Abstract
The most influential theory to explain the pathogenesis of Alzheimer's disease (AD) has been the “Amyloid Cascade Hypothesis” (ACH) first formulated in 1992. The ACH proposes that the deposition of β-amyloid (Aβ) is the initial pathological event in AD leading to the formation of senile plaques (SPs) and then to neurofibrillary tangles (NFTs) death of neurons, and ultimately dementia. This paper examines two questions regarding the ACH: (1) is there a relationship between the pathogenesis of SPs and NFTs, and (2) what is the relationship of these lesions to disease pathogenesis? These questions are examined in relation to studies of the morphology and molecular determinants of SPs and NFTs, the effects of gene mutation, degeneration induced by head injury, the effects of experimentally induced brain lesions, transgenic studies, and the degeneration of anatomical pathways. It was concluded that SPs and NFTs develop independently and may be the products rather than the causes of neurodegeneration in AD. A modification to the ACH is proposed which may better explain the pathogenesis of AD, especially of late-onset cases of the disease.
1. Introduction
Ever since the first description of presenile dementia by Alzheimer in 1907 [1], senile plaques (SPs) and neurofibrillary tangles (NFTs) have been regarded as the “signature” pathological lesions of Alzheimer's disease (AD) [2–4]. AD became a nosological entity in 1910 and was named after Alzheimer by Kraepelin based on the clinical and pathological description of the original cases. Of the two original cases described by Alzheimer, however, both had numerous SPs but only one of the cases had significant numbers of NFTs [5], thus creating a controversy as to the relative significance of the two lesions that still persists today.
Studies of the molecular composition of the SPs played a critical role in the development of hypotheses as to the pathogenesis of AD. Hence, the discovery of β-amyloid (Aβ) as the most important molecular constituent of the SPs [6] resulted in the formulation of the “Amyloid Cascade Hypothesis” (ACH), the most important model of the molecular pathology of AD developed over the last 18 years [7]. Essentially, the ACH proposes that the deposition of Aβ (Figure 1) is the initial pathological event in the disease leading to the formation of NFTs, cell death, and ultimately dementia. Nevertheless, there are observations that are difficult to reconcile with the hypothesis. For example, in transgenic mice, genes overexpressing amyloid precursor protein (APP) do not produce the predicted cascade [8, 9]. Furthermore, SPs and NFTs appear to be separated in the brain both temporally [9, 10] and spatially [11]. The uncertainty as to the significance of SPs and NFTs in AD has led to alternative models being proposed, especially in late-onset cases, based on perturbation of vesicular trafficking at synapses, disruption of the cytoskeletal network, or the distribution of membrane cholesterol [12]. Some authors have even suggested that SPs/NFTs may be the reactive products of neurodegeneration, arising as a consequence of oxidative stress [13], and that the proteins involved in their formation are protective in function [14]. These observations suggest a more complex relationship between SPs and NFTs and the pathogenesis of AD and, therefore, that a reappraisal of the ACH may be necessary.
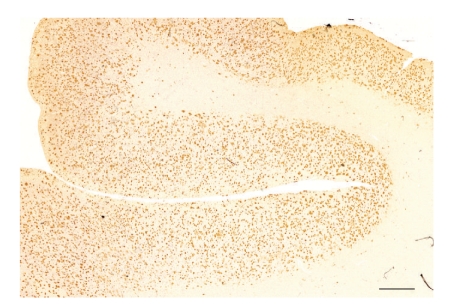
Figure 1.
Extensive β-amyloid (Aβ) deposition in gyri of the temporal lobe in a case of Alzheimer's disease (AD) (Aβ immunohistochemistry, bar = 1 mm).
This paper examines two questions regarding the ACH: (1) is there a relationship between the pathogenesis of SPs and NFTs, and (2) what is the relationship of these lesions to disease pathogenesis? These questions are discussed with reference to (1) studies of the morphology and molecular composition of SPs and NFTs, (2) studies of the effects of gene mutation, (3) studies of head injury patients, (4) experimental studies involving brain lesions and transgenes, and (5) studies of the degeneration of anatomical pathways.
