Abstract
Endocytic recycling is coordinated with endocytic uptake to control the composition of the plasma membrane. Although much of our understanding of endocytic recycling has come from studies on the transferrin receptor, a protein internalized through clathrin-dependent endocytosis, increased interest in clathrin-independent endocytosis has led to the discovery of new endocytic recycling systems. Recent insights into the regulatory mechanisms that control endocytic recycling have focused on recycling through tubular carriers and the return to the cell surface of cargo that enters cells through clathrin-independent mechanisms. Recent work emphasizes the importance of regulated recycling in such diverse processes as cytokinesis, cell adhesion and morphogenesis, cell fusion, and learning and memory.
Introduction
Cells internalize extracellular material, ligands, and plasma membrane proteins and lipids by endocytosis. This removal of membrane from the cell surface is balanced by endosomal recycling pathways that return much of the endocytosed proteins and lipids back to the plasma membrane. The balance between endocytic uptake and recycling controls the composition of the plasma membrane and contributes to diverse cellular processes including nutrient uptake, cell adhesion and junction formation, cell migration, cytokinesis, cell polarity and signal transduction. Since it is estimated that cells internalize their cell surface equivalent one to five times per hour 1, endocytic recycling pathways must be robust and coordinately regulated.
Endocytosis occurs by a variety of mechanisms that can be divided into those that are clathrin-dependent and those that are clathrin-independent 2, 3 (Figure 1). In clathrin-dependent endocytosis (CDE), the cytoplasmic domains of plasma membrane proteins are specifically recognized by adaptor proteins and packaged into clathrin-coated vesicles that are brought into the cell. The receptors for iron-bound transferrin (TfR) and low-density lipoprotein (LDL) particles are classic examples of CDE cargo proteins. CDE is facilitated by numerous accessory proteins, requires the GTPase dynamin for vesicle scission and has been widely studied 2, 3. Clathrin-independent endocytosis (CIE), by contrast, may come in many forms, has been less studied, but is increasingly gaining attention from cell biologists 4, 5. There are also specialized CIE pathways, such as macropinocytosis and phagocytosis, that are actin driven 6 and may, in effect, represent stimulated forms of CIE 7.
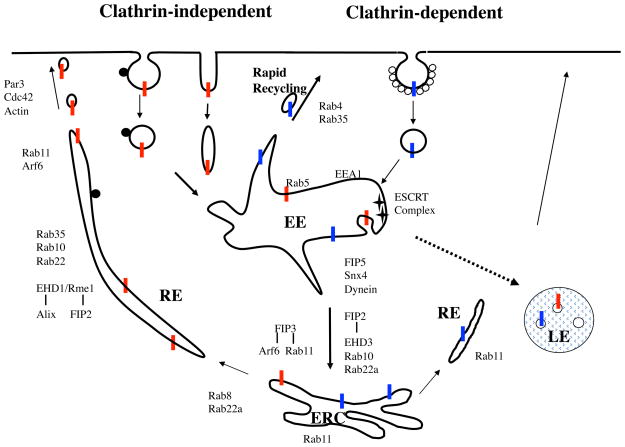
Figure 1. Pathways of endocytosis and endocytic recycling.
Itinerary of cargo proteins entering cells by clathrin-dependent (blue cargo) and clathrin-independent (red cargo) endocytosis. Subsequent routing of cargo to the early endosome, juxtanuclear endocytic recycling compartment (ERC) and recycling endosomes is shown. Some cargo is selected in the early endosome by the ESCRT complex to enter the multivesicular body (MVB) pathway and onto late endosomes (dashed arrow). Clathrin-dependent cargo can recycle back to the cell surface via a rapid recycling pathway that requires Rab4 and Rab35. Both types of cargo can move from the early endosome to endocytic recycling compartment (ERC) by a process requiring Snx4, dynein, EHD3, Rab10, Rab22a and the Rab11 effectors FIP2, -3 and -5. From the ERC, recycling of both types of cargo requires Rab11, and recycling of clathrin-independent cargoes involves the generation of Rab8- and Rab22a-dependent distinctive tubules in addition to many other factors. Clathrin-dependent cargo may also recycle through these different pathways. Rab10, -11, -22 and -35 are associated with the tubular recycling endosome and, along with EHD1/RME-1, Alix and FIP2 are required for recycling. In the periphery, the tubules appear to break up into vesicles prior to fusing with the cell surface, a process requiring Arf6, Rab11, Par3, Cdc42 and cortical actin. This is a composite description of endocytic recycling and all of the components shown here may not be evident in a given cell type.
Regardless of the mode of entry, endocytosed cargo is usually delivered to the early endosome where sorting occurs. Cargo-specific sorting leads to distinct subsequent cargo itineraries (Figure 1). Cargo can be routed from the early endosome to late endosomes and lysosomes for degradation, to the trans-Golgi network (TGN), or to recycling endosomal carriers that bring the cargo back to the plasma membrane. A previous review on endocytic recycling by Maxfield and McGraw specifically focused on CDE cargo recycling 8, and emphasized the default nature of receptor and membrane lipid recycling. In this Review we will focus on endocytic recycling pathways, and in particular those pathways and requirements that have been discovered for recycling of CIE cargo back to the plasma membrane. We do not know yet the extent to which these new pathways and requirements regulate recycling in all cells nor whether CIE and CDE cargo might share recycling pathways. The reader is referred to other reviews for discussions on trafficking to degradative compartments and the formation of multivesicular bodies (MVBs) 9–11 and bidirectional trafficking between endosomes and the TGN 12.
