmental conditions such as infection prevalence, severity, and drug resistance.
2. Methods
2.1 Model Structure
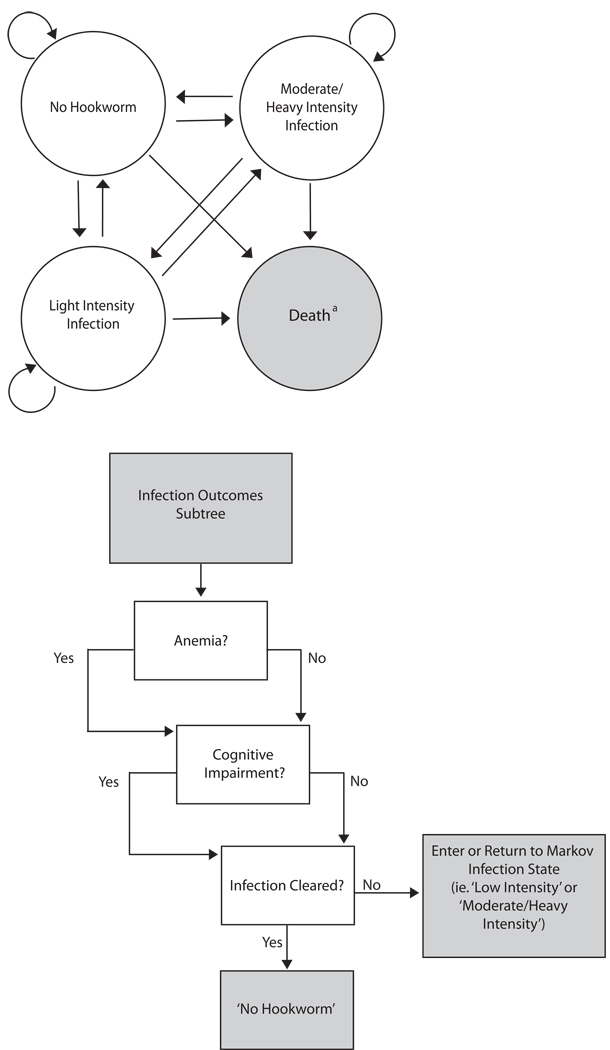
Using TreeAge Pro 2009 (TreeAge Software, Williamstown, Massachusetts), a Markov decision analytic computer simulation model was developed to evaluate the potential cost-effectiveness of introducing hookworm vaccine to Brazil. The model assumed the societal perspective and compared implementing 4 different possible intervention combination strategies: (1) vaccine plus drug treatment, (2) vaccine only, (3) drug treatment only versus (4) no intervention in two different high-risk populations: (1) school-aged children and (2) women of child-bearing age. As illustrated in Figure 1a, the model consisted of the following 4 Markov states:
No Hookworm
Light Intensity Infection
Moderate/Heavy Intensity Infection
Death
FIGURE 1.
Model Structure a) Markov states. Individuals who entered either infection Markov state (‘Moderate/Heavy Intensity’ or ‘Light Intensity’) continued to the outcomes subtree as shown in b. a Simulation terminates when the individual reaches this absorptive state b) Infected individuals had a probability of anemia, cognitive impairment, and the infection being cleared by the end of that year. These probabilities were independent of the presence of each other.
These states are mutually exclusive, i.e., an individual could be in only one state at a given time. Individuals entered the model at age 7 for the school-aged children scenario and at age 13 for the women of reproductive age scenario. During the first year, an individual started in one of three initial states (No Hookworm, Light Intensity Infection, or Moderate/Heavy Intensity Infection), depending on his/her age-specific probabilities of infection as well as heavy intensity infection. The cycle length for the model was 1 year. In other words, an individual stayed in a given state for a year and then each subsequent year had probabilities of remaining in the same state or moving into another state. With each cycle, the individual aged another year and had an age-dependent probability of dying from natural causes (i.e., moving into the Death state), based on Brazil's age-specific crude mortality rate.[32] Individuals continued to cycle in the model until they ended up in the Death state or completed the full 20 cycles. Our choice of this initial baseline time horizon attempted to be conservative about the benefits of a vaccine while encompassing the majority of the time that the target populations would remain in their risk categories, i.e., children in their school age years and women in their reproductive years. Extending this time horizon to an individual’s lifetime would only increase a potential vaccine's benefits.
Although an individual entering either infection state (moderate/heavy or light intensity) could remain in that state (i.e., stay infected) for up to 18 years, 50% of infections resolved within 5 years, based on data from a previous study. [33] Therefore, the majority of infected individuals only remained infected for 5 years or less, particularly if they received annual treatment or the vaccine. Each year, an individual in either infection state then had probabilities of developing anemia, cognitive impairment, or recovering each year, depending on the intensity of the infection, as shown the outcomes subtree in Figure 1b.
Both vaccine and medication had probabilities of altering disease risk. In the vaccine arms, vaccination could decrease not only the risk of hookworm infection but also the probability of higher intensity infection.[27] Once an individual receives medication treatment, re-infection did not occur for 1 year after treatment. Existing infections were therefore a result of lack of efficacy of either the drug treatment or vaccine.
Each simulation run sent 1,000 hypothetical individuals through the model 1,000 times, resulting in a hypothetical cohort of 1,000,000 with every simulated individual accruing a distinctive set of costs and utility decrements. For each simulation, the equation below computed the cost per disability-adjusted life year (DALY) avoided with administration of either drug treatment, vaccine, or both in combination:
| (1) |
Where the denominator yields DALYs averted by the intervention.
Based on World Health Organization (WHO) recommendations, the GDP/capita of Brazil was used to determine thresholds for cost-effectiveness.[34] Strategies were considered highly cost-effective if the ICER was ≤ 1 times the GDP/capita ($8,805/DALY averted), cost-effective if the ICER fell between 1 and 3 times the GDP/capita (between $8,805/DALY and $26,416/DALY averted), and not cost-effective if the ICER exceeded 3 times the GDP/capita.[35]
2.2 Data Inputs
Data on hookworm clinical outcomes came from an extensive MEDLINE search of articles available in English using the following search terms: [hookworm], [hookworm infection], [N. americanus], [anthelmintic drug treatment], and [hookworm vaccine]. Where possible, our model used data specific to Brazil or Latin America and our target populations. Table 1 shows baseline cost (all converted to 2010 values), probability, and DALY model input values and their corresponding references. Infection and anemia probabilities as well as drug delivery costs were age-specific. The baseline scenario was conservative about estimating hookworm treatment costs. Clinic visits and sick leave costs were therefore not included in the cost, since these are not well documented and would only increase the benefit of a vaccine. Our model utilized cost and efficacy values for the drug albendazole, as it is one of the most common and successful anthelminthics used in mass treatment involving hookworm. [17, 36] Children 12 and under were assumed to have been treated with anthelminthics in a school setting, while older children and adults were assumed to have received drug treatment through a community delivery program. All of those passing through the model were assumed to receive treatment once annually. For the baseline scenario, a 3% discount rate converted costs to 2010 values.[37]
TABLE 1.