2. The “Amyloid Cascade Hypothesis” (ACH)
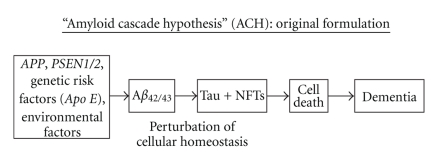
Two key observations resulted in the original formulation of the ACH [7] (Figure 2). First, the discovery of Aβ as the most important molecular constituent of the SPs [6] drew attention to the importance of these amyloid peptides in AD. Second, mutations of the APP gene [15, 16] and, subsequently, of the presenilin genes (PSEN1/2) [17, 18] were directly linked to cases of familial AD (FAD). Hence, the presence of Aβ within SPs was regarded as the residue of the effect of these pathogenic gene mutations and which, via the accumulation of toxic and insoluble Aβ peptides, led to cell death and dementia. Since the pathological phenotype of FAD is similar, apart from age of onset, to that of the more common late-onset, sporadic AD (SAD) [19–21], it was assumed that a similar mechanism, via genetic risk factors and/or environmental factors, could explain the pathogenesis of all cases of AD [22].
Figure 2.
The original Amyloid Cascade Hypothesis (ACH) [7]. Aβ: β-amyloid, APOE: apolipoprotein E, APP: amyloid precursor protein, PSEN1/2: presenilin genes 1 and 2, NFTs: neurofibrillary tangles.
Evidence supporting the ACH comes from several sources. First, experiments using transgenic mice expressing high levels of APP result in Aβ deposition, synaptic loss, and gliosis [23]. Second, FAD caused by the substitution of valine by isoleucine at codon 717 of the APP gene also has significant numbers of NFTs thus supporting a link between APP and the cytoskeleton [24]. Third, cases linked to PSEN1 have greater numbers of SPs and NFTs compared with cases of sporadic AD suggesting that PSEN1 may increase tau deposition [25].
There have been several attempts to establish a mechanism by which the deposition of Aβ directly leads to the formation of NFTs but none have become universally accepted. First, Giasson et al. [26] concluded that Aβ promoted the formation of intracellular tau, although the mechanism of this interaction was uncertain. Second, attempts have been made to show that there is a synergistic interaction between NFTs and Aβ [27, 28]. Third, when fetal rat hippocampal neurons and human cortical neurons were treated with Aβ, fibrillar forms of Aβ could apparently induce tau phosphorylation [29]. It was concluded that amyloid fibril formation might alter the phosphorylation state of tau resulting in the loss of microtubule-binding capacity. Fourth, Pérez et al. [30] showed that Aβ25-35 could induce the aggregation of tau proteins and that a decrease in aggregation of Aβ was induced by tau peptides. Hence, aggregation of tau may be associated with disassembly of Aβ which could explain the lack of spatial correlation of the SPs and NFTs [11].
3. Limitations of the ACH
There are two major objections regarding the ACH as originally formulated. First, SPs and NFTs may be reactive products resulting from neurodegeneration in AD rather than being its cause [31] and, second there is no generally accepted mechanism to explain how the deposition of Aβ leads to the formation of NFTs.
Is the Formation of Aβ and Tau a Reactive Process? —
In survivors of head injury, APP is found in neuronal perikarya and in dystrophic neurites surrounding Aβ deposits, similar pathological features to AD [32]. The processing of APP into Aβ in these cases occurs within the synaptic terminal fold of the axons, the presence of glial cells not being necessary for the conversion. Hence, the production of APP may be a response of the brain to neuronal injury [32]. Subsequently, it was shown that specific neurons in the medial temporal lobe secreted large quantities of APP and that there were more APP-immunoreactive neurons in these areas in head injury patients [33]. Hence, increased expression of APP in head trauma cases may be an acute-phase response to neuronal injury [34], the overexpression of APP leading to the deposition of Aβ. This conclusion is supported by the observation that several acute-phase proteins are localised within the different morphological forms of Aβ deposit, including diffuse, primitive, and classic deposits (Table 1), for example, amyloid-P, complement factors, and α-antichymotrypsin [35]. Furthermore, Regland and Gottfries [36] proposed that, in AD, APP was involved in disease processes secondarily to help maintain cell function. Hence, APP may maintain neuronal growth and survival, and its putative neurotrophic action is supported by the observation that APP shares structural features with the precursor for epidermal growth factor [36]. Furthermore, NFTs may be part of the neurons response to injury [37]. Hence, studies of head injury patients support the hypothesis that Aβ deposition and NFTs formation could be reactive processes.
The results of animal experiments also suggest that the formation of Aβ may be a reactive process. Experimental lesions that damage the nucleus basalis in the brain of the rat elevate APP synthesis in the cerebral cortex suggesting that the production of APP could be a specific response to loss of functional innervation of the cortex [38]. Chemically induced lesions of the brain produce similar results. For example, lesions of the nucleus basalis using N-methyl D-aspartate (NMDA) elevate APP synthesis in cortical polysomes [38], and, in areas of brain damaged by kainite [39], APP695 was recorded in dystrophic neurites near to the lesion. In addition, intrathecal or intraparenchymal injections of a toxin induced APP in hippocampal neurons subsequent to neuronal damage [40].