Clathrin-independent endocytosis pathways
Many cell surface transmembrane proteins lack cytoplasmic sequences for the recruitment and internalization into clathrin-coated vesicles. Over the past several years, many of these proteins have been shown to be internalized by CIE mechanisms. There appears to be several distinct mechanisms of CIE 4 that may reflect, to some extent, the different cell types and cargo molecules monitored. Caveolar endocytosis involves the caveolin coat, is dynamin-dependent and is the mechanism for internalization into cells of glycosphingolipids and some viruses 4, 5. A Cdc42-, Arf1- 13 and actin-dependent mode of CIE has also been described that is dynamin-independent and responsible for endocytosis of fluid-phase markers, GPI (glycosyl phosphatidylinositol)-anchored proteins and bacterial toxins 4, 5. Another dynamin-independent mechanism of CIE, first described in HeLa cells, is associated with the Arf6 GTPase and is responsible for endocytosis of many cell surface integral membrane proteins that lack adaptor protein recognition sequences (see below). Disparate forms of CIE share a requirement for free cholesterol, and proteins and lipids that reside in sphingolipid-rich ‘raft’ membranes appear to be prominent among CIE cargo 4, 5. Recent analysis of recycling of endogenous clathrin-independent cargo proteins is revealing an unexpected complexity within CIE recycling systems.
The Arf6-associated pathway has served as an important experimental paradigm for studies of CIE, allowing identification of many clathrin-independent cargo proteins and elucidation of the itineraries and mechanisms of recycling. Among the endogenous proteins originally shown to enter cells via this CIE mechanism are the major histocompatibility complex class I proteins (MHCI) 14, β-integrin 15, 16 and the GPI-anchored protein CD59 17. A recent proteomics analysis of the Arf6-associated early endosome identified additional cargo proteins, including the ubiquitous glucose transporter Glut1 and other proteins involved in amino acid uptake and cell–matrix interactions 18 (Table 1). Other cargo proteins travelling in this pathway, including Tac, the interleukin 2 receptor α-subunit 14, the MHC-like molecule Cd1a 19, and the β2-adrenergic and M3 muscarinic receptors in the absence of ligand 20, have been identified in transfected HeLa cells (see Table 1). In most cases, following internalization into distinct vesicles, the cargo is transferred to the early endosome where it meets up with clathrin cargo and can then can be routed along degradative route to the lysosome or recycled back to the cell surface.
Table 1.
Cargo proteins in clathrin-independent endocytosis
| Cargo* | Function | Refs |
|---|---|---|
| MHC class I molecule | Immune responses | 14 |
| MHC class II molecule (peptide-loaded) | Immune responses | 22 |
| CD1a | Immune responses | 19 |
| β-integrins | Cell–extracellular matrix interactions and adhesion | 15, 16 |
| E-cadherin | Cell–cell junctions | 91 |
| PMP22§ | Myelination | 123 |
| CD44 (hyaluronan receptor) | Cell–extracellular matrix interactions | 18 |
| CD98 (SLC3A2 and 4F2HC) | Interacts with β-integrins, ICAM1 and the heavy chain of amino acid transporters | 18 |
| CD147 (EMMPRIN and basigin) | Cell–extracellular matrix interactions | 18 |
| ICAM1 (CD54) | Cell–extracellular matrix interactions and adhesion | 23 |
| Syndecan 1 | Cell–extracellular matrix interactions | 21 |
| CD55 (DAF) | GPI-AP; protects cell from complement | 57 |
| CD59 (protectin) | GPI-AP; protects cell from complement | 17 |
| Mucolipin 2§ | TRP-like cation channel | 57 |
| Kir3.4 (GIRK4 and KCNJ5) | Inwardly rectifying potassium channel | 23 |
| GLUT1 (SLC2A1) | Glucose transporter | 18 |
| LAT1 (SLC7A5) | L-type amino acid transporter | 18 |
| β2 adrenergic receptor§ (no ligand) | Signalling receptor | 20 |
| M3 receptor§ (no ligand) | Signalling receptor | 20 |
| MGLUR7§ (no ligand) | Signalling receptor | 124 |
Although this CIE pathway is associated with Arf6, endocytosis is not dependent on Arf6–GTP; instead, it is the recycling from this pathway that requires active Arf6 14. The identification of this pathway was facilitated by the characteristic elaborate tubular endosomal recycling system containing CIE cargo that is visible in HeLa cells. However, mechanistically similar trafficking of such CIE cargo has been observed in a variety of other cells including Cos, MCF7 21, B cells 22, cardiomyocytes 23, hippocampal neurons 23, dendritric cells 22 and the Caenorhabditis elegans intestine 24, 25, indicating wide conservation of such pathways. On the other hand, in some cells, notably CHO cells, Arf6 is associated with and influences the recycling of CDE cargo, such as the TfR 26.
