Abstract
A major issue in lexical processing concerns storage and access of lexical items. Here we make use of the base frequency effect to examine this. Specifically, reaction time to morphologically complex words (words made up of base and suffix, e.g., agree+able) typically reflects frequency of the base element (i.e., total frequency of all words in which agree appears) rather than surface word frequency (i.e., frequency of agreeable itself). We term these complex words decomposable. However, a class of words termed whole-word do not show such sensitivity to base frequency (e.g., serenity).
Using an event-related MRI design, we exploited the fact that processing low-frequency words increases BOLD activity relative to high frequency ones, and examined effects of base frequency on brain activity for decomposable and whole-word items. Morphologically complex words, half high and half low base frequency, were compared to matched high and low frequency simple monomorphemic words using a lexical decision task.
Morphologically complex words increased activation in left inferior frontal and left superior temporal cortices versus simple words. The only area to mirror the behavioral distinction between decomposable and whole-word types was the thalamus. Surprisingly, most frequency-sensitive areas failed to show base frequency effects. This variety of responses to frequency and word type across brain areas supports an integrative view of multiple variables during lexical access, rather than a dichotomy between memory-based access and on-line computation. Lexical access appears best captured as interplay of several neural processes with different sensitivities to various linguistic factors including frequency and morphological complexity.
Keywords: Morphology (Language), fMRI, Word Recognition, Lexical Access
1. Introduction
Complex words are those comprised of multiple morphemes, such as agree+able. For such words, the base verb (agree) can combine with a number of affixes (e.g., agree+ment). An important question in language processing concerns how such morphologically complex words are accessed from the mental lexicon. Much of the behavioral evidence supporting effects of morphological complexity on lexical access comes from the interplay of morphological structure and frequency. It is widely known that an individual word’s frequency of occurrence in the language will affect the speed with which that word can be accessed in a variety of tasks (Forster and Chambers, 1973; Frederiksen and Kroll, 1976; Gernsbacher, 1984; Balota and Chumbley, 1984; Balota and Chumbley, 1985). For morphologically complex words, however, the frequency of the word’s surface form (the word as a whole including its particular affix, e.g., agreeable), as well as the frequency of its base morpheme (that is, the total frequency of all the words containing this component morpheme, e.g., agree + agreed + agreeable + agreement + disagree, etc.), both play an important role in processing (see Alegre and Gordon 1999a, Baayen et al. 1997; Joanisse and Seidenberg 1999). These two types of frequencies are known as the surface frequency (the frequency of a particular word form) and the base frequency (the frequency of the base morpheme, equivalent to the total frequency of all the words containing this morpheme). The effect of the base frequency on response time is referred to as the base frequency effect. A number of studies have manipulated surface and base frequency of complex words independently and have shown that each of these variables affects response times for certain types of words (Taft 1979, Bradley 1980, Vannest and Boland 1999, Bertram et al. 2000). Base frequency effects indicate an influence of morphological complexity on processing; rather than each word containing the base being treated in isolation, the common base across morphologically complex forms affects the processing of all forms sharing that base.
Two types of theoretical accounts have been suggested for the presence/absence of morphological effects in complex word recognition. The classic decomposition view, based on linguistic accounts of rules used for combining morphemes, is that a base frequency effect reflects a process in which complex words are accessed in terms of their component morphemes, which are subsequently combined (Taft 1979, Bradley 1980, Burani and Caramazza 1987, Schreuder and Baayen 1995, Bertram et al. 2000, Vannest et al. 2002, Taft 2004). This type of model specifies either obligatory decomposition (Taft 2004) or a dual-system model in which complex words may be represented as separate morphemes, full forms, or both (Burani and Caramazza 1987, Schreuder and Baayen 1995). An alternative account of these same data (Rueckl, Mikolinski, Raveh, Miner, and Mars, 1997; Joanisse and Seidenberg 1999, Seidenberg and Gonnerman, 2000; Plaut and Gonnerman, 2000; Gonnerman, Seidenberg and Andersen 2007) is that accessing a complex word also activates all the other words in the lexicon that overlap substantially in phonology and meaning (this will usually turn out to be the same set of words that are morphologically related). In either case, accounts of this behavioral difference predict that morphologically complex words associated with robust behavioral base frequency effects should differ from complex words with no associated base frequency effect, as well as from simple words with no morphological complexity.
In the present study, we examine whether there are specialized regions of the brain that support this processing of morphological relationships among word families (words sharing the same base) or, alternatively, whether this kind of processing is done by the same regions that process monomorphemic words (though perhaps with higher levels of activation).
Derivational Morphology and the Base Frequency Effect
In this study, we focus on a particular subset of complex words in English that has shown varying results in behavioral studies: words with derivational affixes. Derivational affixes (such as English –able mentioned above) alter the meaning and grammatical category of the words to which they apply. Typically, particular derivational affixes apply to only a small set of base words; subsets of words in the same grammatical category often take different derivational morphemes to mark the same grammatical and semantic change. For example, in English, some verb forms become nominals by adding –ion (e.g., observation), while others add -ment (e.g., investment) or any of several other nominalizing affixes. Within this group of affixes, lexical access shows robust differences. On the one hand, some derivational affixes, including English - less, -ness and -able, or Dutch -heid, show effects of base frequency. On the other hand, words containing other derivational affixes, like English -ion, do not show a base frequency effect during word recognition. In keeping with the terminology in this literature, we refer to the former as ‘decomposable’ words and to the latter as ‘whole-words’.
A number of linguistic factors contribute to whether a word shows a base frequency effect or not, suggesting the possibility that the distinction might be graded rather than strictly dichotomous. Phonological properties have been proposed to determine the base frequency effect (Bradley, 1980). Typically, phonologically neutral affixes (those that do not cause phonological changes to the words they attach to, e.g., agree - agreeable) show a base frequency effect, whereas non-neutral affixes like -ity (where there can be phonological interaction between base and affix, e.g., serene – serenity) do not (Vannest and Boland, 1999). However, Taft (2004) has demonstrated that the absence of a base frequency effect may not rule out the possibility of explicit representation of individual morphemes (see details below). Moreover, some priming studies show facilitation across the type of word pairs that involve a phonological change (Boudelaa and Marslen-Wilson, 2004), suggesting that their relationship does not go unrecognized. A possible explanation is that a complex word’s semantics is another important linguistic factor (Marslen-Wilson et al. 1994, Feldman et al. 2002, 2004; Bertram et al. 2000b). When the meaning of a complex word can be easily understood from its parts (e.g. adorable), it is more likely to show a base frequency effect than a word whose meaning is not as clearly derived from its parts (e.g. hospitality). Such findings, indicating sensitivity to phonological and semantic structure, are consistent with the view that the base frequency effect reflects co-activation of morphological families during lexical access, or a graded effect of the relevant variables, rather than the presence/absence of a decomposition process. Overall, the behavioral literature on derivationally complex words is extensive and has suggested that a number of variables, typically highly correlated with one another, can influence the base frequency effect. In the present work, we limit ourselves to morphologically complex words either known to lead to robust base frequency effects or not, and we ask how the neural network engaged during lexical access of each type of words differs from that supporting simple, monomorphemic words as well as from each other. We also take advantage of the fMRI method to ask whether neural activation reveals any effect of morphological complexity for ‘whole words’ that behavioral measures do not. This study will thus provide a first window on the neural bases of the base frequency effect or lack thereof.
Models of Morphological Processing
As already noted, two types of accounts have been suggested for the presence/absence of morphological effects in complex word recognition. One class of models suggests that most complex words are processed in terms of their component morphemes, which are subsequently checked for compatibility. On this account, base forms of complex words are stored in the lexicon; inflectional or derivational morphemes are added through a compositional process that is reflected in longer reaction times (Niemi et al. 1994). Full decomposition accounts (Taft 1979, 2004) assume that complex words are always recognized in terms of component morphemes, and base frequency effects arise from the fact that base forms are activated as part of this process. Taft (2004) suggests that even the absence of a base frequency effect does not indicate that no decomposition process occurs. Rather, properties of particular complex words and the other items that surround them in an experimental context (for example, syntactic category ambiguity of the stem, infrequent combinations of stem and suffix, and a context of nonwords made up of real morphemes) may make the recombination stage slower, masking base frequency effects.
Dual-system models (Burani and Caramazza 1987, Schreuder and Baayen 1995) also assume that base frequency effects arise from explicit representation of individual morphemes, but if they are frequent enough, full forms are also represented. Factors such as phonological and semantic transparency may influence whether items are stored in their full form versus constructed from separate morphemes (Bertram et al., 2000). On this account, words with phonologically neutral affixes (such as -able) are influenced by their individual morphemic components, whereas words with non-neutral affixes like -ion are accessed as whole units and therefore do not show a base frequency effect (Schreuder and Baayen 1995, Vannest et al., 2002).
However, as mentioned above, base frequency effects for complex words may be accounted for by mechanisms other than a decomposition process during lexical access. Connectionist models of word recognition (see Rueckl et al., 1997; Joanisse and Seidenberg 1999, Seidenberg and Gonnerman, 2000, Rueckl and Raveh 1999, Raveh 2002) hypothesize that a single mechanism supports recognition of monomorphemic and complex words, without decomposition. According to these models, morphological relationships between words result from overlap in orthographic, phonological and semantic properties of words. For example, Joanisse and Seidenberg (1999) propose such a model for past tense inflection: regular (-ed) past tense word forms have a great deal of phonological similarity with their base forms, so phonological similarity can account for why these forms may be accessed together. Irregular past tenses (e.g. go ➔ went) do not have as much phonological overlap with their base forms, so that semantic information plays a greater role in accessing these past tenses. In this framework, base frequency effects result from frequency-sensitive connections between base words and complex forms that overlap in varying degrees of orthographic, phonological and semantic similarity. Thus, for example, responses to agreeable are facilitated by the frequency of agree more than serenity is facilitated by serene, not because agreeable is accessed as two separate morphemes, but because of the larger degree of orthographic and phonological overlap between agree and agreeable. Rueckl and Raveh (1999) showed that a three-layer network model could quickly learn mappings between orthography and semantics when these mappings contained morphological regularities, whereas random form-to-meaning mappings were learned more slowly. The success of this kind of model suggests that morphological relationships among words could indeed emerge without any explicit representation of a morphological process in the lexicon.