Lesion experiments may also induce pathological changes implicated in the development of NFTs. Denervation of the dopamine pathways and septal lesions affecting both the cholinergic system and γ-aminobutyric acid (GABA) neurons projecting to the dentate gyrus results in a loss of dendritic microtubule-associated protein 2 (MAP2) and the appearance of tau-immunoreactive dentate gyrus granule cells [41]. It was concluded from this experiment that denervation causes transsynaptic changes in dentate gyrus neurons and that these changes may represent a precursor stage to NFTs formation.
Table 1.
Molecular composition of β-amyloid (Aβ) deposits in Alzheimer's disease (AD).
| Deposit subtype | Molecular composition |
|---|---|
| Diffuse Aβ | APP (lacking C terminus), Aβ42/43 apolipoprotein E, α1-antichymotrypsin, HSPG, complement proteins (C1q, C3, C4), amyloid-P |
| Primitive Aβ | APP (N & C-terminal), Aβ42/43, free and conjugated ubiquitin, PHF antigen, phosphorylated tau, chromogranin-A, bFGF, apolipoprotein E, interleukin-6 |
| Classic Aβ | Aβ42/43 “core”, α-synuclein “ring”, Aβ40, actin, tubulin, phosphorylated tau, NF-protein, CAM, chromogranin-A “ring”, α2-macroglobulin, complement proteins “core”, immunoglobulins “core”, amyloid-P, α1-antichymotrypsin, antitrypsin, antithrombin III, apolipoprotein E and D “core”, bFGF, PrP, silicon/aluminium “core”, interleukin-6 “ring” |
Aβ: β-amyloid, APP: amyloid precursor protein, bFGF: basic fibroblast growth factor, CAM: cell adhesion molecule, HSPG: heparan suphate proteoglycan, NF-protein: neurofilament protein, PHF: paired helical filament, and PrP: prion protein.
Is the Formation of NFT Related to Aβ? —
A number of studies have suggested that SPs and NFTs occur in distinct but independently distributed patterns in AD [11, 42]. Studies of the spatial patterns of SPs and NFTs show them to be clustered with, in a significant proportion of cortical areas, a regular distribution of the clusters parallel to the pia mater [43]. The clusters of SPs and NFTs, however, are distributed independently of each other, that is, neither in nor out of phase, which would not support a direct pathogenic link between them. In addition, SPs and NFTs appear to be separated in the brain temporally [10]. Indeed, in the entorhinal cortex, the NFTs may actually precede the appearance of SPs [9].
In transgenic experiments [44], the presence of APP mutations alone or in combination with PSEN1 can induce Aβ deposits in normal brain, but apart from some evidence for hyperphosphorylated tau in neurites associated with the plaques, do not appear to induce tau pathology or a significant inflammatory response. Hence, the presence of tau transgenes in the form of a triple model appears to be necessary to replicate AD pathology.
4. Modification of the ACH
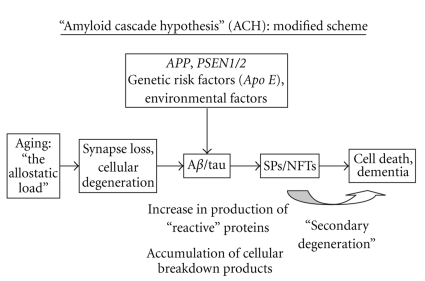
A modification of the original ACH which incorporates these concerns is presented in Figure 3. In this modified hypothesis, the essential trigger to the development of AD is ageing of the brain and associated risk factors such as head trauma, vascular disease, and systemic disease, collectively referred to as the “allostatic load” [45]. These factors exacerbate processes leading to cell death. As neurons degenerate, various proteins are upregulated leading to the formation of extracellular Aβ deposits and intracellular tau, the latter resulting in the development of NFTs. These reaction products may be toxic and initiate a further phase of secondary degeneration that accelerates the neuronal loss leading to dementia. In this modified hypothesis, genetic factors, rather than initiating disease, indirectly influence the formation and composition of peptides formed when neurons degenerate. Hence, the modified ACH incorporates information suggesting a more complex relationship between SPs and NFTs and proposes that the lesions are essentially reactive rather than causal.
Figure 3.