The early endosome: sorting station for cargo
The early endosome is defined as the organelle that receives incoming material from primary vesicles generated by CIE and CDE. The small GTPase Rab5, phosphatidylinositol 3-kinase (PI3K) and its product phosphatidylinositol-3-phosphate (PtdIns3P) mark the early endosome and are required for its function. The lumen of the early endosome is mildly acidic 8, facilitating conformational changes in proteins that can lead to ligand release from receptors. Sorting of membrane proteins and lipids from lumenal content, and the generation of membrane tubules emanating from endosomes, can lead to fast recycling pathways (see below), or to transfer to a later, juxtanuclear, endocytic recycling compartment (ERC) from which recycling endosomes emerge (Fig. 1). Membrane proteins that have been tagged with monoubiquitin are corralled by the ESCRT complex machinery and enter the MVB pathway along with the lumenal content of the early endosome 9–11. For the ‘classic’ CDE cargo proteins, the TfR and the LDL receptor (LDLR), recycling back to the plasma membrane appears to happen by default and does not require any specific cytoplasmic sequences for recognition and sorting 8. By contrast, recycling of CIE cargo and signalling receptors may involve a positive selection process.
Regulators of traffic, and recycling in particular, include GTPases (Rab and Arf proteins) and their effectors (see Box 1 and Table 2), the RME-1 family of C-terminal Eps15 homology domain proteins (EHD1–4) that serve as scaffolding, membrane tubulating, and perhaps membrane fission, proteins27, 28, and motor proteins (Table 3) that transport recycling endosomal carriers. Our understanding of endocytosis and recycling has benefited from genetics studies in yeast32, and more recently from genetic analysis in C. elegans 29–31. In particular, the C. elegans analysis has identified many metazoan-specific regulators of endocytosis and recycling that are now known to perform similar functions in mammalian cells. C. elegans cells also clearly possess and require a morphologically distinct ERC, an organelle yet to be defined in yeast systems 32.
Box 1. Rab and Arf GTPases involved in endocytic recycling.
Low-molecular-weight GTP-binding proteins act as molecular switches to regulate steps that are involved in vesicle carrier formation, movement and fusion with target membranes. These proteins cycle between a GDP-bound ‘off’ state and a GTP-bound ‘on’ state where they interact with and activate effector proteins. Conversion between those states is catalysed by guanine nucleotide exchange factors that convert them to the on state and GTPase-activating proteins that stimulate GTP hydrolysis, returning them to the off state.
There are over 60 Rab proteins in mammals that have distinct localizations and functions in membrane traffic 119, 120. Of the Rab proteins associated with regulation of endosomal traffic (Rab4, -5, -7, -8, -10, -11, -22 and 35), Rab5 and Rab11 have been studied the most. Two effectors of Rab5, phosphatidylinositol 3-kinase and the membrane-tethering protein early endosomal antigen1, lead to the generation of phosphatidylinositol-3-phosphate on the membrane and facilitate early endosomal membrane fusion. Many proteins, named FIPs, have been identified that interact with Rab11, and FIPs in turn interact with many other proteins, serving as scaffolds to organize and coordinate endosomal transport 121.
Of the six mammalian Arf proteins, Arf6 has a distinctive plasma membrane and peripheral localization, whereas the other Arfs cycle between the Golgi and cytosol. However, recent findings have also documented roles for ‘Golgi’ Arfs at the plasma membrane 13, 122. Arf6 activates phospholipase D, generating phosphatidic acid, which is required for endocytic recycling; it also activates phosphatidylinositol 4-phosphate 5-kinase, which generates phosphatidylinositol-4,5-bisphosphate on the distal portions of the recycling endosome and at the plasma membrane. Arf6 also interacts with the Rab11 effector FIP3 to facilitate movement to the endocytic recycling compartment. Arf6 interaction with the JIP3 and JIP4 motor protein scaffolds is important during cytokinesis.
Table 2.
GTPase Regulators of Endocytic Recycling
| Rabs and Arfs | Function | Ref | |
|---|---|---|---|
| Arf6 | CIE recycling pathway | 14 | |
| Rab4 | Early endosome | 8, 34 | |
| Rab8 | Recycling endosome tubules | 61 | |
| Rab10/RAB-10 | Early endosome | 24, 48 | |
| Rab11/RAB-11 | ERC | 8, 53 | |
| Rab22a | Recycling endosome tubules | 47, 53 | |
| Rab35/RAB-35 | Early endosome, RE tubules | 37, 38 | |
| Rab and Arf Effectors | Function | Domains | |
| Rabenosyn5 | Interacts with Rab4, Rab5 EHD1/3, hVPS45 | FYVE,NPF | 43 |
| Rab11-FIP2 | Interacts with Rab11 | C2,NPF | 43 |
| Rab11-FIP3/Arfophilin/Nuf | Interacts with Rab11 | EF hands ERM | 45 |
| Rip11/dRip11/FIP5 | Interacts with Rab11 | C2 | 44 |
| Phospholipase D2 (PLD2) | Cleaves phosphatidylcholine to generate phosphatidic acid (PA) and choline, regulated by Arf6,PIP2 | PX,PH | 55 |
| PIP5K | Phosphatidylinositol-4-phosphate 5-kinase, generates PI4,5P2 from PI4P, activated by Arf6 | 15, 26 | |
| Sec10 | Exocyst subunit, binds Arf6 | 60 | |
| JIP3/JIP4 | Binds Arf6, kinesin light chain and dynactin | Leucine zipper | 59 |
| Rab and Arf Regulators | |||
| RME-4/Connecdenn | Rab35 and AP-2 interacting protein | DENN | 36, 37 |
| EP164C | Rab35 GTPase activating protein | 38 | |
| ASAP | Arf GTPase activating protein | 46 | |
| ACAP1 | Arf6 GTPase activating protein | 58 | |
| Arf-GEP100/Loner | Arf6 nucleotide exchange factor | 105 | |
Table 3.