Neuroscientific Work on Simple versus Complex Words
While many studies have examined behavioral measures of processing derived words, only a handful of studies have addressed the neural bases of derived word processing. Ito et al. (1996) found that Broca's aphasics have difficulty producing a regular derivational affix in Japanese, but not in producing less productive derived forms; they found the opposite pattern for Wernicke's and transcortical aphasics. Vannest, Polk and Lewis (2005) used fMRI to assess the participation of Broca’s area and the basal ganglia in processing derived and inflected words. They found that neural activation increased in Broca’s area and the basal ganglia for inflected words and for derived words that show base frequency effects in behavioral studies relative to monomorphemic words, but not for derived words that do not show base frequency effects (that is, derived words hypothesized to be processed as whole wordforms). Whereas these studies highlight the role of the frontal-basal ganglia network in the processing of derived words, not all studies do so. Using a masked priming paradigm with a lexical decision task to examine derived words, Devlin et al. (2004) found that activation was reduced in the left angular gyrus, left occipitotemporal cortex and the left middle temporal gyrus for base forms when primed with a derivational relative. These same areas were also modulated by semantic and orthographic priming, indicating that the priming from derivational relatives may have been mediated by these factors. Bick, Frost and Goelman (2010) used a similar paradigm in Hebrew and also found morphological priming effects in left frontal and parietal regions, though only the parietal actvation was affected by semantic transparency. Davis, Meunier and Marslen-Wilson (2004) failed to find any effect of morphology in a task requiring synonym monitoring of morphologically complex (inflected and derived) vs. simple English words.
The available studies therefore indicate that, to the extent that an effect of morphological complexity can be observed, it is expressed by an increased recruitment of left language areas, especially the frontal-basal ganglia network and the superior temporal sulcus, during the analysis of morphologically complex words as compared to simple words. However, these findings are not entirely consistent in the specific regions involved or in the precise effects found. In the present study we will investigate this question further, by taking advantage of the known effect of word frequency on brain activation (low frequency processes lead to higher brain activation than high frequency, more automatized processes), in combination with the extensive behavioral evidence of base frequency effects for morphologically complex words, to revisit the question of which areas in the language system that may mediate the processing of complex as compared to simple words.
Neuroscientific Work on Word Frequency Effects
Before asking how manipulations of base frequency might affect neural activation for complex words, it is important to consider what we know about the effects of simple word frequency on neural activation for monomorphemic words. The effect of frequency on neural activation for monomorphemic words has been investigated in a number of studies. The general pattern is increased activation for low frequency words in brain areas associated with lexical processing. Fiez et al. (1999), using PET, found increased activation for naming low versus high frequency words in left superior temporal regions, left supplementary motor regions, and left inferior frontal gyrus (where it interacted with spelling/sound regularity). Using fMRI, Keller et al. (2001) found that reading low as compared with high frequency words in sentence contexts increased activation in a number of regions including the left inferior frontal gyrus, a left superior/middle temporal region, and left and right extrastriate visual cortex; weaker effects were also found in the right hemisphere homologues of these regions. This frequency effect also interacted with sentence complexity (the sentence complexity effect was greater when the sentence contained low frequency words) in left hemisphere language ROI’s. Chee et al. (2003), using a semantic judgment task in fMRI, also found increased activation for low relative to high frequency words in the left inferior frontal gyrus, along with a lesser extent of activation in its right hemisphere homologue, and additional areas of activation in the left anterior cingulate and a left inferior temporal region. Fiebach et al. (2002), using a lexical decision task in fMRI, also showed increased activation for reading low over high frequency words in the left inferior frontal gyrus, anterior insula bilaterally, and in the caudate nucleus and thalamus bilaterally. Recent studies by Kronbichler et al. (2004) and Hauk et al. (2008) made use of fMRI and a silent reading task and parametric variation in word frequency, examining regions that increased in activation with decreasing frequency. These studies found that middle occipital gyrus (Kronbichler et al., 2004) and fusiform gyrus responded to word frequency, as well as left inferior frontal gyrus (Kronbichler et al., 2004 and Hauk et al., 2008). Hauk et al. (2008) also found a relationship with frequency in the insula bilaterally as well as left and right inferior frontal gyrus.
In sum, then, all studies manipulating word frequency indicate greater recruitment of the brain areas that mediate language processing, whether at the lexical level or at the sentence level, for low frequency as compared to high frequency words.
Rationale for the Present Study
The present study compares complex words with high and low base frequency to simple words with matched high and low surface word frequency. This comparison allows us to ask which brain regions show effects of morphological complexity, both independent of frequency (i.e., a main effect of morphological complexity) and interacting with frequency. Of particular interest is the extent to which those brain areas that show a frequency effect for simple words also show a base frequency effect for complex words. The standard view that complex words are decomposed into morpheme+affix, leading to the base frequency effect in behavioral studies, predicts overlapping brain areas responsive to both types of frequency. Based on the existing literature, these may include inferior frontal gyrus, superior/middle temporal regions, extrastriate visual areas, anterior cingulate, insula bilaterally, caudate nucleus or thalamus. Alternatively, some of these regions, which have previously been found to respond to simple word frequency, may not respond to base frequency. This pattern of results would suggest that frequency and morphological structure interact, and contrary to the classical view of decomposition, that the components of complex words are not processed the same way as simple words. The frequency manipulation included in the present design allow us to characterize and differentiate between those brain areas that may reflect compositional behavior and those that do not.
Importantly, then, our study will investigate the details of neural activation during morphological processing by contrasting complex and simple words. In addition, we will investigate the details of neural activation for morphological complexity by exploiting the fact that not all derived words show a base frequency effect. As described earlier, words with derivational affixes that are less productive, more idiosyncratic in meaning, and change the form of base words to which they attach (e.g. -ion, as in locate ➔ location) do not show the usual base frequency effects correlated with morphological complexity and appear to be processed as whole-word units. We therefore will examine the brain systems that mediate the processing of words that do show base frequency effects in behavioral work (called ‘decomposable’), as well as those that do not (called ‘whole-word’). Note that we choose these terms so as not to single out any property (phonological or semantic transparency, productivity) but to represent a cluster of properties that contribute to the finding that some words show morphological effects while others do not. The behavioral literature has often attempted to assess which of the properties of complex words – their phonological transparency, semantic transparency, productivity, or family size – is responsible for the morphological effects. However, some of these properties (e.g., family size) are systematically related to morphological complexity, and several of these properties are not possible to differentiate perfectly in an fMRI study of real English words (see Methods for more discussion of this point). While we attempt to match or differentiate these properties wherever possible, this is not the main focus of the study; rather, we ask whether the properties of complex words act as a cluster of correlated factors that influence their representation and processing, and perhaps, as we investigate here, the neural mechanisms that underlie this processing.
The proposal that ‘decomposable’ words are decomposed into parts, whereas ‘whole-words’ are processed as whole lexical units, like simple words, predicts a greater recruitment of those brain areas sensitive to morphological complexity for ‘decomposable’ as compared to ‘whole-words’. However, we will also be interested in investigating the extent to which the complex morphological structure of ‘whole-words’ differentiates them nonetheless from simple words. While behavioral evidence has suggested that ‘whole-words’ are no different than simple words, it is possible that patterns of neural activation may reveal a more graded role of morphological complexity than has previously been revealed, with ‘whole-words’ showing activation patterns somewhere in between those of decomposable words and those of simple monomorphemic words, in line with their intermediate morphological status.
Finally, we will analyze our fMRI data with the goal of decoupling the differences in response time to high frequency and low frequency words from differences in fMRI activation. A common finding in fMRI studies is that the level of BOLD signal increases with increased task difficulty, which often corresponds to increases in the time required to perform the task (i.e. longer response times, see Dassonville et al. 1998, Huettel et al. 2001, Huettel et al. 2004). Consequently, it becomes difficult in data analysis to interpret higher BOLD signal in a particular brain region when response times differ between two conditions: is this signal increase due to the relevant cognitive operations involved in the task, or due to perceptual or motor processing that increases with longer response time? Stowe et al. (2004), for example, suggest that it may be impossible to separate cognitive/language functions from motor processes in subcortical areas and the cerebellum. In an effort to distinguish differences due to frequency or morphological structure versus response time in our data, a regression analysis will be used to identify the BOLD activation level in various brain regions that vary directly with response times in the task. All further analyses of frequency and morphology effects will be performed after variation due to perceptual and motor increases associated with longer response time is removed, resulting in a more accurate assessment of these effects independent of response preparation/execution times.
2. Experimental Procedure
Materials
Materials consisted of 80 ‘decomposable’ derived words with -able -ness, or –less (40 with high base frequency and 40 with low base frequency) and 80 ‘whole-word’ derived words with –ity or –ation (40 with high base frequency and 40 with low base frequency). Also included were 80 monomorphemic words (40 high frequency and 40 low frequency) (frequency counts from MRC psycholinguistic database, Coltheart, 1981). Note that base and surface frequency are closely correlated for monomorphemic words in English in general, and also in our set of items. While a few studies have begun to investigate the independent influence of base frequency on monomorphemic words (e.g. Schreuder and Baayen 1997), we designed our monomorphemic items with a contrast in surface frequency, in accord with much of the established literature on frequency effects for these items.