Data inputs for model variables
| Variable | Mean | Source | |
|---|---|---|---|
| Probability | |||
| Risk of Hookworm Infection | |||
| 0–9 years old | 0.60 | [13] | |
| 10–19 years old | 0.76 | [13] | |
| 20–59 years old | 0.73 | [13] | |
| 60 years old and older | 0.70 | [13] | |
| Risk of Hookworm Infection being Heavy Intensity | |||
| 0–2 years old | 0.00 | [52] | |
| 3–5 years old | 0.05 | [52] | |
| 6–10 years old | 0.08 | [52] | |
| 11–15 years old | 0.08a | [52] | |
| 16–20 years old | 0.10 | [52] | |
| 21–30 years old | 0.13 | [52] | |
| 31–40 years old | 0.12 | [52] | |
| 41–50 years old | 0.03 | [52] | |
| 51 years old and older | 0.23 | [52] | |
| Anemia | |||
| School-age Children | 0.01 | [11] | |
| Women of Reproductive Age | 0.30 | [9] | |
| Anemia from Heavy Intensity Infection | |||
| 5–19 years old | 0.17 | [53] | |
| 20 years old and older | 0.09 | [53] | |
| Anemia from Light Intensity | 0.03 | [53] | |
| Infection | |||
| Albendazole Cure Rate b | 0.76 (Range: 0.57 to 0.95) | [36] | |
| Albendazole Egg Reduction b | 0.93 (Range: 0.79 to 0.99) | [36] | |
| Crude Mortality Rate | 0.01 | [54] | |
| Costs (2010 US$) | |||
| Community | 0.63 | [12, 16] | |
| School | 0.05 | [1, 12] | |
| Albendazole | 0.06 | [1] | |
| Effectiveness | |||
| Life Expectancy | |||
| 0 –12 months old | 71.70 | [32] | |
| 1–4 years old | 72.00 | [32] | |
| 5–9 years old | 68.10 | [32] | |
| 10–14 years old | 63.20 | [32] | |
| 15 –19 years old | 58.30 | [32] | |
| 20–24 years old | 53.7 | [32] | |
| 25 –29 years old | 49.1 | [32] | |
| 30–34 years old | 44.6 | [32] | |
| 35–39 years old | 40.0 | [32] | |
| 40–44 years old | 35.6 | [32] | |
| 45–49 years old | 31.2 | [32] | |
| 50–54 years old | 27.0 | [32] | |
| 55–59 years old | 23.0 | [32] | |
| 60–64 years old | 19.2 | [32] | |
| 65–69 years old | 15.8 | [32] | |
| 70–74 years old | 12.6 | [32] | |
| 75–79 years old | 9.9 | [32] | |
| 80–84 years old | 7.5 | [32] | |
| 85–89 years old | 5.6 | [32] | |
| 90–94 years old | 4.0 | [32] | |
| 95–99 years old | 2.8 | [32] | |
| 100 years old and older | 2.1 | [32] | |
| DALY Disability Weights | |||
| Anemia | 0.024 | [38] | |
| Heavy Intensity Infection | 0.006 | [38] | |
| Cognitive Impairment | 0.024 | [38] | |
| Misc | |||
| Hemoglobin Cut-offs for Anemia (g/dL) | |||
| Children | 11.5 | [42] | |
| Women of Reproductive Age | 12.0 | [42] | |
Extrapolated from 6–10 age group
Triangular distribution
World Health Organization (WHO) reports and statistics provided disability weights and age-specific life expectancy estimates for Brazil. [32, 38] For the baseline scenario, a 3% discount rate adjusted future DALYs to present 2010 values, as consistent with the Global Burden of Disease Study (WHO.[37, 39–40] Triangular distributions accounted for the variation in reported drug cure rates and egg reduction rates and captured the asymmetric distribution. A previous report suggests that nutritional status and infection duration largely determines the chances of cognitive impairment, however no specific data exists on the exact relationship between these factors and cognition.[6] Therefore, to remain conservative about the benefits of vaccination, our model assumed that cognitive impairment could only occur (50% probability) after an individual was infected for at least two years.
Productivity losses from hookworm infection were a product of a reduced production ability (i.e., an individual being unable to be as productive while working) that resulted from anemia and depended on the infected individual’s hemoglobin concentration (studies have suggested a relationship between hemoglobin and cognitive impairment).[5, 41] Initial hemoglobin levels were normally distributed (mean: 12.8g/dL for school-age children and 12.5g/dL for women of reproductive age; standard deviation: 0.989). Age-specific and infection intensity-specific probabilities (Table 1) determined whether an individual had anemia. If an individual had anemia, then a probability draw from the distribution of anemic hemoglobin values. [9, 42] The following equation from Shastry then translated the hemoglobin level to productivity losses:
| (2) |
Where:
Ha represents the hemoglobin concentration for the individual. [9]
H is the comparator: the hemoglobin threshold for anemia 11.5 g/dL for school-age children and 12 g/dL for women of child-bearing age,
1.5g/dL is the fluctuation in hemoglobin level that would have no additional effect on productivity.[9]
2.3 Sensitivity Analysis
Sensitivity analyses varied hookworm infection prevalence, i.e., risk of infection (baseline: 100% of age-specific rates; range: 25% to 100% of age-specific rates), drug cure rate and egg reduction rate (50% to 100% of baseline values) to model resistance, the probability of vaccine preventing infection (baseline: 60%; range: 30% to 60%), the probability of vaccine preventing heavy intensity infection (baseline: 80%; range 40% to 80%), and vaccination cost [baseline: $30; range: $1 or 1.83 Brazilian reals (BRL) to $100 or 184 BRL] to represent, for example, different vaccination prices, the requirement for booster shots, and various administration strategies such as providing vaccine during Child Health Days.[43] Sensitivity analyses also varied costs associated with treatment by +/− 50% (of baseline values in Table 1) to account for less expensive medications and additional administration costs. Additional explorations examined the effects of varying discount rate (baseline: 3%; range 0% to 10%), the presence or absence of the half-cycle correction (accounting for the onset of morbidity throughout the year), and the duration of vaccine protection (5 years versus 20 years) as well as patient compliance with follow-up vaccination if the protection is finite. These analyses assumed that 50% of those initially vaccinated received the booster at the 5 year time point; those not receiving the booster lost all protection and reverted to the baseline unvaccinated risk of infection.
3. Results
3.1 Overall Results
The vaccination plus drug treatment (combination) strategy dominated (i.e., was less costly and more effective) all other explored strategies in a wide range of scenarios for both target populations, only failing to dominate when vaccine efficacy was low (≤30% in prevent infection, <40% in reducing egg production) and/or vaccine cost was high (>$100).