Motors, Adaptors, and Membrane Remodeling Proteins.
| Motors | Function | Ref | |
|---|---|---|---|
| Myosin Vb | Actin based, barbed end directed | 110 | |
| Myosin VI | Actin based, pointed end directed | 88 | |
| Dynein | Microtubule based minus end directed | 50, 59 | |
| Dynactin (p150glued, p50) | Dynein adaptor | 59 | |
| KLC (kinesin light chain) | Microtubule based plus end directed | 59 | |
| Others | Function | Domains | |
| RME-1/EHD1-4 | Lipid binding, tubulation (fission?) ATPase | EH | 27 |
| Syndapin/Pacsin | Membrane tubulation and actin remodeling | F-BAR,SH3 | 25, 68 |
| Alix/ALX-1 | ? | Bro1, V | 25 |
| Par3/PAR-3 | Polarity protein | PDZ | 29, 98–100 |
| Par6/PAR-6 | Polarity protein | PDZ | 29, 98–100 |
| Cdc42/CDC-42 | Small GTPase (Rho family) | 29, 98–100 | |
| aPKC/PKC-3 | Atypical protein kinase C Ser/Thr kinase | 29, 98–100 | |
| Myoferlin | Synaptotagmin family | C2 | 107 |
| KIBRA/WWC1 | Binds Snx4 and dynein | WW,C2 | 50 |
| SNX4 | Binds KIBRA | PX, coiled coil | 50 |
| Erk7 | Serine-threonine kinase | 7 | |
| Ras7 | Ras GTPase | 7 | |
| Src7 | SH3, SH2, tyrosine kinase | 7 | |
| Rac | Rho-family GTPase | 7, 104–106 | |
| MCOLN2 | Transient receptor potential superfamily cation channel | 57 | |
| Kir3.4 | Subunit of inward rectifier K(+)channel | 23 | |
| Clathrin | coat protein | 2 |
Rapid recycling route
The existence of a fast recycling route back to the plasma membrane from either the early endosome or an earlier stage has been documented for the TfR 8 and glycosphingolipids 33. Early studies identified Rab4 as being important for recycling of the TfR 8, 34 and glycosphingolipids 33 from early endosomes (Fig. 1). The literature is not clear on the precise role of Rab4 in recycling, as expression of dominant-negative Rab4 inhibits fast recycling, but small interfering RNA (siRNA)-mediated knock down of Rab4 increases rapid recycling, perhaps by blocking early endosome-to-ERC transport 35, 36. Recent reports of RNA interference (RNAi) and knockout studies have indicated that Rab35 is as an important regulator of rapid recycling. Studies revealed that Rab35 localizes to the plasma membrane as well as early endosomes and is required for rapid recycling of the mammalian TfR 37 and the C. elegans LDLR-like yolk receptor 38. Furthermore, in worms recruitment of RAB-35 (also known as RME-5) to the early endosome is dependent on RME-4, a DENN-domain protein related to mammalian Connecdenn. RME-4 binds to GDP-associated RAB-35 and adaptor protein 2 (AP2), thus recruiting RAB-35 to clathrin-coated pits 38, 39. Another study demonstrated that overexpression of EP164C, a Rab35 GTPase-activating protein (GAP), or depletion of Rab35, impaired TfR recycling and the formation of the immunological synapse 40, highlighting a role for rapid recycling in T cell function. The localization of Rab35 to incoming clathrin-coated vesicles links this rapid recycling route with CDE.
Rab35 is also associated with Arf6- and EHD1-positive tubular recycling endosomes that carry CIE cargo back to the plasma membrane 22. Whether Rab35 functions on tubular recycling endosomes or is only a passenger is not known. Rab35 was also found to be required for efficient abscission, breaking the final bridge linking daughter cells after cytokinesis (see below) 37.
‘Slow’ recycling route
The so-called ‘slow’ recycling route is the one that is typically measured experimentally when measuring recycling, and involves the transport of cargo proteins from the early endosome to the ERC, and from the ERC to the plasma membrane. In many tissue culture cells, the ERC is localized centrally near the microtubule organizing centre and Golgi complex, but this is not always the case, especially in polarized cells. The current prevailing model of geometric based, iterative sorting posits that during its maturation the early endosome elaborates tubules that become the ERC, whereas the main body of the early endosome becomes the MVB 8. Evidence of this transformation has come during live imaging of the early endosome losing Rab5 and acquiring Rab1141. The ERC is molecularly defined by the presence of Rab11 and/or EHD1, and morphologically as a tubular compartment largely devoid of fluid.