While the fMRI design required a fairly large number of distinct words in each of these categories (so that there would be no repeated presentations of a word across the experiment), there were not enough words in English of the relevant types to permit us to match words perfectly on all the many dimensions that have been studied in the behavioral literature. Stimulus selection proceeded with the following constraints in mind: (1) Stay within the domain of derivational morphology where the base frequency effect has been well documented behaviorally (i.e. effects of decomposable versus whole-word base frequency); (2) Vary base frequency between high and low, and match all items for surface frequency; (3) Do not repeat stimuli, and still assemble enough words to perform an event-related fMRI study; (4) Match for length and other variables as closely as possible without altering the frequency matching.
Word length was matched as closely as possible across word types, given the frequency constraints and the number of different words required by the fMRI design. As Table 1 illustrates, length was closely matched between decomposable and whole word items; however, such a close match could not be achieved with monomorphemic items without compromising the frequency match. Since this was the core of our study, we privileged frequency matching over length matching.
Table 1.
Mean frequency for each condition as calculated from the MRC Psycholinguistic Database; word length in characters; family size (including derived forms only, based on Schreuder and Baayen, 1997).
| Word Type: | “decomposable” -able, -less, -ness | “whole-word” -ity, -ation | Monomorphemic | |||
|---|---|---|---|---|---|---|
| High Base freq. | Low Base freq. | High Base freq. | Low Base freq. | High freq. | Low freq. | |
| Surface frequency | 6.95 (5.4) | 6.05 (7.7) | 7.8 (7.8) | 7.25 (6.2) | 202.73 (117.1) | 9.88 (11.2) |
| Base frequency2 | 204.13 (118.6) | 28.08 (19.6) | 208.1 (137.1) | 20.95 (14.7) | 214.18 (121.7) | 13.88 (15.2) |
| Length (characters) | 9 (1.3) | 9 (1.1) | 9.28 (1.0) | 9.1 (1.5) | 6.55 (0.8) | 6.6 (0.7) |
| Family Size | 6.2 (5.2) | 4.3 (3.1) | 6.3 (6.2) | 3.0 (2.3) | 3.8 (4.4) | 1.8 (2.3) |
The role of base vs. surface frequency is not as clear for monomorphemic words as it is for complex ones. In English, monomorphemic words will appear much more often as surface forms than as part of an inflected or derived form, making their base and surface frequency closely correlated.
Complex words were matched for surface frequency, which was quite low for both types of complex words (Table 1). Both groups of derived words included items that involved a spelling change from base to suffix form (envy --> enviable): 19 in the decomposable group and 30 in the whole-word group. Decomposable words were all phonologically transparent; among whole-word items, 9 items in the low base frequency set and 2 in the high base frequency set were phonologically transparent. In addition, family size was correlated with base frequency in our stimulus set, as is the case for most complex words in English. Although we will refer to base frequency effects in the remainder of the paper, family size may also play a role in the base frequency effects we describe.
Taft (2004) suggests that syntactic ambiguity of the base may interfere with base frequency effects because responses to words with high base frequency may be slowed by competition between forms associated with multiple syntactic categories, making a decision about which suffixes may combine with a particular base more difficult. Unfortunately, it was impossible to remove syntactically ambiguous stems since a substantial number of words in English are used in multiple syntactic categories, especially higher frequency words. Indeed, this relationship with frequency was present in our data: among high-frequency monomorphemic words, 13/40 could be used in multiple syntactic categories; in the low-frequency group, only 6/40. Among decomposable words, 21/40 of the bases in the high-frequency group were syntactically ambiguous, and only 9/40 in the low-frequency group. In whole-word words the statistics were similar: 13/40 of the bases in the high-frequency group and only 6/40 in the low-frequency group. In our stimuli, then, the greatest amount of syntactic ambiguity was in the high base frequency decomposable words, where we most expect base frequency to speed processing. Therefore, syntactic ambiguity may work against the frequency effects we predict, but will not bias our results.
In order to make a lexical decision task possible, nonwords were presented as well, the data from which are not analyzed here. Nonword items included 60 pronounceable nonwords, 60 nonwords with nonsense bases and the same real suffixes present in the real word items (e.g. blurkable), and 40 nonwords with real base words and nonce suffixes (e.g. cooliben). The purpose of the nonword stimuli was to make it impossible to make the lexical decision strictly on the basis of the base or suffix alone. Taft (2004) has shown that a preponderance of nonwords made up of real bases and suffixes in illegal combinations makes determining the combinability of base and suffix more difficult and may therefore wash out base frequency effects, so we avoided this type of nonword in our stimuli.
Participants
Participants were 22 right-handed native speakers of English (8 males), ages 18–30 with normal or corrected-to-normal vision and no history of reading disabilities or neurological disorders. For reasons detailed below, only 18 of these (7 males) were included in the analysis. Each subject gave informed written consent according to guidelines of the University of Rochester Research Subjects Review Board.
Procedure
Words and nonwords were presented in counterbalanced order in an event-related design. The event-related design is particularly appropriate for a lexical decision task because it allows items to be presented in a variable order without being grouped into blocks. While block designs may increase sensitivity to changes in fMRI signal, repetition of a particular type of item may result in a behavioral strategy that does not reflect normal processing. Presenting a lexical decision task in a block design would result in repetitive ‘yes’ and ‘no’ responses, and is not the standard behavioral paradigm for this task.
Words/nonwords of each morphology and frequency condition (high and low frequency simple words, high and low frequency ‘decomposable’, high and low frequency ‘whole word’, along with pronounceable nonwords with and without real morphemes) were divided evenly over four runs of the MRI scanner, with a total of 100 stimuli per run. Order of presentation of each run was counterbalanced across subjects. Stimuli were presented in 36 pt Arial font in black capital letters on a 30% grey background. Each word/nonword was preceded by a ready signal of three asterisks presented for 500 ms, then the word/nonword was presented for 500 ms. Participants made their YES/NO lexical decision response using a keypad positioned in their right hand. The interstimulus interval varied from 4–12 seconds (in 1-second increments), resulting in a jittered presentation rate suitable for event-related fMRI designs (Miezin et al. 2000). Each scanner run was 8.5 minutes long. During the inter-stimulus interval, a visual fixation cross was presented (null events), during which a resting baseline level of activation was established.
fMRI methods
This experiment was carried out using a Siemens Trio 3 T scanner at the Rochester Center for Brain Imaging, University of Rochester. We used a standard clinical quadrature radiofrequency head coil; foam padding was used to restrict head motion. Gradient-echo, echoplanar image acquisition was used to detect susceptibility-based (BOLD) contrast. Thirty contiguous oblique axial slices were obtained per acquisition, with flip angle 90 degrees, 30 msec effective TE, a TR of 2 seconds, FOV 256 mm and a 64 × 64 matrix, resulting in a voxel size of 4 × 4 × 4 mm. Each imaging protocol concluded with an 8.5 min T1-weighted structural MRI (MP-RAGE sequence), TR = 1960 msec, TE = 3.93 msec, 176 slices in a 256 × 256 matrix, voxel size 1 × 1 × 1 mm).
fMRI data processing and analysis
K-space data was reconstructed using a 2D FFT, then, using the AFNI software package (Cox, 1996), slice timing correction was performed (using Fourier interpolation in AFNI’s 3dTshift program) to align the acquisition time of all 30 slices to the same timepoint, as well as head movement correction using a rigid body (6-parameter) model in AFNI’s 3dvolreg program. Three participants who exhibited more than 2 mm of head movement in any direction were discarded from subsequent analysis. An additional participant was excluded from analysis due to a malfunction of the response device (behavioral lexical decision data could not be collected for this participant). Then, using the FLIRT program in the FSL 3.3 software package (Jenkinson et al., 2002) each subject’s movement-corrected images were skull-stripped and normalized to a to the standardized space of the International Consortium for Brain Mapping (ICBM152). Subsequent to this, the images were spatially smoothed with a Gaussian kernel having a full width at half maximum of 2× the voxel dimensions (8mm), using AFNI’s 3dmerge program.
Statistical analyses were performed using AFNI’s 3dDeconvolve software program. For each subject, voxel-wise multiple regression was performed using a general linear model approach. The expected hemodynamic response to each word was modeled by a 1 sec event convolved with a gamma function. The full regression model included regressors for correct responses to each combination of word type (simple, decomposable, whole-word) and frequency level (low, high) as well as a regressor for nonwords and error trials. (Error rates were extremely low, see behavioral results below). An initial 3-way mixed-effects ANOVA (AFNI program 3dANOVA3) with subjects treated as a random effect and word type and frequency treated as fixed-effects factors was then run. From this analysis, group-level activation maps were obtained for each of the six word conditions (contrasted with the fixation baseline). These were thresholded at a voxel-wise alpha of p < 0.005 (z threshold = 2.58) and a minimum cluster size of 24 voxels resulting in a corrected brain-wise alpha value of p < 0.05 (determined using Monte-Carlo simulations via AFNI’s AlphaSim program). As described below, these maps were then used to ensure that only voxels showing positive activation with language stimuli be included in all further analyses.
In order to test our hypotheses about the interaction between frequency and morphological processing on activation in the language comprehension system, all analyses focused on regions where effects of word frequency (Fiez et al. 1999, Keller et al. 2001, Chee et al. 2003, Fiebach et al. 2002) or morphological processing (Jaeger et al. 1996, Laine et al. 1999, Beretta et al. 2003, Shtyrov et al. 2004, Devlin et al. 2004, Vannest et al. 2005) have previously been reported. These include the inferior frontal gyrus, insula, supplementary motor area, superior, middle and inferior temporal gyri, the angular gyrus, inferior occipital cortex, fusiform gyrus, the caudate nucleus, thalamus and cerebellum. These regions of interest were first defined both in the left and right hemispheres using the anatomical parcellations of the ICBM single-subject template performed by Tzourio-Mazoyer et al. (2002). Then to further increase the sensitivity of our analysis to relevant difference in BOLD signal among our different word conditions, these regions were further restricted through use of a functionally-defined mask. This mask was created by taking the union of all six group maps described above (3 word type × 2 frequency, p-corrected<.05). All further analyses were limited to voxels in our anatomical regions of interest that were also part of this union mask. This masking ensures that only areas activated in performance of the task (relative to the low-level baseline) were considered, therefore preventing possible contamination from deactivation when comparing between conditions. Appendix 1 includes a list of the areas included and their extent in number of voxels, along with MNI coordinates for the cluster centroid of the ROI after application of the mask.