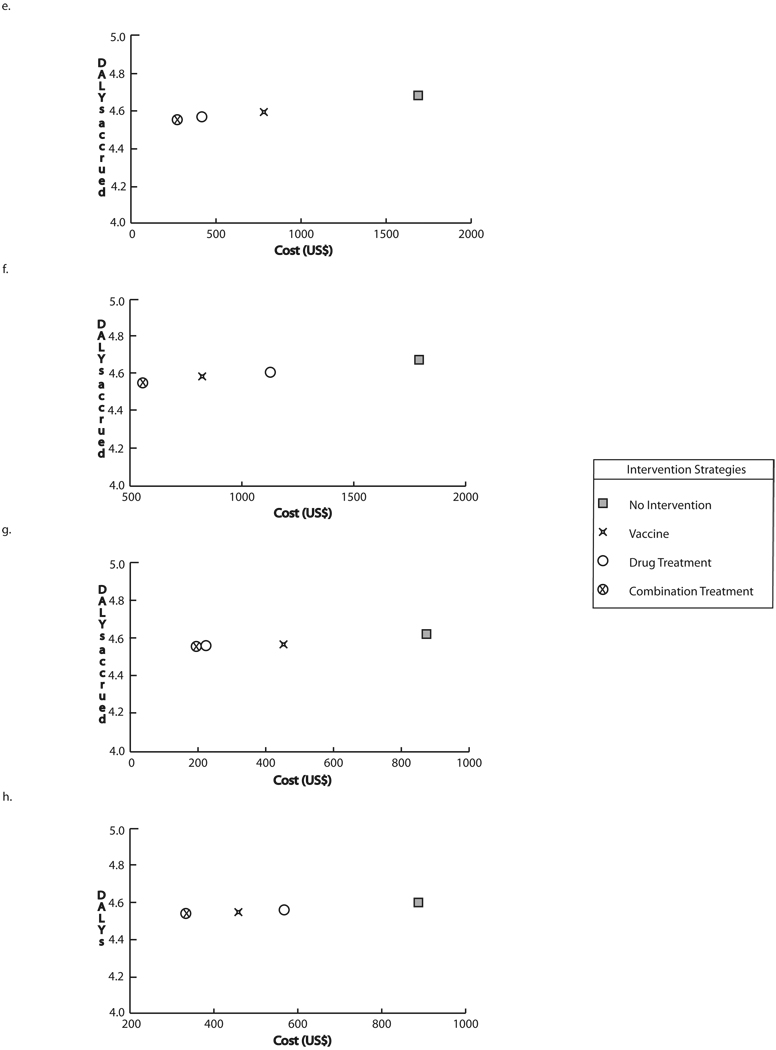
3.2 School-age Children
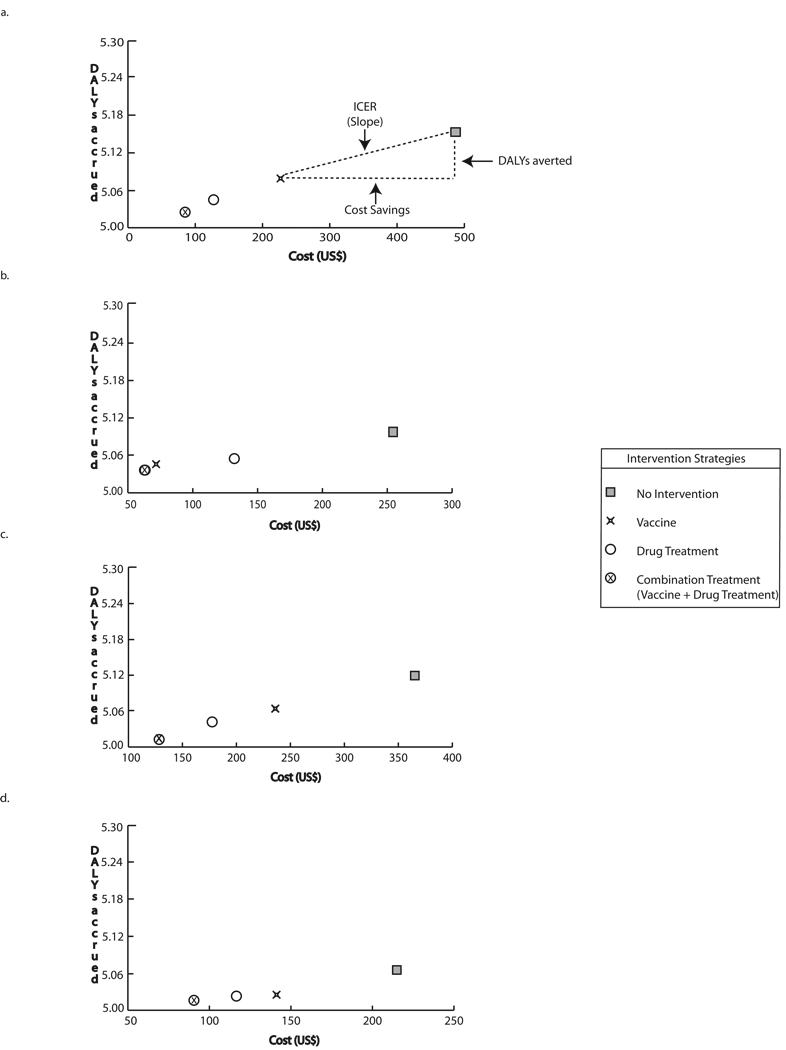
Figure 2 a–h features a number of graphs, each displaying school-age children scenario results for different circumstances assuming optimal vaccine efficacy (60% infection prevention, 80% prevention of heavy intensity infection). Each graph plots the cost and DALYs accrued for each strategy, with the position along the vertical (y) axis representing the DALYs accrued and the position along the horizontal (x) axis representing the cost. The further to the left on the graph a strategy is, the less costly it was. The lower a strategy was on the y-axis, the fewer DALYs resulted when the strategy was implemented. The horizontal and vertical dashed lines on Figure 2a represent the cost savings and DALYs averted respectively by vaccination compared to no intervention; the ratio of these two components (slope) produce the ICER, depicted as a diagonal line. When one strategy is both lower and to the left of a second strategy, it was economically dominant over that second strategy (i.e., using the first strategy instead of the second will save costs as well as provide health benefits).
FIGURE 2.