Traffic to the ERC
A number of proteins have been shown to be important for the juxtanuclear positioning of the ERC and for transport of endocytosed cargo to the ERC (see Fig. 1 and Tables 2 and 3). One of these proteins, EHD4, is important for export from the early endosome to the ERC and late endosome 42. Another member of the same family, EHD3, binds to the NPF (Asn-Pro-Phe) motifs in two Rab effectors, rabenosyn5 and Rab11-FIP2, suggesting a role as a linker between the Rab5-associated early endosome and the Rab11-associated ERC 43. Another Rab11 effector, FIP5, is important for the movement of TfR from the early endosome to the ERC. Loss of FIP5 inhibits transport of TfR from the early endosome to the ERC, which seems to lead to enhanced recycling of TfR, presumably via the fast recycling route directly from the early endosome to the plasma membrane 44. The Rab11 effector FIP3 (also known as arfophilin or Nuf) interacts with both Rab11 and Arf6, is important for the juxtanuclear positioning of the ERC 45 and plays a clear role in cytokinesis (see below). Additionally, ASAP, an Arf GAP, also binds to FIP3 and influences ERC positioning 46. Rab22a is also important for movement of cargo from the early endosome to the ERC, and Rab22 depletion inhibits slow TfR recycling 47. RAB-10 in C. elegans and polarized epithelial cells from mammals has been implicated in transport of CIE and CDE cargo between early endosomes and recycling endosomes 24, 48
An important function of the transport of cargo from the early endosome to the ERC may be to prevent its entry into degradative compartments. The sorting nexins serve this function in yeast 49, and in mammalian cells sorting nexin 4 (SNX4) associates with tubular-vesicular elements on the early endosome and ERC. Loss of SNX4 causes the TfR to be sorted to the late endosome for degradation 50. SNX4 interacts with the dynein motor through a linker protein KIBR, facilitating the movement of the early endosome/ERC to the juxtanuclear region. Depletion of KIBR results in a peripheral distribution of TfR, but does not lead to TfR degradation, suggesting that SNX4 coordinates the sorting of TfR away from the late endosome and, together with dynein, the movement towards the ERC 50.
Interestingly, another study found that depletion of the AP2 complex led to increased routing of CIE cargos MHCI and β-integrin to degradative compartments, and reduced their recycling 51, similar to the block of TfR observed with SNX4 depletion (see above). Since AP2 depletion inhibits internalization of TfR but not that of MHCI or β-integrin, these results raise the possibility that input from CDE at the early endosome might be required for recycling of CIE cargo proteins. For example, CDE cargo, such as TfR and the proteins that associate with it, may facilitate the movement of CIE cargo from early endosomes to the ERC for their subsequent recycling. Further studies will be needed to determine the extent to which CDE and CIE pathways are interdependent.
From the ERC to the plasma membrane
There are probably a number of distinct recycling pathways from the ERC back to the plasma membrane. In HeLa cells the bifurcation from the ERC is distinctive. TfR is recycled back to the plasma membrane in recycling endosomes that are separate from the tubular recycling endosomes that carry CIE cargo back to the plasma membrane 14, 17, 52. Although both recycling pathways require Rab11 function 53, this latter, more elaborate recycling system, which we will refer to as the CIE recycling pathway, is subject to many more regulatory elements (including Arfs, Rabs and polarity proteins), as will be discussed below. Analysis in C. elegans also indicates genetically separable recycling for a model CDE cargo and a model CIE cargo, suggesting the conservation of bifurcated export from the ERC 25.
Arf6 and recycling from the ERC
The CIE recycling pathway was originally described to include Arf6-associated tubular endosomes that emanate from the juxtanuclear ERC carrying recycling clathrin-independent cargo such as MHCI back to the plasma membrane. The tubules align along microtubules, and recycling back to the plasma membrane depends on both microtubules and actin 14, 53, 54. Importantly, it is the recycling of this membrane back to the plasma membrane where Arf6 exerts its effect. Arf6 is required, at least in part, for the activation of phospholipase D (PLD) 55. PLD2 is present on the tubular recycling endosome and the lipid products of PLD, phosphatidic acid (PA) and diacylglycerol (DAG), are important for recycling function 55. PA has been implicated in membrane fission and so may promote release of recycling carriers, whereas DAG has been implicated in membrane fusion and so may promote fusion of such carriers with the plasma membrane. Arf6 also activates phosphatidylinositol 4-phosphate 5-kinase (PIP5-kinase), an enzyme that generates phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). PtdIns(4,5)P2 is present both at the cell surface and on the distal portions of the tubular endosome 15. PtdIns(4,5)P2 is important for the recruitment to the membrane of many proteins involved in vesicle formation, fusion and actin polymerization. Indeed, it is this Arf6-directed endocytic recycling back to the plasma membrane that modulates the cortical actin cytoskeleton for cell spreading, cell migration, wound healing and metastasis 26, activities ascribed to Arf6 function.
Several proteins found associated with the tubular endosomes may regulate the activation of Arf6. Inhibition of extracellular signal-regulated kinase (Erk) inhibits Arf6 activation, causes a build up of CIE recycling tubules and inhibits recycling 56. Activities of other signaling molecules (Ras, Src, Rac) localized to these tubules may also alter Arf6 activation and/or recycling 7. The cation channel mucolipin 2 (MCOLN2) is associated with the tubular recycling endosome and its overexpression results in increased activation of Arf6, whereas its depletion results in a decrease in recycling of CD59 57.
In addition to these global requirements for Arf6 in CIE recycling, three studies have implicated Arf6 in cargo-specific sorting and recycling functions. The recycling of syndecan 1, a heparin sulfate proteoglycan, and the fibroblast growth factor (FGF) receptor, which traffics with syndecans, requires Arf6, PtdIns(4,5)P2 (produced by Arf6-mediated activation of PIP5-kinase) and syntenin, a PDZ domain-containing protein that binds to the C terminus of syndecans. Expression of syntenin mutants that cannot bind to PtdIns(4,5)P2 causes the accumulation of syndecan and the FGF receptor in endosomes, and blocks their recycling back to the plasma membrane. As a consequence, cell spreading is impaired 21. In another example, a cytoplasmic acidic cluster motif that is present on inwardly rectifying potassium channels (Kir3.4) binds to the Arf6 guanine nucleotide exchange factor (GEF) EFA6, leading to the activation of Arf6 and increased presence of Kir3.4 at the plasma membrane 23. Another intriguing observation is that the Arf6 GAP ACAP1, which hydrolyses GTP and thus inactivates Arf6, binds to clathrin, and both ACAP1 and clathrin were found to be important for the recycling of integrin and Glut4 58, although exactly how and where these components function to sort such diverse cargo is not clear.