After ROIs were defined according to the method described above, the analyses proceeded in a two-stage process. In an effort to distinguish signal increases due to the relevant cognitive operations involved in the task from increases due to perceptual or motor processing involved with longer response time, a regression analysis was used to assess the role of each participant’s mean response time (correct responses only) in each word condition as a predictor of each participant’s mean percent BOLD signal change in each ROI. This approach identified various brain regions where the BOLD activation level varied directly with response times in the task. All further analyses of frequency and morphology effects did not include variation associated with longer response time by being performed on each participant’s residual mean BOLD response in each ROI extracted during this first regression analysis.
Using these residuals, a 3 × 2 mixed-effects ANOVA (AFNI program 3dANOVA3) with subjects treated as a random effect and fixed-effect factors word type (decomposable, whole-word and simple) and frequency (high and low) was performed to assess the relative sensitivity of these areas to variation in morphological complexity and in frequency and their interaction. For regions where significant effects of word type were found in the ANOVA, planned contrast analyses were performed. These contrasts examined the effect of morphological complexity by comparing each of two complex word conditions (decomposable and whole-word) to simple. All results discussed below are p < 0.05 (corrected as described above) unless otherwise noted.
Behavioral response time data was analyzed using separate 3 × 2 ANOVAs with word type (decomposable, whole-word and simple) and frequency (high and low) as factors, and items and participants, respectively, as independent variables.
3. Results
Behavioral data
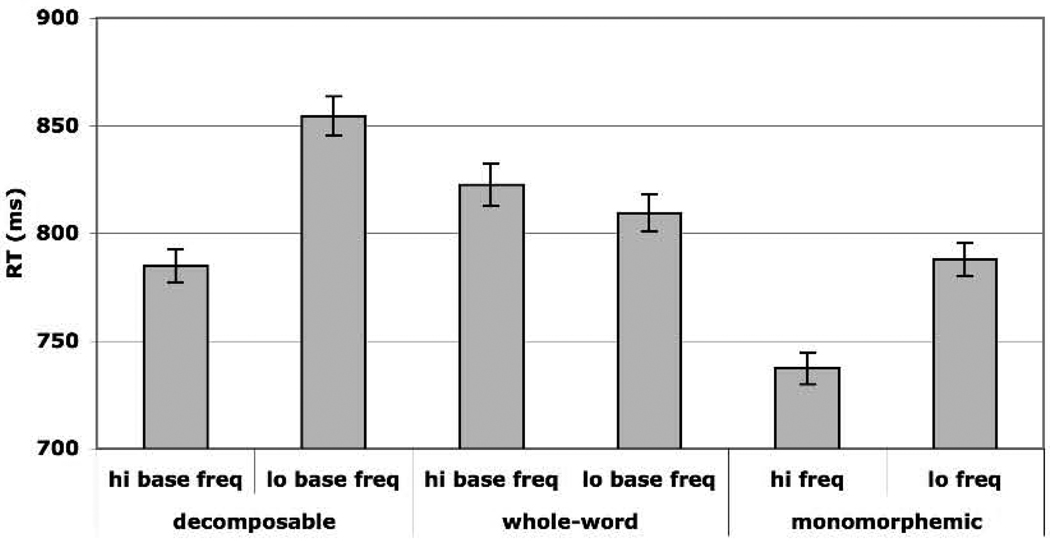
The reaction times obtained for different types of items in the lexical decision task are shown in Figure 1 (for correct responses only). The ANOVA showed a main effect of frequency [F1(1,17)=22.12, p<.05; F2(1, 239)=13.79, p<.05], a main effect of word type [F1(2, 34)=44.47, p<.05; F2(2, 238)= 14.61, p<.05], and an interaction between frequency and word type [F1(2, 34)=14.85, p<.05; F2(2, 238)= 6.35, p<.05]. Planned comparisons revealed that, as in previous studies, ‘decomposable’ words showed an effect of base frequency, with faster response times to high than low base frequency words [t1(17)=6.27, p<.05; t2(78)=4.03, p<.05]. ‘Whole-words’ did not show such an effect (p>.3). Simple words also showed a significant word frequency effect [t1(17)=7.86, p<.05, t2(78)=4.59, p<.05]. Reaction times and error rates for each condition are listed in Table 2.
Figure 1.
Mean lexical decision response times for each condition (error bars correspond to standard error of the mean).
Table 2.
Response times and error rates for each word type and frequency condition
| Word Type: | “decomposable” -able, -less, -ness | “whole-word” -ity, -ation | Monomorphemic | |||
|---|---|---|---|---|---|---|
| High Base freq. | Low Base freq. | High Base freq. | Low Base freq. | High freq. | Low freq. | |
| RT (standard error) | 784.9 (7.8) | 854.5 (9.0) | 822.5 (9.6) | 809.5 (8.5) | 737.2 (7.3) | 787.7 (7.7) |
| Error rate (standard error) | 4.3 (1.1) | 7.0 (2.1) | 6.0 (1.6) | 5.7 (1.2) | 0.7 (.4) | 5.7 (1.3) |
fMRI data
Analysis 1: Response time as a significant predictor of BOLD response
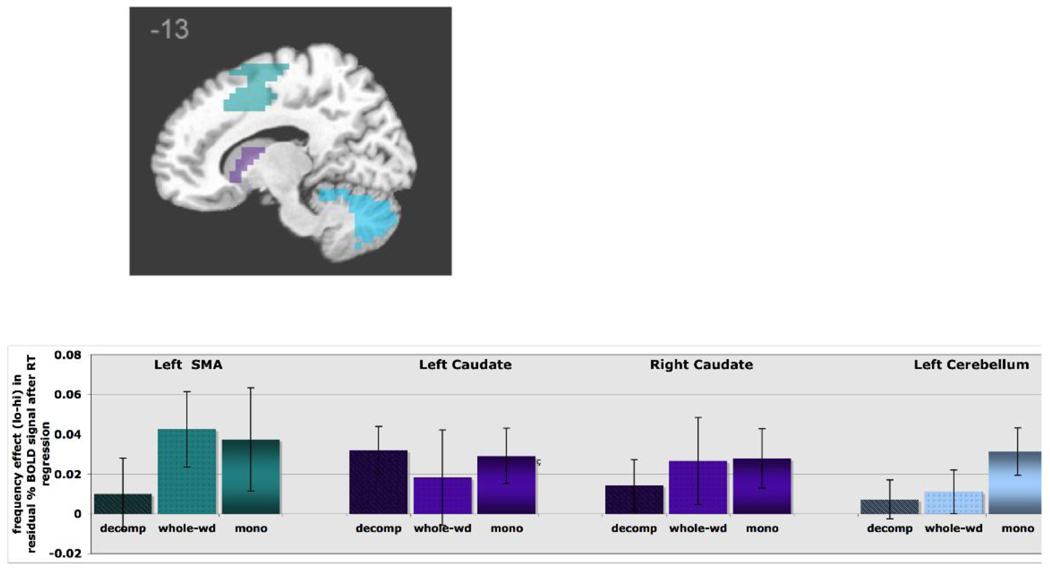
Those regions where lexical decision response time was a significant predictor of BOLD signal change are presented in Table 3, along with relevant statistics. These were the left SMA, right middle temporal gyrus, right inferior occipital cortex, the caudate nucleus, thalamus, and the cerebellum bilaterally. The SMA may reflect involvement in the preparation and execution of motor aspects of the button-press response that was made with the contralateral hand (Banich 1997, Carlson 1998, Dassonville et al. 1998) and has previously shown increases in fMRI activation with increased motor response time (Dassonville et al. 1998, Richter et al. 2000), particularly in tasks where the motor response is unpredictable. The caudate and cerebellum may also reflect motor processing (Carlson 1998, though see Stowe 2004 for discussion). Activation in both the thalamus (Gitelman et al. 1999, Huettel et al. 2001) and occipital areas (Huettel et al. 2001, Mohamed et al. 2004) may be modulated with increased visual attention and perceptual processing. Overall, the areas where response time was a significant predictor of BOLD signal are those involved in perceptual or motor processing (Carlson 1998).
Table 3.
ROIs where response time is a significant predictor of BOLD percent signal change.
| Region | r | Regression p-value |
|---|---|---|
| L SMA | 0.310 | 0.001 |
| R Inferior Occipital | 0.310 | 0.001 |
| R Caudate | 0.340 | 0.0001 |
| L Caudate | 0.192 | 0.046 |
| R Thalamus | 0.281 | 0.003 |
| L Thalamus | 0.262 | 0.006 |
| R cerebellum | 0.251 | 0.009 |
| L cerebellum | 0.37 | 0.0001 |
Analysis 2: Frequency and Word Type Effects
As described above, 3×2 ANOVA of word type (decomposable, whole-word and simple) and frequency (low versus high) was performed on the residual BOLD response in each ROI after regression analysis. These analyses determine the relative sensitivity of these areas to variation in frequency and in morphological complexity, above and beyond differences in response time (see footnote 1 for the same analysis done without regressing reaction times).
2.1 Effects of Frequency
Regions reported in Table 4 showed either a main effect of frequency or an interaction between frequency and word type.
Table 4.