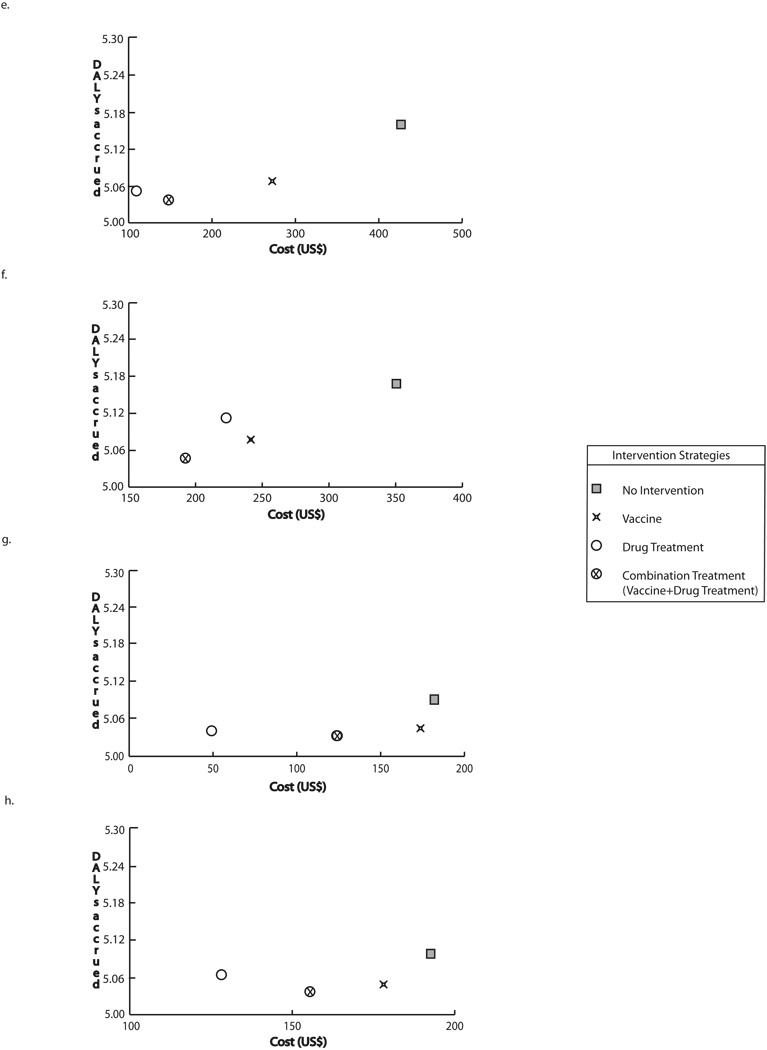
Intervention costs and DALYs accrued through strategies targeting school-age children at optimal vaccine efficacy a) Baseline prevalence and drug cure rate, vaccine cost = $30 b) Baseline prevalence and 50% of baseline drug cure rate, vaccine cost = $30 c) 50% of baseline prevalence and drug cure rate, vaccine cost = $30 d) 50% of baseline prevalence and baseline drug cure rate, vaccine cost = $30 e) Baseline prevalence and drug cure rate, vaccine cost = $100 f) Baseline prevalence and 50% of baseline drug cure rate, vaccine cost = $100 g) 50% of baseline prevalence, vaccine cost = $100 h) 50% of baseline prevalence and drug cure rate
As can be seen, in all cases, there was a clear hierarchy of strategies with the combination strategy (i.e., vaccine plus drug treatment) being the most economically favorable followed by drug treatment alone then vaccine alone then no intervention. In fact, this strategy was economically dominant over all of the strategies lower in this hierarchy. Therefore, the combination strategy, employing both vaccine and drug treatment, was dominant, saving both costs and DALYs, over all other strategies. Using drug treatment alone dominated over the vaccine alone and no intervention. Finally, vaccine alone dominated over no intervention, i.e., implementing a vaccine was clearly better (saves costs and provides health benefits) than doing nothing.
The difference in x-axis values represents the cost difference and the difference in y-axis values represents the effectiveness difference between two strategies. Therefore, in the baseline scenario when vaccine cost was $30 and risk of infection as well as drug cure rate remained at baseline, utilizing the combination strategy instead of no intervention saved $402 (740 BRL) and 0.13 DALYs per case. Adding vaccine to the drug treatment saved $42 (77 BRL) and 0.02 DALYs per case. The addition of drug treatment to vaccination saved $142 (261 BRL) and 0.05 DALYs per case. Utilizing the vaccine instead of no intervention saved $260 (478 BRL) and 0.07 DALYs per case. These results remained fairly robust when ranging vaccine efficacy (both for preventing hookworm infection and preventing higher intensity infections), drug efficacy, and hookworm prevalence or risk when vaccine price was $30, but became increasingly sensitive to these parameters when price was increased to $100.
Under many circumstances (i.e., when the vaccine price did not exceed $30), this combined strategy was economically dominant over no intervention as well as both drug and vaccine only strategies (Table 2). In other words, implementing this strategy saved costs as well as provided health benefits, providing strong evidence supporting its adoption. Even when the combined strategy was not economically dominant, the cost per DALY saved is below $10,000, well below the willingness to pay value derived using the per capita gross domestic product (GDP) in Brazil. [34] This result was robust to varying hookworm infection risk as well as the efficacy of the vaccine and anthelminthic drug.
TABLE 2.
Cost-Effectiveness of hookworm control strategies targeting school-age children
| Baseline Infection Prevalence | |||
|---|---|---|---|
| Drug CR 76% | Drug CR 38% | ||
| Vaccine Cost $30 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | Combination a | Combination | |
| 30% | Combination | Combination | |
| Vaccine Cost $100 | |||
| Vaccine Prevention | |||
| 60% | $2,655 (4,885 BRL) b, c | Combination | |
| 30% | $8,807 (16,205 BRL) | $510 (938 BRL) | |
| 50% Infection Prevalence | |||
| Drug CR 76% | Drug CR 38% | ||
| Vaccine Cost $30 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | Combination | Combination | |
| 30% | $5,532 (10,179 BRL) | Combination | |
| Vaccine Cost $100 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | $9,496 (17,473 BRL) | $1,001 (1,842 BRL) | |
| 30% | $28,552 (52,536 BRL) | Drug/Combination d | |
Combination treatment strategy was economically dominant over all other strategies
Incremental cost-effectiveness ratio (ICER) value of combination treatment compared to no intervention
1 US$ = 1.84 BRL (2010)
While drug treatment dominated the no intervention strategy, combination treatment dominated the strategy involving the vaccine only.
When the prevalence of hookworm infection was reduced to 50% of the baseline, combination treatment failed to dominate in all scenarios, but remained highly cost-effective in most cases (Table 2). At a reduced infection probability, administering drug treatment only dominated over all other intervention strategies when the probability of vaccine preventing infection was 30% and the probability of reducing infection intensity was additionally decreased to 40% while drug cure rate and egg reduction rate remained at baseline.
As the price of vaccine increases to $100, the cost-effectiveness of the interventions become increasingly sensitive to both vaccine and drug efficacy probabilities (i.e. vaccine prevention, vaccine prevention of heavy intensity infection, drug cure rate, and drug egg reduction rate) as shown in Table 2. Scenarios using a baseline probability of hookworm infection show complete dominance of combination treatment only when vaccine prevention is kept at 60% and the drug cure rate is reduced to 50% of its baseline value.
In scenarios where combination treatment fails to dominate all other interventions, the cost per DALY avoided using this strategy increases with decreasing probability that the vaccine will prevent infection or heavy intensity infection. Alternatively, this cost decreases with decreasing drug cure rate and egg reduction rate, making it more cost-effective when drug efficacy is reduced. Costs associated with avoiding disability through a combination strategy increase with reducing hookworm infection prevalence. In many cases, avoiding 1 DALY is 3 or more times more costly when the probability of infection is 50% of the baseline.