Two additional Arf6 effectors, the c-Jun N-terminal interacting kinases 3 and 4 (JIP3 and JIP4), and the Sec10 subunit of the exocyst may play distinct roles in regulating endosomal trafficking and the target site of recycling in particular (Table 2). JIP3 and JIP4 are scaffolding proteins that bind to the plus-end-directed motor kinesin and the minus-end-directed motor dynein, and Arf6 may control JIP binding to these two motors, and thus control direction of transport along microtubules 59. This interaction is important during cytokinesis (see below) and may also function along the CIE pathway in interphase cells. Arf6 interaction with the exocyst appears to influence the target site at the plasma membrane receiving recycling CIE cargo 60.
Other regulators of recycling
The ERC is itself defined by the presence of Rab11 and the many proteins that interact with Rab11. Since the ERC is the site where slow recycling of TfR occurs, interfering with Rab11 or its interactors inhibits recycling and often alters positioning of the ERC within the cell. The recycling of CIE cargo MHCI 53 andβ-integrin 16 is also dependent on Rab11 function. Other Rabs appear to be associated with the ERC or are involved in differentiation of the ERC, to form the recycling tubules that carry CIE cargo back to the plasma membrane. Although Rab22a appears to be important for early endosome-to-ERC transport 47, it is also critical for the biogenesis of the recycling endosome tubules, and for the recycling of MHCI 53 and Cd1a 19 back to the plasma membrane. Rab8 is also present on CIE tubules where it may function with Arf6 to promote recycling and cortical actin-driven plasma membrane protrusions 61.
Specific sorting of cargo for endosomal recycling is especially evident in polarized epithelial cells. The AP1-B adaptor is specifically important for targeting proteins to the basolateral surface 62. Furthermore, proteins destined for apical or basolateral recycling sort out into distinct domains in the recycling endosome 63. Exactly how recycling pathways in polarized cells are similar or distinct from that in non-polarized cells remains to be determined. The variety of pathways observed in non-polarized cells could be indicative of the distinct trafficking pathways in polarized cells.
The genetic screen for endocytosis mutants in C. elegans has been instrumental in expanding our understanding of recycling from the ERC. The first endocytosis mutant from that screen, RME-1 and its mammalian counterpart EHD1 are required for recycling of MHCI and, in some cells, TfR 64–66. EHD1 may bind to phosphatidylinositol-4-phosphate (PtdIns4P) and PtdIns(4,5)P2, and the ability of EHD1 to bind to PtdIns4P seems to be important for its association with the tubular aspects of recycling endosomes 67. EHD1 also binds to rabenosyn5 and Rab11-FIP2 43, hence coupling Rab5 with Rab11 effectors. Furthermore, EHD1 can bind to syndapins (also known as pacsins), proteins believed to serve as links between membrane traffic and membrane-associated actin. Syndapins colocalize with EHD1/RME-1 on the CIE recycling tubules in mammals and C. elegans 25, 68. Structural data from the related protein EHD2 from mouse suggests that RME-1-family proteins may function like dynamin, assembling in ring structures around membranes and promoting fission 28. Thus, EHD1/RME-1 might represent part of the fission apparatus for release of recycling cargo carriers.
C. elegans ALX-1 (known as Alix or Bro1 in mammals and yeast, respectively) was identified as an RME-1-binding protein that is required for recycling of human Tac (CIE cargo) but not human TfR (CDE cargo) cargo25. This was an unexpected finding as Alix/Bro1 proteins in mammals and yeast are known to be associated with the ESCRT complex during MVB biogenesis. However, the new study found that ALX-1 in worms was also associated with a subset of RME-1-positive endosomes and was required for the recycling of the CIE cargo protein Tac, expressed in the worm, as a cargo marker of the CIE pathway. Mutation of the RME-1-binding site in ALX-1 produced a version of the protein that could still function in MVB and late endosomal processes, but lacked its recycling function 25. Overexpression of truncated human Alix in HeLa cells strongly inhibited the recycling of MHCI 25. The MVB regulator HRS has also been implicated in the recycling process and, like Alix, is associated with tubular endosomes in vivo, suggesting that some proteins known to act in receptor degradation have dual functions 69, 70
Another surprising set of proteins to come from the C. elegans screens were the polarity proteins Par3, Par6 and Cdc42 29. Cdc42 colocalizes with RME-1- and EHD1-labelled endosomes in worms and HeLa cells, respectively, and expression of dominant-negative forms of Par6 and Cdc42 led to an inhibition of MHCI recycling, with no effect on TfR recycling. On the other hand, the inhibitory forms of Par6 and Cdc42 diminish TfR endocytosis with no effect on MHCI endocytosis. This suggests a coordinate regulation of CDE and CIE pathways, with important implications for understanding how these pathways function in the organism.
Endocytic recycling function in organisms
Cell division, cell migration, cell junction formation and maintenance, cell–cell fusion events, control of neuronal signalling during learning and memory, and cell polarity appear to all share certain molecular underpinnings. In particular, each of these processes depends on accurate and efficient endocytic recycling. These are heavily studied and complex areas of research, each deserving of its own review. In the interest of space, we have taken specific examples of the involvement of recycling in these processes to illustrate the contributions of recycling pathways to these complex processes.