ROIs that show frequency effects in the 3×2 ANOVA on mean residual percent change (after RT regression).
| P level for main effect of frequency |
P level for freq. X wordtype interaction |
|
|---|---|---|
| L SMA | 0.04 | |
| R Insula | 0.002 | |
| L Insula | 0.031 | |
| L Inferior Occiptal | 0.039 | |
| R Caudate | 0.08 | |
| L Caudate | 0.06 | |
| R Thalamus | 0.006 | 0.017 |
| L Thalamus | 0.003 | |
| L Cerebellum | 0.039 |
2.1.1 Regions that show a main effect of frequency
Among the regions responsive to frequency were left SMA, left cerebellum, thalamus bilaterally and the caudate nucleus (marginally significant, p = 0.06 for the left caudate and p = 0.08 for the right). As expected, these areas showed overall greater activation for low frequency relative to high frequency words (see Figure 2). Importantly, while activation in these areas was correlated with response time, the effect of frequency persisted even in this analysis in which response time was regressed out. Thus these areas, perhaps involved in the perceptual and motor aspects of the task, were also sensitive to frequency above and beyond their association with increased response times.
Figure 2.
ROIs that display a main frequency effect (low>high) after removal of the effect of response time: left SMA, bilateral caudate nucleus and left cerebellum. For each ROI, the frequency effect is graphed as a function of word type.
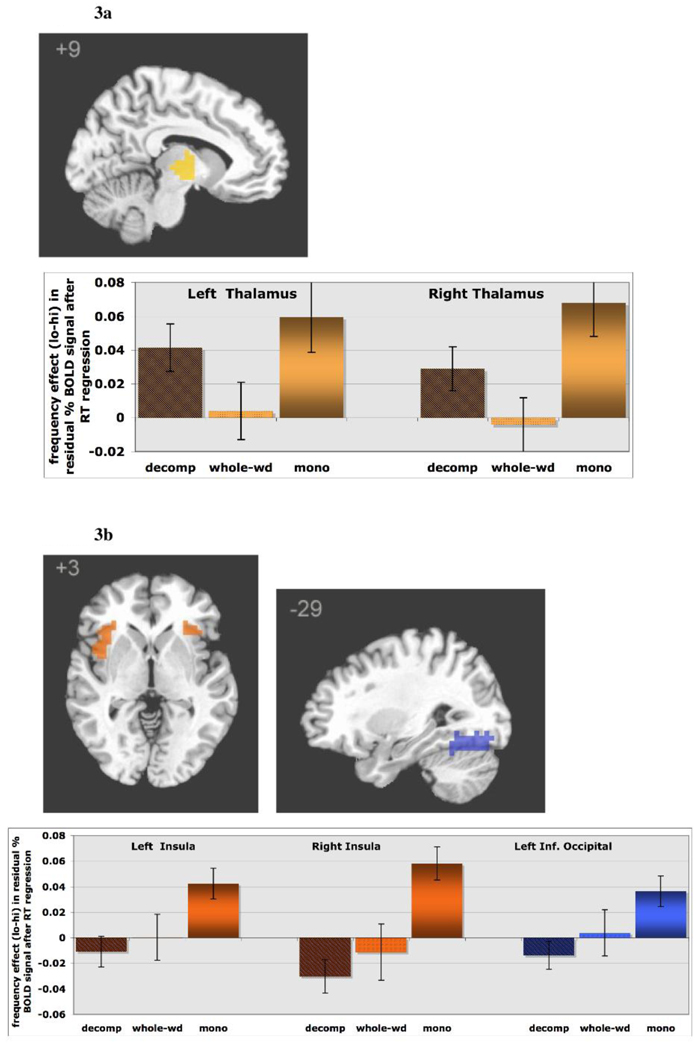
2.1.2 Regions that show an interaction of frequency and word type
Of the areas that showed a main effect of frequency, only the thalamus showed an interaction between frequency and word type. The pattern of effect was similar for left and right thalamus, though the interaction reached significance only on the right. Importantly, the interaction reflects a pattern of activation highly consistent with the behavioral data, with significant effects of frequency (low > high) for ‘decomposable’ words and simple words but little effect of frequency for ‘whole-words’ (Figure 3). Of all the language regions considered, the thalamus was the only area in which activation correlated with the base-frequency effect seen behaviorally.
Figure 3.
ROIs that display a frequency by wordtype interaction after removal of the effect of response time. For each ROI, the frequency effect is graphed as a function of word type. (a) The thalamus showed a pattern of frequency effects parallel with the behavioral data: significant effects for decomposable and monomorphemic words, but not for ‘whole-word’ words. (b) The insula bilaterally and left inferior occipital region showed an effect of frequency for monomorphemic words only.
Two additional areas that did not show a main effect of frequency did show an interaction of frequency and word type: the insula bilaterally and the left inferior occipital gyrus. These areas displayed a large frequency effect for simple words that is not observed for the two types of complex words. This suggests that these areas are sensitive to word frequency in simple words, but not base frequency in complex words.
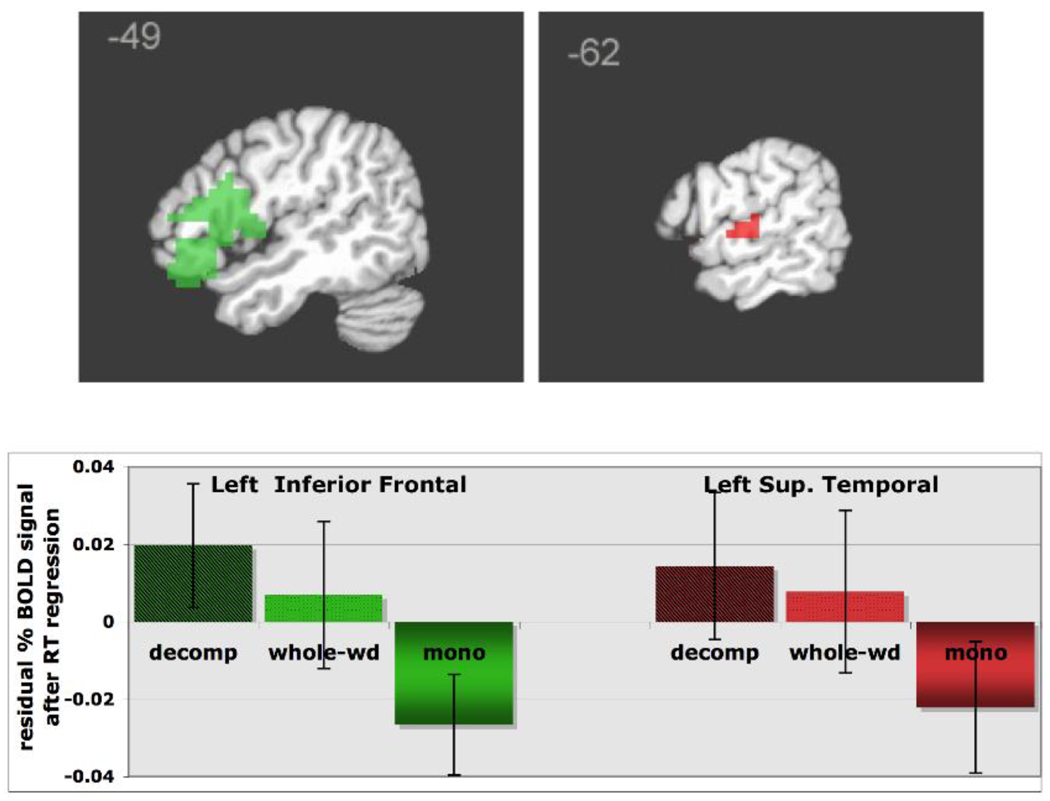
2.2 Effects of Word Type
A number of regions showed greater recruitment for complex words (whole-word and decomposable) than simple words. These include the left inferior frontal gyrus and left superior temporal gyrus, as well as the right inferior temporal gyrus and left angular gyrus (AG, p = 0.07 - Table 5). The source of this main effect was assessed by contrasting each of the two complex word conditions (decomposable and whole-word) with simple words in separate comparisons.
Table 5.
ROIs that show effects of wordtype in the 3×2 ANOVA on mean residual percent change (after RT regression).
| P level for main effect of wordtype |
P level for planned contrast decomp. > mono |
P level for planned contrast whole >mono |
|
|---|---|---|---|
| L Inferior Frontal | 0.003 | 0.005 | .02 |
| L Superior Temporal | 0.014 | 0.011 | .054 |
| R Inferior Temporal | 0.033 | ||
| L Angular | 0.07 |
Of those regions that showed a main effect of word type in the ANOVA, two regions showed increases for ‘decomposable’ words relative to simple words: the left inferior frontal gyrus and left superior temporal gyrus (Figure 4). Interestingly, ‘whole-words’ showed similar, though smaller, increases in BOLD signal in these two regions relative to simple words (Figure 4).
Figure 4.
ROIs that display a main effect of wordtype (complex > simple): left inferior frontal gyrus and left superior temporal gyrus. For each ROI, the mean residual percent signal change (after RT regression) is graphed as a function of word type.
Note that while all of the complex words elicited longer response times than simple words, this variation has been removed from the fMRI data in this analysis. None of the regions displaying effects of morphological complexity resulted in BOLD signal that was significantly correlated with response time, further suggesting sensitivity to morphological complexity above and beyond differences in response time1.