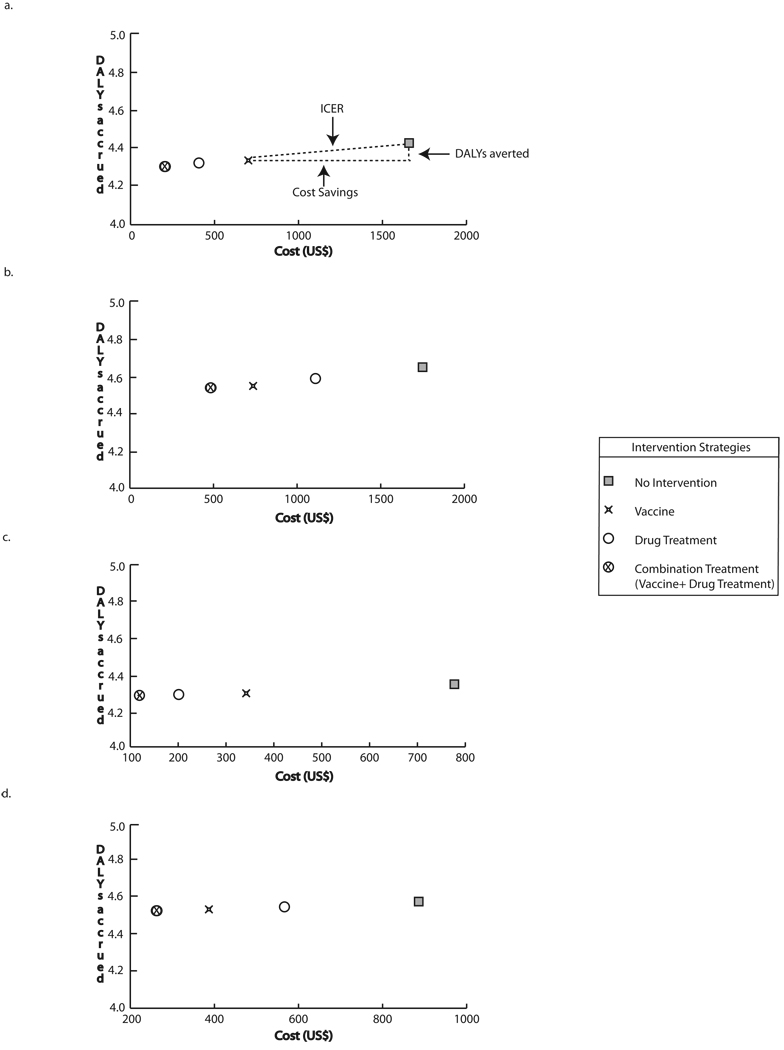
3.3 Women of Reproductive Age
Figure 3 a–h compares the cost and effectiveness of the different strategies for various women of reproductive age scenarios. In the baseline scenario for this group, incorporating the vaccine into an existing drug treatment initiative saved $208 (383 BRL) and 0.02 DALYs per case (represented by the horizontal and vertical distance between the two interventions, respectively); introducing a vaccination only strategy instead no intervention saved $957 (1,761 BRL) and 0.09 DALYs per case. Compared to no intervention, combination treatment saved $1,459 (2,685 BRL) and 0.12 DALYs per case. The addition of drug treatment to utilizing the vaccine saved $502 (924 BRL) and 0.03 DALYs per case.
FIGURE 3.
Intervention costs and DALYs accrued through strategies targeting women of reproductive age at optimal vaccine efficacy a) Baseline prevalence and drug cure rate, vaccine cost = $30 b) Baseline prevalence and 50% of baseline drug cure rate, vaccine cost = $30 c) 50% of baseline prevalence and drug cure rate, vaccine cost = $30 d) 50% of baseline prevalence and baseline drug cure rate, vaccine cost = $30 e) Baseline prevalence and drug cure rate, vaccine cost = $100 f) Baseline prevalence and 50% of baseline drug cure rate, vaccine cost = $100 g) 50% of baseline prevalence, vaccine cost = $100 h) 50% of baseline prevalence and drug cure rate, vaccine cost = $100
In comparison to the school-age children model, the cost-effectiveness of combination treatment for women of reproductive age was much less sensitive to infection prevalence, as this strategy dominated all others when vaccine cost did not exceed $30 (Table 3). Administering vaccine and drug treatment in combination dominated all other alternative strategies when infection prevalence remained at baseline and vaccine price was as high as $100. However, the drug treatment only intervention was not dominated by the combination treatment strategy when infection prevalence was 50% of baseline or less, vaccine cure rate was no greater than 30%, and drug cure rate remained at baseline. For scenarios where the vaccine plus drug treatment strategy failed to dominate all other interventions, the cost needed to avoid 1 DALY using combination treatment decreased with decreasing drug efficacy.
TABLE 3.
Cost-Effectiveness of hookworm control strategies targeting women of reproductive age
| Baseline Infection Prevalence | |||
|---|---|---|---|
| Drug CR 76% | Drug CR 38% | ||
| Vaccine Cost $30 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | Combination a | Combination | |
| 30% | Combination | Combination | |
| Vaccine Cost $100 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | Combination | Combination | |
| 30% | Combination | Combination | |
| 50% Infection Prevalence | |||
| Drug CR 76% | Drug CR 38% | ||
| Vaccine Cost $30 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | Combination | Combination | |
| 30% | Combination | Combination | |
| Vaccine Cost $100 | |||
| Vaccine Efficacy (Prevention) | |||
| 60% | Combination | Combination | |
| 30% | $7,964 (14,654 BRL) b | $4,648 (8,552 BRL) | |
Combination treatment strategy was economically dominant over all other strategies
Incremental cost-effectiveness ratio (ICER) value of combination treatment compared to no intervention
3.4 Sensitivity Analyses
Sensitivity analyses demonstrated model results to be relatively insensitive to drug treatment and delivery costs, discount rate, and the inclusion or exclusion of a half-cycle correction. The combination strategy dominated all other strategies as long as the vaccine cost was ≤ $30 and infection risk was ≥ 50% of the baseline (age-specific) rates.