Recycling in cytokinesis
Plasma membrane remodelling during cytokinesis is known to depend on the regulation of endosomal membrane traffic. As cultured cells round up prior to cell division, endocytic recycling function is temporarily blocked while endocytosis proceeds, leading to the loss of plasma membrane area and the accumulation of cell surface-derived membrane in the endosomal system 71. Soon thereafter, the contractile ring forms and the cytokinetic furrow ingresses. In plants, cytokinesis proceeds via the de novo synthesis of plasma membrane by vesicle transport and fusion at the center of a mother cell (reviewed in Ref. 72). Although animal cell cytokinesis was initially thought to be mechanistically distinct from that of plant cells, it is now clear that in animal cells vesicular transport is also critical for cytokinesis, as first evidenced by the production of multinucleate cells after depletion of a particular SNARE protein 73–75. Vesicles that are important for animal cell cytokinesis are derived from recycling endosomes and the Golgi complex, contributing membrane to the advancing cleavage furrow 76, 77. Recycling endosomes are also closely associated with the late step of cytokinesis, called abscission, the process that breaks the final thin connection between daughter cells (see Fig. 2). In particular, the recycling endosome-specific GTPases Rab11 and Arf6 are required for cytokinesis in several organisms, including vertebrates and invertebrates 78–80.
Figure 2. Recycling and cytokinesis.
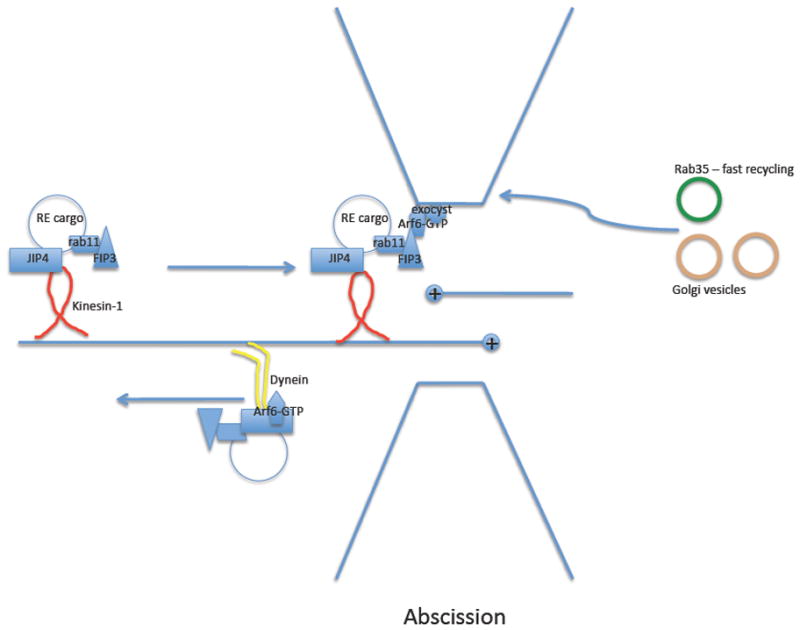
During anaphase, membrane is added to the cleavage furrow by recycling endosomes and Golgi-derived vesicles. During abscission, as depicted, the removal of ECT2 (not shown) from the central spindle complex allows binding of CYK-4 (not shown) to FIP3, which helps to recruit Rab11–GTP- and FIP3-positive recycling endosomes to the intercellular bridge. Transport to the bridge is mediated, at least in part, through association of kinesin-1 with recycling endosome adaptor protein JIP4. At the midbody, Arf6–GTP promotes fusion via interaction with FIP3 and the exocyst. Arf6–GTP also interacts with JIP4, promoting exit from the intercellular bridge via motor switching to the dynactin–dynein complex. Rab35–GTP also contributes to abscission, probably through contribution of early endosome (EE)-derived vesicles and appears to be important for phosphatidylinositol-4,5-bisphosphate accumulation at the midbody.
The association of recycling endosomes with the cleavage furrow, and the donation of membrane to the furrow, depends on Rab11 79. In cultured mammalian cells, Arf6 appears to have less effect on furrowing but is important for severing the final intercellular bridge during abscission 80, 81. In Drosophila melanogaster spermatocytes, Arf6 is required during the early and late phases of cytokinesis, but somatic cell cytokinesis can proceed without Arf6 82. Another key recycling endosomal protein in cytokinesis is FIP3 (Nuf in D. melanogaster), the Rab11 and Arf6 effector 79, 83. In cultured mammalian cells, FIP3 knockdown inhibits abscission, whereas in D. melanogaster embryos the loss of Nuf inhibits membrane addition to ingressing furrows 79, 83.
In flies, RhoGEF2 activates RhoA and is important for actin polymerization on furrowing membranes 84, 85. Recruitment of RhoGEF2 to the cleavage furrow by Nuf appears to be a key function of Nuf 86. RhoGef2 may be associated with vesicles trafficked to the furrow by Nuf 85. In mammals, ECT2, in association with centralspindlin (a complex composed of Cyk-4 and MKLP1), activates RhoA to promote furrowing 87. During telophase ECT2 is lost from the centralspindlin complex, allowing Cyk-4 to recruit Rab11–FIP3, promoting tethering of recycling endosomes to the midbody (Fig 2) 87.