4. Discussion
Behavioral Data
Our lexical decision response time data, revealing a frequency effect for monomorphemic words, a base frequency effect for ‘decomposable’ words, and no base frequency effect for ‘whole-word’ items, is consistent with previous work (Bradley 1980, Vannest and Boland 1999, Vannest et al., 2002). As mentioned above, models of morphological processing have different accounts for the presence and absence of base frequency effects in these different types of words. Obligatory decomposition accounts (Taft 1979, 2004) assume that all complex words are accessed as separable morphemes and base frequency effects arise from direct access of the base. In cases where base frequency effects do not arise, this model assumes that they are masked by slowing at a later stage in processing where morphemes are recombined and a lexical decision is made. Dual-system models (Schreuder & Baayen, 1995; Burani and Caramazza 1987) assume that both individual morphemes and full forms are represented; factors such as semantic and phonological transparency influence how a complex word is accessed. While the dual-system models differ in the exact details of the level of processing where base frequency effects arise, they account for our behavioral results by assuming that ‘whole-word’ items are accessed as full-forms, ‘decomposable’ items as separable morphemes. Finally, single-system models (Rueckl, et al., 1997; Joanisse and Seidenberg 1999, Seidenberg and Gonnerman, 2000; Plaut and Gonnerman, 2000; Gonnerman et al., 2007) suggest that base frequency effects arise not from any explicit representation of morphemes in the lexicon, but from co-activation of words that are similar in form and meaning (which happen to be morphological family members). The activation of more (and more frequent) related items results in faster response times. For ‘whole-word’ items, the there is a lesser degree of phonological and semantic similarity with other words, so response times are not speeded even for frequent bases. All of these models can account for the behavioral results that we observe.
fMRI data
Our results highlight the variety of responses that different brain regions may exhibit when processing words of different frequency and morphological complexity. As expected, response time was a significant predictor of activation level in a number of areas, all previously found to be involved in perceptual and/or motor processing. Even after removal of the effects of response time, however, robust frequency effects remained in a large network of regions previously implicated in language processing. This finding indicates that the modulation of activation of these areas by frequency cannot be wholly attributed to the typically confounding effect of longer lexical decision response times, and as such these results emphasize the importance of these regions in lexical access. Surprisingly, the base frequency effect was reflected in the activation of only one brain area, the thalamus. In contrast, the insula bilaterally and the left inferior occipital gyrus showed a large frequency effect solely for simple words, suggesting that these areas are not sensitive to manipulations of base frequency in complex words. Finally, an effect of word complexity was observed in two key traditional language processing areas, the left inferior frontal gyrus and left superior temporal gyrus. These areas responded more strongly to morphologically complex words than to morphologically simple ones, confirming that these areas are sensitive to morphological complexity, regardless of frequency. These results are discussed in turn below.
The left SMA and the right inferior occipital cortex as well as the caudate nucleus, thalamus, and cerebellum bilaterally showed levels of BOLD response that were significantly predictable from response times in the lexical decision task. All of these regions are involved in some aspects of perceptual or motor processing, so these results are not unexpected. The involvement of SMA in the preparation and execution of motor responses is well established (Banich 1997, Carlson 1998, Dassonville et al. 1998), and the correlation between increased motor response time and increases in fMRI signal has also been previously reported in the SMA (Dassonville et al. 1998, Richter et al. 2000). The caudate and cerebellum have also been implicated in motor processing, whether at the level of response planning or rehearsal during short term memory tasks (Carlson 1998; Fiez 1996, Fiez et al. 1996, 1999), or as a self-monitoring mechanism for performance in both cognitive and motor domains (Stowe 2004). Apart from these predominantly motor areas, the thalamus and the occipital cortex also showed levels of BOLD responses that were correlated with lexical decision time. These areas may be playing a role in perception of the stimuli, as activation in both the thalamus and occipital areas are known to be modulated with increased perceptual processing as well as attentional load (Gitelman et al. 1999, Huettel et al. 2001, Mohamed et al. 2004). Overall, this analysis reveals that, although changes in reaction time account for part of the variability in the strength of the BOLD signal, they do so significantly only for a limited set of areas that are known to mediate sensory or motor processes.
After the modulation in BOLD signal due to response time variation was removed, effects of frequency and of morphological complexity were observed in a number of language-related regions. The effect of longer response times for lower-frequency items is one of the most robust behavioral findings in psycholinguistics, and its neural signature has been observed in a number of previous studies (Fiez et al. 1999, Keller et al. 2001, Chee et al. 2003, Fiebach et al. 2002). In the present results, a few regions (the left SMA, caudate nucleus, and left cerebellum) showed sensitivity to both word frequency (for simple words) and base frequency (for morphologically complex words) differences with, as expected, larger BOLD signal for low than high frequency items. Although low frequency items may lead to higher BOLD activation than high frequency items because they take more time to process, the present analysis ensured that the frequency effects observed were due to differences above and beyond mere differences in processing time, by regressing out variance associated with reaction times. The higher activation for low frequency items is consistent with a graded view of the effect of frequency on lexical access, whereby lower frequency items have to overcome greater competition during lexical access than high frequency items. Reaching the threshold for decision making is therefore more computationally demanding for low frequency items, resulting in longer RTs and greater levels of activation.
Importantly, frequency interacted with morphological status, and did so differently in different brain areas. Although the behavioral data replicated the well-known base frequency effect, levels of BOLD signal mirrored this behavioral effect only in the thalamus. As in the behavioral results, activation in the thalamus reflected frequency effects for ‘decomposable’ and simple words, but not for ‘whole-words’ (Figure 3a). Fiebach et al. (2002), using a lexical decision task, have also reported an effect of frequency on BOLD response in the thalamus. However, only frequency of simple words was manipulated in their study; complex words were not investigated. The present finding that the base frequency of complex words affects thalamus recruitment for ‘decomposable’ words but not for ‘whole-words’ establishes that this brain area is sensitive to the properties that distinguish these two word types, such as phonological and semantic transparency among others. Damage to the thalamus has been documented to result in dysfunctions of lexical retrieval, particularly in semantic errors during naming (Crosson 1999, Bhatnagar and Mandybur 2005). Crosson (1999) described two patients with thalamic lesions who had particular difficulties in naming low-frequency words. Based on these results and others implicating the thalamus in verbal and nonverbal working memory and selective attention tasks, the thalamus has been suggested to play a role in language processing as a selective engagement or gating mechanism that interacts with the cortical regions mediating lexical retrieval and verbal working memory (Radinovic et al. 2003, Manoach et al. 2003, Chee et al. 2004, Gazzaley et al. 2004, Radanovic and Scaff 2003), as well as syntactic and semantic processing at the sentence level (Wahl et al, 2008). On this view, the greater thalamus activation for low frequency items may reflect the extra computational demands for retrieval of lower frequency items in lexical decision. The finding that thalamus recruitment is modulated by base frequency for ‘decomposable’ but not ‘whole-words’ may also be understood in terms of computational demands during lexical access. The greater transparency of ‘decomposable’ words may allow greater support from their base forms during lexical access, as compared to ‘whole-words’ that would less readily engage their related items because they have less overlap in phonology and semantics (Rueckl, Mikolinski, Raveh, Miner, and Mars, 1997; Joanisse and Seidenberg 1999, Seidenberg and Gonnerman, 2000). Future studies are needed to understand why this effect is be limited to the thalamus; Wahl et al. suggest that the selective engagement mechanism makes specific connections between interacting cortical regions, in this case, those associated with lexical access and/or morphological processing such as the temporal lobe and the inferior frontal gyrus respectively.
The other regions responding to frequency in the present study (bilateral insula, left inferior occipital gyrus) showed a different pattern of recruitment. They were primarily sensitive to the differences in word frequency for simple words, but showed little sensitivity to the differences in base frequency for complex words, even ‘decomposable’ ones. Levels of BOLD activation suggest sustained recruitment for all conditions except for the high frequency simple words. The sensitivity of these brain areas to frequency during lexical access is not entirely new. For example, Fiebach et al. (2002) reported a frequency effect in the insula bilaterally when simple word frequency was manipulated. The lack of sensitivity of these areas to base frequency, especially ‘decomposable’ ones, is surprising since base frequency effects are observed for those words. However, the left inferior occipital region may be involved in the early visual processing of letter strings, and as such its sensitivity to frequency may be based on the frequency of the orthographic structure of the whole word. Detecting the base frequency of ‘decomposable’ words is dependent on a system which is sensitive to the internal morphological structure of wordforms and so may not be detectable at this level of processing. The insula has also been proposed to reflect more automatic aspects of processing (Raichle et al. 1994, Van Turennout 2003, Ruz et al. 2005) and may be involved in rapid, automatized aspects of lexical access that, like the occipital region, only operate on whole wordforms and is not sensitive to their internal morphological structure. Together, although this pattern of results will have to be confirmed, these results are consistent with the proposal that different brain areas are differentially affected by the interaction of frequency and morphological complexity, and that the pattern of effects observed through brain imaging unveils a richer and more intertwined relationship between frequency and morphology than that suggested by the behavioral data.
Finally, two of the key traditional language areas, LIFG and LSTG, displayed sensitivity to morphological structure. The finding of a morphological effect in these areas is consistent with a number of previous neuroimaging studies that have found increased activation for complex words, with several studies documenting LIFG recruitment (Laine et al. 1999, Jaeger et al. 1998, Tyler et al. 2005, Joanisse and Seidenberg 2005), and two documenting LSTG (Tyler et al. 2005, Shtyrov 2003). ‘Whole-words’ showed similar, though smaller, increases in BOLD signal in these two regions relative to simple words (Figure 4). This finding is in contrast with the behavioral data, which show no effect of morphological complexity for ‘whole-words’ (that is, there is no base frequency effect for these words in lexical decision RT). This increase in activation for ‘whole-words’ has two possible interpretations: The first possibility is that it is related to morphological complexity, despite the lack of a base frequency effect in the behavioral data. The second possibility is that the activation level increase is actually an effect of frequency: if the ‘whole-words’ are truly being accessed as simple forms, they are actually all low-frequency words (because the mean surface frequency across all ‘whole-word’ items is low compared to the mean frequency of the simple words). This difference may elicit increases in activation for ‘whole-word’ items relative to higher-frequency simple words. The morphological complexity account seems more plausible, since these same regions responded to morphological complexity for ‘decomposable’ words, but not to frequency for any word type. Such an interpretation is consistent with full-decomposition model such as Taft (1979, 2004), where all complex words are accessed as separable morphemes and response times are influenced by the subsequent recombination/decision stage. A close look at the BOLD activation level in these areas suggests greatest activation for ‘decomposable’ over ‘whole-words’, which in turn show greater activation than simple words. This suggests that these regions exhibited graded activation as a function of the words’ degree of phonological and semantic transparency (i.e. whole-word items contain suffixes that can make a phonological change to the base, e.g., serene – serenity, and they have also been suggested to be less semantically transparent, e.g., hospital – hospitality). This gradient of activation suggests that these areas reflect the influence of the base morpheme on the processing of complex words, a process believed to be, at least in part, influenced by linguistic factors such as phonological transparency and semantic transparency. The influence of these factors is incorporated in both dual-system and single-system models. For decomposable words, where there is a clear relationship between the suffixed word and its base, the base and other words that contain it can all be accessed, leading to increased computational demand. For whole-word items, this relationship is less clear, and the base and other morphological relatives may be less likely to be accessed.