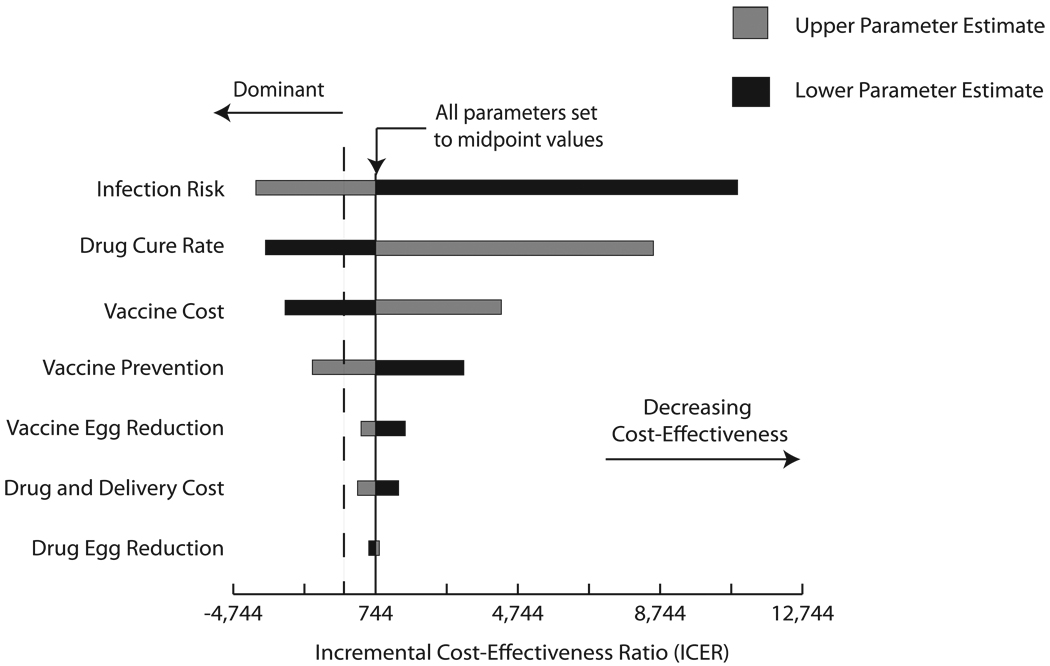
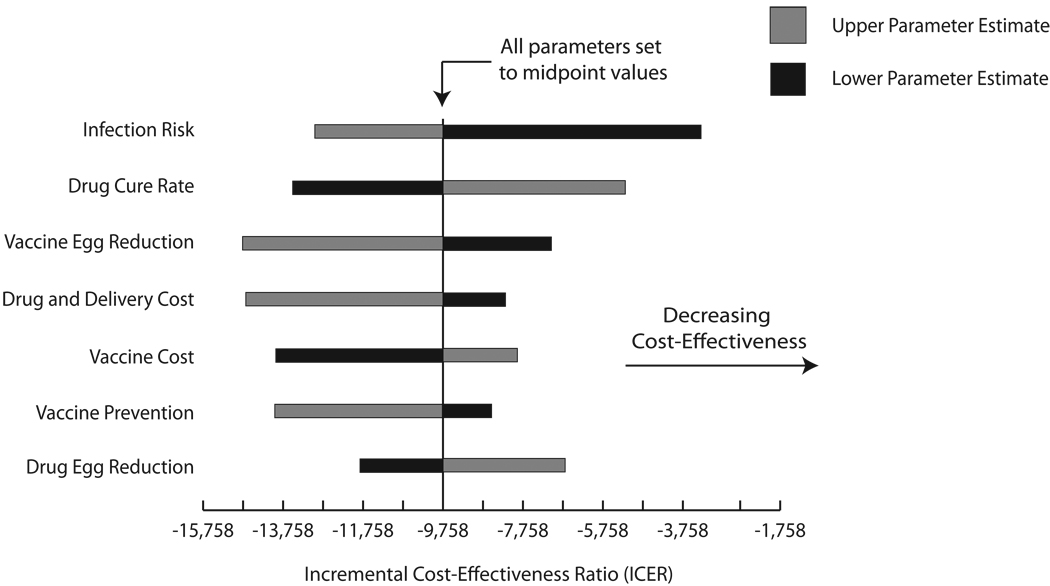
Tornado diagrams in Figure 4 illustrate the results of one-way sensitivity analyses in the experiments comparing vaccination plus drug treatment versus drug treatment alone for both target populations: school-age children (Figure 4a) and women of reproductive age (Figure 4b). The central axis on each graph marks the ICER when all parameters are set to their midpoint values evaluated through sensitivity analyses. The horizontal bars profile how the ICER changes as each parameter ranges from its highest to lowest values, with the grey portion representing the parameter’s higher values and the black the lower values. For example, adding vaccination grows more cost-effective when infection risk increases. By contrast, adding vaccination becomes less cost-effective as drug cure rate increases. For both target populations, infection risk appears to have the greatest impact on the ICER of adding vaccination. While there is a clear order of parameter impact for the school-age children, all parameters had relatively similar effects on the ICER of adding vaccination to drug treatment for the women of reproductive age scenarios. Moreover, all horizontal bars in Figure 4b are left of an ICER of zero, emphasizing that adding vaccination to drug treatment remained economically dominant across the full range of one-way sensitivity analyses in the women of reproductive age population.
FIGURE 4.
Tornado Diagrams a) School-age Children and b) women of reproductive age. The central axis represents the resulting ICER when all parameters listed were set to their midpoint value of their explored ranges: infection risk (range: 25% – 100% of age-specific probability), drug cure rate (range: 50% – 100% of baseline distribution), drug egg reduction (range: 50% – 100% of baseline distribution), vaccine cost (range: $1 – $100), vaccine efficacy in preventing infection (range: 30 – 60%) vaccine efficacy in reducing egg production (range: 40 – 80%), drug and delivery costs (range: 50% – 150% of age-specific costs). Table 1 shows baseline values for each parameter. The dashed vertical line marks where the ICER = 0. Negative ICERs occur when adding vaccination to drug treatment economically dominates drug treatment alone.
3.5 Requiring a Five-Year Booster Vaccination
For our booster and vaccine protection duration exploration, combining vaccination and drug treatment continued to dominate all other strategies for vaccination cost up to $1 (a total of $2 for both doses) in the school-age children population and up to $10 (total of $20 for both doses) in women of reproductive age. At higher vaccination costs, vaccination plus drug treatment continued to dominate over the vaccine only and no intervention strategies, but did not dominate drug treatment alone. However, adding vaccine to drug treatment remained cost-effective for the same scenarios.
4. Discussion
While the substantial global morbidity burden of hookworm has spurred hookworm vaccine development efforts, better understanding the potential economic value and role of a hookworm vaccine can assist public health officials, scientists, manufacturers, and other key decision makers. Results suggest that a hookworm vaccine would be strongly cost-effective (and in many situations economically dominant) especially when combined with a drug treatment program over a range of vaccine efficacies, vaccine costs, and hookworm attack rates. It is fairly compelling that vaccination is still cost-effective at fairly low vaccine efficacies (as low as 30%) and hookworm attack rates (25% of baseline prevalence). It is also noteworthy that even a fairly costly vaccine ($100 per patient) is still cost-effective.
The model has demonstrated that incorporating vaccination into current hookworm drug treatment strategies targeting school-age children and women of child-bearing age may yield benefit at minimal cost. While interventions were cost-effective for both school-age children and women of child-bearing age, the coverage and therefore economic return for targeting school-age children may be greater. Therefore, this group may be an initial target for vaccination initiatives with subsequent expansion to women of reproductive age. This may not always be the case, and may change with varying environmental conditions and infection risk among these groups. Less additional benefit may be seen with the initiation of vaccination in regions where infection prevalence is still able to be controlled through drug treatment. Low cost of anthelminthic drugs currently available make the cost-effectiveness of local vaccine distribution contingent upon the current drug efficacy present within the community.
An effective hookworm vaccine could have a substantial market. The models’ results highlight the substantial burden of hookworm in Brazil. [18] However, hookworm is a major problem in many other parts of the world. China is estimated to represent over a quarter of all hookworm infections worldwide, with approximately 203 million cases. [44] A hookworm vaccine may be beneficial in high prevalence regions such as Sub-Saharan Africa and East Asia and the Pacific Islands, where infections are estimated to occur in 29% and 26% of the population respectively. [44–45] Vaccination campaigns in these regions may provide greater health benefits at a lower economic burden, as the total number of those infected is reported to be higher in these geographical areas than in Brazil.
The considerable potential benefits of a hookworm vaccine support further investment into its development. Realizing that the market may support relatively high vaccine price points could encourage more vaccine developers to pursue this arena. Higher price points with reasonable adoption could translate into ample revenues, justifying upfront investment into research and development.