A modification of the Amyloid Cascade Hypothesis (ACH). Aβ: β-amyloid, APOE: apolipoprotein E, APP: amyloid precursor protein, PSEN1/2: presenilin genes 1 and 2, NFTs: neurofibrillary tangles, and SPs: senile plaques.
5. Discussion
5.1. Predictions of the Modified ACH
The modified ACH suggests that it is ageing and the diseases associated with ageing that provide the “trigger” initiating the “cascade” of events leading to AD rather than the initial deposition of Aβ. The modified hypothesis makes a number of predictions. First, the hypothesis predicts that significant signs of neuronal degeneration in AD should precede those of Aβ deposition and the effect of Aβ is secondary rather than primary in causing neurodegeneration. Second, it predicts that the pathogeneses of SPs and NFTs are not directly linked and the two lesions essentially arise independently. Third, in transgenic experiments, the effect of the transgene will be age-dependent. In a model which incorporates an APP, V717I mutation, for example, there was an age-related loss of pyramidal neurons in the hippocampus CA sectors included at sites devoid of plaque deposition [46] consistent with this prediction.
5.2. Predictions of the Modified ACH
First, the modified hypothesis suggests that SAD is not a disease linked primarily to defective genes but a complex syndrome dependant on the rate of ageing and indirectly influenced by genetic risk factors and the environment. Second, the hypothesis questions whether the presence, distribution, and molecular determinants of SPs and/or NFTs (Table 1) should continue to play a primary role in the pathological diagnosis of AD. There are two problems that need to be considered. If SPs/NFTs are the products of brain degeneration and not its cause, then they may represent relatively late stages in pathogenesis. Hence, there may be cases of AD that are difficult to classify because they may have insufficient numbers of SPs and NFTs or exhibit early developmental stages of these pathologies. In addition, if SPs and NFTs represent the consequences of specific types of neurodegeneration rather than being characteristic of a particular disease, then there are likely to be many cases that show combinations of pathological features; that is, there will be a considerable degree of overlap between different disorders. Numerous examples of such cases have been reported in the literature, for example, dementia with Lewy bodies (DLB) with associated AD pathology, Creutzfeldt-Jakob disease (CJD) with AD, and Pick's disease (PkD) with AD, and these cases are often difficult to classify within the existing system [47]. Third, assuming that the role of SPs and NFTs in the pathogenesis of AD is at least controversial, should significant effort continue to be devoted to immunotherapy and other treatments designed to remove Aβ from the brain? Such treatments could be beneficial in limiting the degree of secondary degeneration induced by Aβ. Nevertheless, Aβ might be beneficial to the nervous system by promoting neurogenesis [48] and having a range of other protective functions [49]. Hence, excessive removal of Aβ could reduce chelation within the brain and result in enhanced oxidative stress [13].
6. Conclusions
Since 1992, the ACH has played an influential role in explaining the etiology and pathogenesis of Alzheimer's disease (AD). It proposes that the deposition of β-amyloid (Aβ) is the initial pathological event in AD leading to the formation of senile plaques (SPs), and then to neurofibrillary tangles (NFTs), death of neurons, and ultimately dementia. There are, however, two limitations of the ACH: (1) SP and NFT may develop independently, and (2) SPs and NFTs may be the products rather than the causes of neurodegeneration in AD. A modification to the ACH is proposed which may better explain the pathogenesis of AD, especially in late-onset cases of the disease. The modifications to the ACH make a number of predictions which could be usefully investigated.