It remains unclear whether the LIFG and LSTG play similar or different roles in this access. Tyler et al. (2005) propose that LIFG and LSTG play different roles in the processing of complex words. The LIFG is seen to support the analysis and production of complex morpho-phonological sequences in any type of complex words, whereas the LSTG supports mapping of inputs onto stored representations of word meaning and is sensitive to increased processing demands made by a more complex semantic analysis for morphologically complex forms. This view, however, is not shared by all: increases in neural activity in these areas may reflect a general demand for verbal processing resources that affects multiple language brain areas. Just et al. (1992; 1996, see also Mason et al. 2003) note that both anterior and posterior language areas have been implicated in semantic processing (Bookheimer 2002, Martin 2003), and increases for complex words in both LIFG and LSTG may reflect a compositional semantic analysis that is applied when processing complex words.
Overall, our results demonstrate the sensitivity of lexical access mechanisms in the brain to the morphological complexity of words, above and beyond variation in response time. Traditional language processing areas showed increases in activation with increasing morphological complexity. This result suggests that the processing of morphological relationships among words is done by the same regions that process simple words, but with increased levels of activation. This is in contrast to the notion that distinct brain systems come on-line for the processing of morphologically complex words. In addition, we identified a network of areas sensitive to word frequency. Whereas activation in sensory or motor areas was clearly driven by differences in reaction time, a core set of areas remained sensitive to frequency differences after reaction times were regressed out. Surprisingly however, only the thalamus showed the predicted base frequency effect; other areas either did not differentiate between complex and simple words or only showed frequency effects for simple words. This variety of brain responses to word frequency across brain areas provides a more nuanced picture of the role of frequency during lexical access than that suggested by behavioral work, and perhaps a more complex picture of the differential roles of these brain areas in processing various aspects of lexical items. Although further work is needed to determine the specific roles of brain regions that respond to base frequency, surface frequency and morphological structure in complex words, the present results demonstrate that subtle differences in words’ morphological complexity (semantic and phonological transparency, the relationship between its base and surface frequency) affect the neural mechanisms used to recognize them in a more graded fashion than what behavioral measures have suggested so far.
Acknowledgements
This research was supported by the National Institutes of Health (DC04418 to D.B.; DC00167 to E.L.N.), by the Packard Foundation and the James S. McDonnell Foundation (D.B.). AJN is supported by the Canada Research Chairs program.
Appendix 1: List of ROIs and their size in voxels, MNI coordinates for ROI centroid
| Region | Number of voxels included in ROI |
Centroid (MNI coordinates) | ||
|---|---|---|---|---|
| x | y | z | ||
| R SMA | 83 | 1.9 | −8.0 | 57.5 |
| L SMA | 195 | −7.9 | −2.0 | 60.2 |
| R inferior frontal | 123 | 43.8 | −25.5 | 8.5 |
| L inferior frontal | 384 | −49.0 | −23.1 | 7.7 |
| R Insula | 32 | 31.9 | −21.1 | −1.0 |
| L Insula | 106 | −39.9 | −13.0 | −0.6 |
| L Superior Temporal | 22 | −64.7 | 16.9 | 8.9 |
| L Middle Temporal | 66 | −54.6 | 54.1 | 0.8 |
| R Inferior Temporal | 24 | 46.7 | 54.5 | −17.3 |
| L Inferior Temporal | 119 | −50.4 | 55.2 | −15.1 |
| R Inferior Occipital | 74 | 34.8 | 79.5 | −8.9 |
| L Inferior Occipital | 128 | −38.0 | 83.3 | −8.3 |
| R fusiform | 101 | 34.4 | 66.0 | −16.0 |
| L fusiform | 121 | −34.2 | 63.2 | −15.3 |
| L Angular | 30 | −32.9 | 58.7 | 43.6 |
| R Caudate | 60 | 11.2 | −7.5 | 9.5 |
| L Caudate | 46 | −14.4 | −8.6 | 10.4 |
| R Thalamus | 78 | 9.5 | 14.7 | 6.8 |
| L Thalamus | 111 | −12.3 | 17.4 | 5.6 |
| R cerebellum | 744 | 22.8 | 64.8 | −28.2 |
| L cerebellum | 659 | −28.2 | 66.8 | −29.4 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ANOVA results when the data was analyzed without regressing out response time were highly similar. Main effects of frequency were found in the left SMA, bilaterally in the cerebellum, thalamus and caudate nucleus. As in the analysis above, the thalamus showed an interaction of frequency and wordtype along with the main effect of frequency replicating the behavioral data. The insula bilaterally and the left inferior occipital gyrus also showed an interaction of frequency and wordtype as described above. Regions that showed a main effect of word type were left inferior frontal gyrus, left superior temporal gyrus, and the right inferior temporal gyrus. All ps<.05.
References
- Abdel Rahman R, van Turennout M, Levelt WJM. Phonological Encoding Is Not Contingent on Semantic Feature Retrieval: An Electrophysiological Study on Object Naming. Journal of Experimental Psychology; Learning, Memory and Cognition. 2003;29(5):850–860. doi: 10.1037/0278-7393.29.5.850. [DOI] [PubMed] [Google Scholar]
- Alegre M, Gordon P. Frequency effects and the representational status of regular inflections. Journal of Memory and Language. 1999a;40:41–61. [Google Scholar]
- Baayen RH, Lieber R. Productivity and English word-formations: a corpus-based study. Linguistics. 1991;29:801–843. [Google Scholar]
- Baayen RH, Dijkstra TJ, Schreuder R. Singulars and plurals in Dutch: Evidence for a parallel dual-route model. Journal of Memory and Language. 1997;37:94–117. [Google Scholar]
- Balota DA, Chumbley JI. Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology Human Perception and Performance. 1984;10(3):340–357. doi: 10.1037//0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- Balota DA, Chumbley JI. The locus of word-frequency effects in the pronunciation task: Lexical access and/or production? Journal of Memory and Language. 1985;24(1):89–106. [Google Scholar]
- Banich MT. Neuropsychology: The Neural Bases of Mental Function. New York: Houghton Mifflin; 1997. [Google Scholar]
- Beretta A, Campbell C, Carr T, Huang J, Schmitt L, Christianson K, Cao Y. An ER-fMRI investigation of morphological inflection in German reveals that the brain makes a distinction between regular and irregular forms. Brain and Language. 2003;85:67–92. doi: 10.1016/s0093-934x(02)00560-6. [DOI] [PubMed] [Google Scholar]
- Bertram R, Schreuder R, Baayen RH. The balance of storage and computation in morphological processing: The role of word formation type, affixal homonymy, and productivity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(2):1–23. doi: 10.1037//0278-7393.26.2.489. [DOI] [PubMed] [Google Scholar]
- Bhatnagar SC, Mandybur GT. Effects of intralaminar thalamic stimulation on language functions. Brain and Language. 2005;92(1):1–11. doi: 10.1016/j.bandl.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bick AS, Frost R, Goelman G. Imaging implicit morphological processing: evidence from Hebrew. Journal of cognitive neuroscience. 2010;22(9):1955–1969. doi: 10.1162/jocn.2009.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T, Barch D, Carter C, Cohen J. Conflict monitoring and cognitive control. Psych Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley D. Lexical representation of derivational relation. In: Aronoff M, Kean M, editors. Juncture. Saratoga, CA: Anna Libri.; 1980. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Laudanna A, Romani C. Lexical access and inflectional morphology. Cognition. 1988;28:297–332. doi: 10.1016/0010-0277(88)90017-0. [DOI] [PubMed] [Google Scholar]
- Carlson N. Physiology of behavior. 6th ed. Boston: Allyn and Bacon; 1998. [Google Scholar]
- Chee M, Westphal C, Goh J, Graham S, Song A. Word frequency and subsequent memory effects studies using event-related fMRI. Neuroimage. 2003;20:1042–1051. doi: 10.1016/S1053-8119(03)00335-5. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical functions in language: a working model. Brain Lang. 1985;25:257–292. doi: 10.1016/0093-934x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus. Brain and Cognition. 1999;40(2):414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis S, Zhu X, Ugurbil K, Kim S, Ashe J. Effects of movement predictability on cortical motor activation. Neurosci Res. 1998;32:65–74. doi: 10.1016/s0168-0102(98)00064-9. [DOI] [PubMed] [Google Scholar]
- Davis MH, Meunier F, Marslen-Wilson WD. Neural responses to morphological, syntactic and semantic properties of single words. An fMRI study. Brain and Language. 2004;89:439–449. doi: 10.1016/S0093-934X(03)00471-1. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Morphology and the internal structure of words. Proc Nat Acad Sci USA. 2004;101(41):14984–14988. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Fiebach C, Friederici A, Muller K, von Cramon Y. fMRI Evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14(1):11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Cerebellar contributions to cognition. Neuron. 1996;16(91):13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife E, Balota DA, Schwarz J, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. Journal of Neuroscience. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Forster KI, Chambers SM. Lexical access and naming time. Journal of Verbal Learning and Verbal Behavior. 1973;12(6):627–635. [Google Scholar]
- Frederiksen JR, Kroll JF. Spelling and sound: Approaches to the internal lexicon. Journal of Experimental Psychology: Human Perception and Performance. 1976;2(3):361–379. [Google Scholar]