Certainly, bringing a hookworm vaccine to market will involve surmounting technical challenges. A candidate hookworm vaccine that has completed a phase I clinical trial has proven safe and immunogenic among a population previously unexposed to hookworm (N. americanus); however, extrapolating these vaccine characteristics to the exposed population has been difficult.[18] A second phase I trial is currently underway in Brazil, where study participants are more likely to have been previously exposed to hookworm. [30] This vaccine contains the Na-Asp-2 antigen, targeting the larval form of N. americanus, the most prevalent type of hookworm in this region.[46–47] Its suspected mechanisms of action include reducing larval penetration of human tissue and preventing the developmental progression of larvae into adults. [30] Other candidate antigens currently at the forefront of the pre-clinical development are Na-GST-1 and Na-APR-1, which would likely be combined into a bivalent vaccine, target adult hookworm and may serve an additional therapeutic purpose. [48] A vaccine containing these antigens may reduce adult worm blood-feeding and worm fecundity, potentially limiting the occurrence of anemia and infection duration. It has been suggested that an optimal vaccine would contain multiple antigens targeting both adult and larval stages. [30]
Nonetheless, our study suggests that vaccine efficacy does not need to be very high to offer substantial economic value (Even a vaccine with an efficacy as low as 30% can be very cost-effective.) In other words, vaccine developers do not necessarily have to design the "perfect" vaccine that provides high levels of protection. A weaker vaccine would be far favorable to having no vaccine available at all.
The model endeavored to remain very conservative about the benefits of a hookworm vaccine. For instance, calculations of disability avoided through vaccination of women of reproductive age did not take into consideration adverse birth outcomes such as child mortality and severe malnutrition. Many clinical trials evaluating the safety and benefit of administering anthelminthic drugs during pregnancy have observed no affect on maternal anemia status or low birth weight following drug treatment. [49] The questionable benefit of anthelminthics to improve birthing outcomes, such as average birth weight or maternal hemoglobin levels, may potentially mean that pregnant women remain at high risk for severe adverse effects of hookworm infection even in the presence of drug treatment and strongly advocates for vaccination of women prior to becoming pregnant.[3] A review of literature reveals a study that found the presence of N. americanus in one patient’s breast milk, raising the possibility of breast milk as a potential source of hookworm infection.[50] However, since no other studies have confirmed this as a possible route of transmission, our study did not include this possibility. Our model also did not include hospitalization costs because our review of the literature revealed no reporting of hospitalization incidence from hookworm infection.
Although available age-specific prevalence data is limited, we attempted to validate our model by comparing the model-generated hookworm infection prevalence when individuals completed the 7th year (cycle) with the overall prevalence reported within a Brazilian community. The model predicted that approximately 33% of surviving individuals in each target population were infected at this time point, less than the total reported prevalence.[51] Our model therefore may conservatively underestimate infection risk thereby potentially underestimating the economic and health benefits of vaccination. Additionally, vaccination may prevent the occurrence of other co-morbidities for which hookworm infected individuals are placed at higher risk. Finally, as our evaluation of a booster assumed no protection was provided to those non-compliant to the booster as well as a conservative (50%) compliance rate, these results suggest that a vaccine with a fairly short duration of protection may remain cost-effective at lower vaccine and booster prices.
5. Limitations
All computer models make simplifying assumptions and cannot represent all possible outcomes of hookworm infection, the presence of drug resistance, or other complications of hookworm control. Additionally, they cannot account for the vast diversity in the socio-demographic and health characteristics of the adult population. For instance, anemia probabilities used are age and not gender specific are therefore likely to underestimate the anemia burden due to hookworm among women of reproductive age. Our model used a one-year cycle to match the time frames for reported infection risks in the literature (available studies do not report infection risk on a monthly basis). A one-year cycle does not allow an individual to be reinfected more than once in a year, which could occur. However, this limitation errs on the side of being conservative about the benefits of a vaccine. Our model focused on the individual, and therefore did not consider herd immunity. Increasing vaccine coverage could decrease the risk of infection and, therefore, only enhance the benefits and therefore cost-effectiveness of the vaccine. Although model assumptions and data inputs were drawn from extensive review of the literature, the sources may vary in quality and input values may not hold under all conditions.
6. Conclusions
As development of hookworm vaccine proceeds, it is essential for vaccine developers and manufacturers, policy makers, and other public health officials to understand the potential costs and benefits of such a vaccine. The study suggests that such a vaccine would provide not only cost savings, but potential health benefits to both populations modeled. In fact, the most cost-effective intervention strategy may be to combine vaccine and drug treatment strategies. Findings warrant future studies that explore the implications of the introduction of a hookworm vaccine into other countries.
ACKNOWLEDGEMENTS
This study was supported by the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation and the National Institute of General Medical Sciences Models of Infectious Agent Study (MIDAS) grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of latin america and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2(9):e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooker S, Hotez PJ, Bundy DA. Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl Trop Dis. 2008;2(9):e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bundy DA, Chan MS, Savioli L. Hookworm infection in pregnancy. Trans R Soc Trop Med Hyg. 1995 Sep–Oct;89(5):521–522. doi: 10.1016/0035-9203(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 5.Jardim-Botelho A, Raff S, Rodrigues Rde A, Hoffman HJ, Diemert DJ, Correa-Oliveira R, et al. Hookworm, Ascaris lumbricoides infection and polyparasitism associated with poor cognitive performance in Brazilian schoolchildren. Trop Med Int Health. 2008 Aug;13(8):994–1004. doi: 10.1111/j.1365-3156.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 6.Belizario VY, Jr, de Leon WU, Lumampao YF, Anastacio MB, Tai CM. Sentinel surveillance of soil-transmitted helminthiasis in selected local government units in the Philippines. Asia Pac J Public Health. 2009 Jan;21(1):26–42. doi: 10.1177/1010539508327245. [DOI] [PubMed] [Google Scholar]
- 7.Sakti H, Nokes C, Hertanto WS, Hendratno S, Hall A, Bundy DA, et al. Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Trop Med Int Health. 1999 May;4(5):322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 8.Wakelin D. Helminths. Curr Opin Infect Dis. 2000 Oct;13(5):465–469. doi: 10.1097/00001432-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Shastry GKWD. How Much of Cross-Country Income Variation Is Explained by Health? Journal of the European Economic Association. 2003;1(2/3) [Google Scholar]
- 10.Edgerton VR, Gardner GW, Ohira Y, Gunawardena KA, Senewiratne B. Iron-deficiency anaemia and its effect on worker productivity and activity patterns. Br Med J. 1979 Dec 15;2(6204):1546–1549. doi: 10.1136/bmj.2.6204.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva: World Health Organization; Anemia. 2004