References
- 1.Alzheimer A. On a peculiar disease of the cerebral cortex. Allgemeine Zeitschrift fur Psychiatrie und Psychish-Gerichtlich Medicin. 1907;64:146–148. [Google Scholar]
- 2.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Archives of Neurology. 1985;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 3.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 4.Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 1999;58(11):1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Graeber MB, Kösel S, Egensperger R, et al. Rediscovery of the case described by Alois Alzheimer in 1911: historical, histological and molecular genetic analysis. Neurogenetics. 1997;1(1):73–80. doi: 10.1007/s100480050011. [DOI] [PubMed] [Google Scholar]
- 6.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochemical and Biophysical Research Communications. 1984;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 7.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 8.Mudher A, Lovestone S. Alzheimer’s disease—do tauists and baptists finally shake hands? Trends in Neurosciences. 2002;25(1):22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 9.Duyckaerts C. Looking for the link between plaques and tangles. Neurobiology of Aging. 2004;25(6):735–739. doi: 10.1016/j.neurobiolaging.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Mann DMA, Younis N, Jones D, Stoddart RW. The time course of pathological events in Down’s syndrome with particular reference to the involvement of microglial cells and deposits of β/A4. Neurodegeneration. 1992;1:201–215. [Google Scholar]
- 11.Armstrong RA, Myers D, Smith CUM. The spatial patterns of plaques and tangles in Alzheimer’s disease do not support the ‘Cascade hypothesis’. Dementia. 1993;4(1):16–20. doi: 10.1159/000107291. [DOI] [PubMed] [Google Scholar]
- 12.Drouet B, Pinçon-Raymond M, Chambaz J, Pillot T. Molecular basis of Alzheimer’s disease. Cellular and Molecular Life Sciences. 2000;57(5):705–715. doi: 10.1007/s000180050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atwood CS, Obrenovich ME, Liu T, et al. Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β. Brain Research Reviews. 2003;43(1):1–16. doi: 10.1016/s0165-0173(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 14.Smith MA, Casadesus G, Joseph JA, Perry G. Amyloid-β and τ serve antioxidant functions in the aging and Alzheimer brain. Free Radical Biology and Medicine. 2002;33(9):1194–1199. doi: 10.1016/s0891-5849(02)01021-3. [DOI] [PubMed] [Google Scholar]
- 15.Chartier-Harlin MC, Crawford F, Houlden H, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature. 1991;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 16.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 17.Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 18.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong RA, Nochlin D, Bird TD. Neuropathological heterogeneity in Alzheimer’s disease: a study of 80 cases using principal components analysis. Neuropathology. 2000;20(1):31–37. doi: 10.1046/j.1440-1789.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Haupt M, Kurz A, Pollmann S, Romero B. Alzheimer's disease: identical phenotype of familial and non-familial cases. Journal of Neurology. 1992;239(5):248–250. doi: 10.1007/BF00810345. [DOI] [PubMed] [Google Scholar]
- 21.Nochlin D, Van Belle G, Bird D, Sumi SM. Comparison of the severity of neuropathologic changes in familial add sporadic Alzheimer’s disease. Alzheimer Disease and Associated Disorders. 1993;7(4):212–222. [PubMed] [Google Scholar]
- 22.Styczyńska M, Strosznajder JB, Religa D, et al. Association between genetic and environmental factors and the risk of Alzheimer’s disease. Folia Neuropathologica. 2008;46(4):249–254. [PubMed] [Google Scholar]
- 23.Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 24.Lantos PL, Luthert PJ, Hanger D, Anderton BH, Mullan M, Rossor M. Familial Alzheimer’s disease with the amyloid precursor protein position 717 mutation and sporadic Alzheimer’s disease have the same cytoskeletal pathology. Neuroscience Letters. 1992;137(2):221–224. doi: 10.1016/0304-3940(92)90408-y. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd CE, Gregory GC, Vickers JC, et al. Positional effects of presenilin-1 mutations on tau phosphorylation in cortical plaques. Neurobiology of Disease. 2004;15(1):115–119. doi: 10.1016/j.nbd.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Giasson BI, Lee VMY, Trojanowski JQ. Interactions of amyloidogenic proteins. NeuroMolecular Medicine. 2003;4(1-2):49–58. doi: 10.1385/NMM:4:1-2:49. [DOI] [PubMed] [Google Scholar]
- 27.Smith MA, Siedlak SL, Richey PL, et al. Tau protein directly interacts with the amyloid β-protein precursor: implications for Alzheimer's disease. Nature Medicine. 1995;1(4):365–369. doi: 10.1038/nm0495-365. [DOI] [PubMed] [Google Scholar]