- Gazzaley A, Rissman J, Desposito M. Functional connectivity during working memory maintenance. Cognitive, Affective and Behavioral Neuroscience. 2004;4(4):580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA. Resolving 20 years of inconsistent interactions between lexical familiarity and orthography, concreteness, and polysemy. Journal of Experimental Psychology: General. 1984;113(2):256–281. doi: 10.1037//0096-3445.113.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Hay J. Doctoral dissertation. Northwestern University; 2000. Causes and consequences of word structure. [Google Scholar]
- Huettel S, Guzeldere G, McCarthy G. Dissociating the Neural Mechanisms of Visual Attention in Change Detection Using Functional MRI. Journal of Cognitive Neuroscience. 2001;13(7):1006–1018. doi: 10.1162/089892901753165908. [DOI] [PubMed] [Google Scholar]
- Huettel S, Song A, McCarthy G. Functional magnetic resonance imaging. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Jaeger J, Lockwood A, Kemmerer D, Van Valin R, Jr., Murphy B, Khalak H. A Positron Emission Tomographic study of regular and irregular verb morphology in English. Language: Journal of the Linguistic Society of America. 1996;72(3):451–497. [Google Scholar]
- Jaeger J, Van Valin R, Jr., Lockwood A. Response to Seidenberg and Hoeffner. Language. Journal of the Linguistic Society of America. 1998;74(1):123–128. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Seidenberg MS. Impairments in verb morphology after brain injury: A connectionist model. Proc Natl Acad Sci USA. 1999;96:7592–7597. doi: 10.1073/pnas.96.13.7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse MF, Seidenberg MS. Imaging the past: Neural activation in frontal and temporal regions during regular and irregular past-tense processing. Cognitive, Affective and Behavioral Neuroscience. 2005;5(3):282–296. doi: 10.3758/cabn.5.3.282. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psych Review. 1992;99(1):122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller T, Eddy W, Thulborn K. Brain activation modulated by sentence comprehension. Science. 1996;274(5284):114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Varma S. Computational modeling of high-level cognition and brain function. Human Brain Mapping. 1999;8(2–3):128–136. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<128::AID-HBM10>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Carpenter PA, Just MA. The neural bases of sentence comprehension: A fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11(3):223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kerr DL, Gusnard DA, Snyder AZ, Raichle ME. Effect of practice on reading performance and brain function. Neuroreport. 2004;15(4):607–610. doi: 10.1097/00001756-200403220-00007. [DOI] [PubMed] [Google Scholar]
- Kohn S, Mevold J. Effects of morphological complexity on phonological output deficits in fluent/nonfluent aphasia. Brain and Language. 2000;73:323–346. doi: 10.1006/brln.2000.2179. [DOI] [PubMed] [Google Scholar]
- Laine M, Rinne J, Krause B, Teras M, Sipila Left hemisphere activation during processing of morphologically complex word forms in adults. Neuroscience Letters. 1999;271(2):85–88. doi: 10.1016/s0304-3940(99)00527-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Greve DN, Lindgren KA, Dale AM. Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. Neuroimage. 2003;20(3):1670–1684. doi: 10.1016/j.neuroimage.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Incoccia C, Pizzamiglio L, Sabatini U, Castriota-Scanderbeg A, Burani C. The right hemisphere involvement in the processing of morphologically derived words. Journal of Cognitive Neuroscience. 2003;15(3):364–371. doi: 10.1162/089892903321593090. [DOI] [PubMed] [Google Scholar]
- Martin RC. Language processing: Functional organization and neuroanatomical basis. Annual Rev of Psych. 2003;54:55–89. doi: 10.1146/annurev.psych.54.101601.145201. [DOI] [PubMed] [Google Scholar]
- Mason R, Just MA, Keller T, Carpenter PA. Ambiguity in the brain: What brain imaging reveals about the processing of syntactically ambiguous sentences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(6):1319–1338. doi: 10.1037/0278-7393.29.6.1319. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15(2):260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 Pt 1):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mohamed M, Yousem D, Tekes A, Browner N, Calhoun V. Correlation between the amplitude of cortical activation and reaction time A functional MRI Study. AJR. 2004;183:759–765. doi: 10.2214/ajr.183.3.1830759. [DOI] [PubMed] [Google Scholar]
- Niemi J, Laine M, Tuominen J. Cognitive morphology in Finnish: Foundations of a new model. Language and Cognitive Processes. 1994;9(3):423–446. [Google Scholar]
- Price CJ, Indefrey P, van Turennout M. The neural architecture underlying the processing of written and spoken word forms. In: Brown C, Hagoort P, editors. The Neurocognition of Language. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Pugh KR, Shaywitz BA, Constable RT, Shaywitz SA, Skudlarski P, Fulbright RK, Brone RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Azambuja M, Mansur LL, Porto CS, Scaff M. Thalamus and language: interface with attention, memory and executive functions. Arquivos de Neuro Psiquiatria. 2003;61(1):34–42. doi: 10.1590/s0004-282x2003000100006. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Scaff M. Speech and language disturbances due to subcortical lesions. Brain and Language. 2003;84(3):337–352. doi: 10.1016/s0093-934x(02)00554-0. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex. 1994;4(1):8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Richter W, Somorjai R, Summers R, Jarmasz M, Menon RS, Gati JS, Georgopoulos AP, Tegeler C, Ugurbil K, Kim SG. Motor area activity during mental rotation studied by time-resolved single-trial fMRI. Journal of Cognitive Neuroscience. 2000;12(2):310–320. doi: 10.1162/089892900562129. [DOI] [PubMed] [Google Scholar]
- Ruz M, Wolmetz ME, Tudela P, McCandliss B. Two brain pathways for attended and unattended words. NeuroImage. 2005;27:852–861. doi: 10.1016/j.neuroimage.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Sereno J, Jongman A. Processing of English inflectional morphology. Memory and Cognition. 1997;25:425–437. doi: 10.3758/bf03201119. [DOI] [PubMed] [Google Scholar]
- Sach M, Seitz RJ, Indefrey P. Unified inflectional processing of regular and irregular verbs: a PET study. NeuroReport. 2004;15:533–537. doi: 10.1097/00001756-200403010-00030. [DOI] [PubMed] [Google Scholar]
- Schreuder R, Baayen RH. Modeling morphological processing. In: Feldman L, editor. Morphological aspects of language processing. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1995. pp. 131–154. [Google Scholar]
- Schmitt B, Schiltz K, Zaake W, Kutas M, Munte TF. Electrophysiological analysis of the time course of conceptual and syntactic encoding during tacit picture naming. Journal of Cognitive Neuroscience. 2001;13(4):510–522. doi: 10.1162/08989290152001925. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Shelton J, Caramazza A. Grammatical class in lexical production and morphological processing: Evidence from a case of fluent aphasia. Cognitive Neuropsychology. 2000;17(8):665–682. doi: 10.1080/026432900750038281. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Pulvermuller F, Naatanen R, Ilmoniemi R. Grammar processing outside the focus of attention: an MEG study. Journal of Cognitive Neuroscience. 2003;15(8):1195–1206. doi: 10.1162/089892903322598148. [DOI] [PubMed] [Google Scholar]
- Stowe LA, Paans AM, Wijers AA, Zwarts F. Activations of ‘motor’ and other non-language structures during sentence comprehension. Brain and Language. 2004;89(2):290–299. doi: 10.1016/S0093-934X(03)00359-6. [DOI] [PubMed] [Google Scholar]
- Taft M. Recognition of affixed words and the word frequency effect. Memory and Cognition. 1979;7(4):263–272. doi: 10.3758/bf03197599. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Post B, Randall B, Marslen-Wilson WD. Temporal and frontal systems in speech comprehension: An fMRI study of past tense processing. Neuropsychologia. 2005;43(13):1963–1974. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ullman M, Corkin S, Coppola M, Hickok G, Growdon J, Koroshetz W, Pinker S. A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9(2):266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Ullman M. The declarative/procedural model of lexicon and grammar. Journal of Psycholinguistic Research. 2001a;30(1):37–69. doi: 10.1023/a:1005204207369. [DOI] [PubMed] [Google Scholar]
- Vannest J, Boland JE. Lexical Morphology and lexical access. Brain and Language. 1999;68:324–332. doi: 10.1006/brln.1999.2114. [DOI] [PubMed] [Google Scholar]
- Vannest J, Bertram R, Jarvikivi J, Niemi J. Counterintuitive cross-linguistic differences: More morphological computation in English than in Finnish. Journal of Psycholinguistic Research. 2002;31:83–106. doi: 10.1023/a:1014934915952. [DOI] [PubMed] [Google Scholar]
- Vannest J, Polk TA, Lewis RL. Dual-route processing of complex words: New fMRI evidence from derivational suffixation. Cognitive, Affective, and Behavioral Neuroscience. 2005;5(1):67–76. doi: 10.3758/cabn.5.1.67. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Hagoort P, Brown CM. Electrophysiological evidence on the time course of semantic and phonological processes in speech production. Journal of Experimental Psychology: Learning, Memory and Cognition. 1997;23(4):787–806. doi: 10.1037//0278-7393.23.4.787. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Hagoort P, Brown CM. The Time Course of Grammatical and Phonological Processing During Speaking: Evidence from Event-Related Brain Potentials. Journal of Psycholinguistic Research. 1999;28(6):649–676. doi: 10.1023/a:1023221028150. [DOI] [PubMed] [Google Scholar]
- Wahl M, Marzinzik F, Friederici AD, Hahne A, Kupsch A, Schneider GH, Saddy D, Curio G, Klostermann F. The human thalamus processes syntactic and semantic language violations. Neuron. 2008;59:695–707. doi: 10.1016/j.neuron.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Woods R, Grafton S, Holmes C, Cherry S, Mazziotta J. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]