- 12.Hotez PJ, Bundy DAP, Beegle K. Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis. Disease Control Priorities in Developing Countries. 2nd ed. Disease Control Priorities Project. 2006 [Google Scholar]
- 13.Jardim-Botelho A, Brooker S, Geiger SM, Fleming F, Souza Lopes AC, Diemert DJ, et al. Age patterns in undernutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Trop Med Int Health. 2008 Apr;13(4):458–467. doi: 10.1111/j.1365-3156.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming FM, Brooker S, Geiger SM, Caldas IR, Correa-Oliveira R, Hotez PJ, et al. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop Med Int Health. 2006 Jan;11(1):56–64. doi: 10.1111/j.1365-3156.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 15.Montresor A, Zin TT, Padmasiri E, Allen H, Savioli L. Soil-transmitted helminthiasis in Myanmar and approximate costs for countrywide control. Trop Med Int Health. 2004 Sep;9(9):1012–1015. doi: 10.1111/j.1365-3156.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt H. The cost of delivering and sustaining a control programme for schistosomiasis and soil-transmitted helminthiasis. Acta Trop. 2003 May;86(2–3):267–274. doi: 10.1016/s0001-706x(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 17.Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli AF, Savioli L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl Trop Dis. 2008;2(3):e126. doi: 10.1371/journal.pntd.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatelli L, Ghani AC, Rodrigues LC, Hotez PJ, Brooker S. Modelling heterogeneity and the impact of chemotherapy and vaccination against human hookworm. J R Soc Interface. 2008 Nov 6;5(28):1329–1341. doi: 10.1098/rsif.2007.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva: WHO; Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. 2002 [PubMed]
- 20.De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J. Failure of mebendazole in treatment of human hookworm infections in the southern region of Mali. Am J Trop Med Hyg. 1997 Jul;57(1):25–30. doi: 10.4269/ajtmh.1997.57.25. [DOI] [PubMed] [Google Scholar]
- 21.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81(5):343–352. [PMC free article] [PubMed] [Google Scholar]
- 22.Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004 Oct;34(11):1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Smits HL. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev Anti Infect Ther. 2009 Feb;7(1):37–56. doi: 10.1586/14787210.7.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Bungiro R, Cappello M. Hookworm infection: new developments and prospects for control. Curr Opin Infect Dis. 2004 Oct;17(5):421–426. doi: 10.1097/00001432-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Diemert DJ, Bethony JM, Hotez PJ. Hookworm vaccines. Clin Infect Dis. 2008 Jan 15;46(2):282–288. doi: 10.1086/524070. [DOI] [PubMed] [Google Scholar]
- 26.Hotez PJ, Bethony JM, Oliveira SC, Brindley PJ, Loukas A. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Rev Vaccines. 2008 Aug;7(6):745–752. doi: 10.1586/14760584.7.6.745. [DOI] [PubMed] [Google Scholar]
- 27.Bethony JM, Simon G, Diemert DJ, Parenti D, Desrosiers A, Schuck S, et al. Randomized, placebo-controlled, double-blind trial of the Na-ASP-2 hookworm vaccine in unexposed adults. Vaccine. 2008 May 2;26(19):2408–2417. doi: 10.1016/j.vaccine.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 28.Sabin Vaccine Institute. Human Hookworm Vaccine Initiative Overview. 2010 [cited 2010; Available from: http://www.sabin.org/vaccine-development/vaccines/hookworm.
- 29.World Health Organization. Initiative for Vaccine Research: Parasitic Diseases. 2010 [cited 2010; Available from: http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index2.html.
- 30.Bethony JM, Loukas A, Hotez PJ, Knox DP. Vaccines against blood-feeding nematodes of humans and livestock. Parasitology. 2006;133 Suppl:S63–S79. doi: 10.1017/S0031182006001818. [DOI] [PubMed] [Google Scholar]
- 31.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010 Apr 1;28(16):2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Life Tables for WHO Member States. 2006
- 33.Beaver PC. Light, long-lasting Necator infection in a volunteer. Am J Trop Med Hyg. 1988 Oct;39(4):369–372. doi: 10.4269/ajtmh.1988.39.369. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of State BoWHA. Background Note: Brazil. 2010 [cited; Available from: http://www.state.gov/r/pa/ei/bgn/35640.htm.
- 35.World Health Organization. Choosing Interventions that are Cost Effective (WHO-CHOICE): Cost-effectiveness thresholds. 2009
- 36.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008 Apr 23;299(16):1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 37.Shepard DS. In: Cost-effectiveness in Health and Medicine. Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. New York: Oxford University Press; 1996. J Ment Health Policy Econ 1999 Jun 1;2(2):91-2. [Google Scholar]
- 38.World Health Organization. Global Burden of Disease 2004 Update: Disability Weights for Diseases and Conditions. Geneva: 2004
- 39.World Health Organization. Health statistics and health information systems: Disability weights, discounting and age weighting of DALYs. 2010 [cited 2010; Available from: http://www.who.int/healthinfo/global_burden_disease/daly_disability_weight/en/index.html.
- 40.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006 May 27;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 41.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 42.de Benoist BME, Egli I, Cogswell M. Geneva: WHO Global Database on Anaemia; Worldwide Prevalence of Anaemia 1993–2005. 2005
- 43.Santos LM, Paes-Sousa R, Silva Junior JB, Victora CG. National Immunization Day: a strategy to monitor health and nutrition indicators. Bull World Health Organ. 2008 Jun;86(6):474–479. doi: 10.2471/BLT.07.043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003 Dec;19(12):547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3(8):e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pullan RL, Bethony JM, Geiger SM, Correa-Oliveira R, Brooker S, Quinnell RJ. Human helminth co-infection: no evidence of common genetic control of hookworm and Schistosoma mansoni infection intensity in a Brazilian community. Int J Parasitol. 2010 Mar 1;40(3):299–306. doi: 10.1016/j.ijpara.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson MS, Pickering DA, Tribolet L, Cooper L, Mulvenna J, Oliveira LM, et al. Neutralizing antibodies to the hookworm hemoglobinase Na-APR-1: implications for a multivalent vaccine against hookworm infection and schistosomiasis. J Infect Dis. 2010 May 15;201(10):1561–1569. doi: 10.1086/651953. [DOI] [PubMed] [Google Scholar]
- 49.Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD005547.pub2. CD005547. [DOI] [PubMed] [Google Scholar]
- 50.Setasuban P, Punsri W, Meunnoo C. Transmammary transmission of Necator americanus larva in the human host. Southeast Asian J Trop Med Public Health. 1980 Dec;11(4):535–538. [PubMed] [Google Scholar]
- 51.Quinnell RJ, Pullan RL, Breitling LP, Geiger SM, Cundill B, Correa-Oliveira R, et al. Genetic and household determinants of predisposition to human hookworm infection in a Brazilian community. J Infect Dis. 2010 Sep 15;202(6):954–961. doi: 10.1086/655813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traub RJ, Robertson ID, Irwin P, Mencke N, Andrew Thompson RC. The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Health. 2004 Jun;9(6):688–701. doi: 10.1111/j.1365-3156.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 53.Brooker S, Jardim-Botelho A, Quinnell RJ, Geiger SM, Caldas IR, Fleming F, et al. Age-related changes in hookworm infection, anaemia and iron deficiency in an area of high Necator americanus hookworm transmission in south-eastern Brazil. Trans R Soc Trop Med Hyg. 2007 Feb;101(2):146–154. doi: 10.1016/j.trstmh.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 54.UNICEF. Brazil: 2007. [cited 2010; Available from: http://www.unicef.org/infobycountry/brazil_statistics.html. [Google Scholar]