- 28.Oyama F, Shimada H, Oyama R, Titani K, Ihara Y. β-amyloid protein precursor and τ mRNA levels versus β-amyloid plaque and neurofibrillary tangles in the aged human brain. Journal of Neurochemistry. 1993;60(5):1658–1664. doi: 10.1111/j.1471-4159.1993.tb13388.x. [DOI] [PubMed] [Google Scholar]
- 29.Busciglio J, Lorenzo A, Yeh J, Yankner BA. β-Amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14(4):879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 30.Pérez M, Cuadros R, Benítez MJ, Jiménez JS. Interaction of Alzheimer’s disease amyloid β peptide fragment 25-35 with tau protein, and with a tau peptide containing the microtubule binding domain. Journal of Alzheimer’s Disease. 2004;6(5):461–467. doi: 10.3233/jad-2004-6501. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong RA, Cairns NJ, Lantos PL. Are pathological lesions in neurodegenerative disorders the cause or the effect of the degeneration? Neuropathology. 2002;22(3):133–146. doi: 10.1046/j.1440-1789.2002.00446.x. [DOI] [PubMed] [Google Scholar]
- 32.Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. β-amyloid precursor protein (βAPP) as a marker for axonal injury after head injury. Neuroscience Letters. 1993;160(2):139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie JE, Gentleman SM, Roberts GW, Graham DI, Royston MC. Increased numbers of βAPP-immunoreactive neurones in the entorhinal cortex after head injury. NeuroReport. 1994;6(1):161–164. doi: 10.1097/00001756-199412300-00041. [DOI] [PubMed] [Google Scholar]
- 34.Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. β amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. Journal of Neurology Neurosurgery and Psychiatry. 1994;57(4):419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalaria RN, Perry G. Amyloid P component and other acute-phase proteins associated with cerebellar A β-deposits in Alzheimer’s disease. Brain Research. 1993;631(1):151–155. doi: 10.1016/0006-8993(93)91202-4. [DOI] [PubMed] [Google Scholar]
- 36.Regland B, Gottfries CG. The role of amyloid β-protein in Alzheimer’s disease. Lancet. 1992;340(8817):467–469. doi: 10.1016/0140-6736(92)91780-c. [DOI] [PubMed] [Google Scholar]
- 37.Renkawek K, De Jong WW, Merck KB, Frenken CWGM, Van Workum FPA, Bosman GJCGM. αB-crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathologica. 1992;83(3):324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- 38.Wallace WC, Bragin V, Robakis NK, et al. Increased biosynthesis of Alzheimer amyloid precursor protein the in cerebral cortex of rats with lesions of the nucleus basalis of Meynert. Molecular Brain Research. 1991;10(2):173–178. doi: 10.1016/0169-328x(91)90108-a. [DOI] [PubMed] [Google Scholar]
- 39.Kawarabayashi T, Shoji M, Harigaya Y, Yamaguchi H, Hirai S. Expression of APP in the early stage of brain damage. Brain Research. 1991;563(1-2):334–338. doi: 10.1016/0006-8993(91)91558-i. [DOI] [PubMed] [Google Scholar]
- 40.Kalaria RN, Bhatt SU, Perry G, Lust WD. The amyloid precursor protein in ischaemic brain injury and chronic hypoperfusion. In: Proceedings of the 7th International Study Group on Pharm Mem Dis Assoc with Aging; 1993; pp. 291–294. [DOI] [PubMed] [Google Scholar]
- 41.Torack RM, Miller JW. Immunoreactive changes resulting from dopaminergic denervation of the dentate gyrus of the rat hippocampal formation. Neuroscience Letters. 1994;169(1-2):9–12. doi: 10.1016/0304-3940(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 42.Hyman BT, Tanzi RE. Amyloid, dementia and Alzheimer’s disease. Current Opinion in Neurology and Neurosurgery. 1992;5(1):88–93. [PubMed] [Google Scholar]
- 43.Armstrong RA, Myers D, Smith CUM. The spatial patterns of β/A4 deposit subtypes in Alzheimer’s disease. Acta Neuropathologica. 1993;86(1):36–41. doi: 10.1007/BF00454896. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong RA. Plaques and tangles and the pathogenesis of Alzheimer’s disease. Folia Neuropathologica. 2006;44(1):1–11. [PubMed] [Google Scholar]
- 45.Carroll BJ. Ageing, stress and the brain. Novartis Foundation Symposium. 2002;242:26–45. [PubMed] [Google Scholar]
- 46.Schmitz C, Rutten BPF, Pielen A, et al. Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer’s disease. American Journal of Pathology. 2004;164(4):1495–1502. doi: 10.1016/S0002-9440(10)63235-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong RA, Lantos PL, Cairns NJ. Overlap between neurodegenerative disorders. Neuropathology. 2005;25(2):111–124. doi: 10.1111/j.1440-1789.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 48.López-Toledano MA, Shelanski ML. Neurogenic effect of β-amyloid peptide in the development of neural stem cells. Journal of Neuroscience. 2004;24(23):5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HG, Casadesus G, Zhu X, Takeda A, Perry G, Smith MA. Challenging the amyloid cascade hypothesis: senile plaques and amyloid-β as protective adaptations to Alzheimer disease. Annals of the New York Academy of Sciences. 2004;1019:1–4. doi: 10.1196/annals.1297.001. [DOI] [PubMed] [Google Scholar]