It remains to be determined exactly how RhoA-promoted actin dynamics influences furrowing. Actin could act as a track for vesicle movement, ultimately promoting localized fusion. This idea is supported by evidence that motor protein myosin VI is important for cytokinesis 88. Another possible explanation for the Nuf and RhoGEF2 phenotypes is that Nuf recruits RhoGEF2 to recycling endosomes, and RhoGEF2 activity promotes recycling endosome-derived vesicle budding. Actin polymerization is required for recycling of CIE cargo back to the plasma membrane14, 53 and may be associated with membrane budding events, presumably providing membrane tension that contributes to vesicle scission 89.
The microtubule-based spindle is key to many aspects of cell division, and recycling endosomes are clearly associated with microtubules and microtubule-based motors 90. Association of recycling endosomes with the cleavage furrow requires molecular motor activity associated with the spindle. As noted above, JIP3 and JIP4 are proposed effectors of Arf6 59, and new evidence indicates that during cytokinesis Arf6 activity acts as a switch that controls recycling endosome movement to the intercellular bridge 59. The LZII (leucine zipper 2) domain of JIP proteins was found to interact with two dynactin (dynein activator complex) components, p150glued and p50, kinesin-1 component KLC and Arf6–GTP. p150glued and KLC compete for binding to the LZII domain of JIP proteins, and the addition of the constitutively active mutant of Arf6 enhanced the association of dynactin and antagonized KLC binding. Thus, it was proposed that Arf6 activation couples kinesin-to-dynein motor switching (Fig 2).
Recycling of E-cadherin in cell adhesion and morphogenesis
Cell adhesion and morphogenesis also depends on endocytic recycling pathways. As mentioned above, E-cadherin can be taken up from the cell surface by CIE 91. Once internalized, the decision whether to degrade or recycle E-cadherin appears to be a critical one for cells undergoing adhesion and morphogenesis. Both Rab11 and Arf6, as well as sorting nexin 1 (Snx1), are thought to be regulators of E-cadherin recycling from endosomes to the plasma membrane 92–94. Snx1 promotes E-cadherin trafficking along the retrograde route to the Golgi, prior to transport through the Rab11- and Arf6-regulated recycling endosomes 93, 94. The exocyst is also required for E-cadherin recycling 95.
Tracheal development in D. melanogaster is a particularly interesting example of how morphogenesis is regulated by modulation of recycling activity. The D. melanogaster trachea is a complex set of branched epithelial tubes that allow gas exchange in the animal. During development, the trachea change shape. Initially, all tracheal branches are large diameter ‘multicellular’ tubes. As development proceeds, cells in most branches undergo a rearrangement, termed intercalation, which causes the branches to decrease in diameter. However, cells in the major tracheal branch, known as the dorsal trunk, are prevented from undergoing intercalation. The decision of whether cells intercalate, or not, is regulated by multiple signalling inputs 96. Recent work indicates that increased accumulation of Rab11 in the dorsal trunk, concomitant with transcriptional upregulation of its effector Rip11 (a downstream effect of Wingless signalling), increases junctional accumulation of D. melanogaster E-cadherin. It was proposed that such an increase in junctional E-cadherin in the dorsal trunk inhibits cell intercalation in this branch. Conversely, the absence of Rip11 and lower levels of Rab11 and junctional E-cadherin in other tracheal branches allows intercalation to occur 97.
Another regulator of cell adhesion during development is the Cdc42–Par6–Par3–atypical PKC (aPKC) complex. In C. elegans cells and in human HeLa cells, these proteins were found to regulate uptake of clathrin-dependent cargo, and were found to regulate the recycling of cargo taken up independently of clathrin 29. Interestingly several groups working in D. melanogaster have recently described a requirement for Cdc42, Par6 and aPKC in the regulation of adherens junctions. One study followed cellular rearrangements during neuroectoderm development 98. About one-third of the cells in this tissue layer rearrange their adherens junctions and ingress to become neuroblasts, whereas the other cells remain in the epithelium and become epidermal cells. Cdc42, Par3, Par6 and aPKC activity appeared to be specifically required to maintain junction integrity during the ingression process98. Apical polarity protein Crumbs and apically endocytosed styryl dye FM 4–64 were abnormal in cells lacking Cdc42 or Par proteins, accumulating in enlarged HRS-positive endosomes. These phenotypes likely indicate a defect in apical recycling of Crumbs and FM 4–64, or increased uptake and reduced early to late endosome transport in the absence of Cdc42or Par activity 98, 99.
Around the same time, a related pair of studies in D. melanogaster examined the role of Cdc42, Par6 and aPKC in development of the dorsal thorax of pupae 100, 101. In this case, Cdc42 and its effectors Par6 and aPKC, but not Par3, were required to maintain E-cadherin at adherens junctions. Junctional dynamics downstream of Cdc42–Par6–aPKC was mediated by the only D. melanogaster member of the CIP4/TOCA (Cdc42 interacting protein 4; transducer of Cdc42-dependent actin assembly) protein family. CIP4/TOCA proteins contain F-BAR domains that bind and remodel lipid bilayers. These proteins also regulate actin dynamics via their SH3 domains, which binds to N-Wasp, thereby activating Arp2/3. In this case, CIP4 also associated, directly or indirectly, with dynamin. The primary defect appeared to be at the level of scission of plasma membrane-derived tubules, thereby removing E-cadherin from the cell surface, a process that may be facilitated by local actin polymerization. Taken together, these three studies emphasize the importance of polarity proteins in developmentally controlled membrane traffic, and suggest that some of these same proteins can function at the level of the plasma membrane and at the level of endosomes, depending on the developmental context and the particular cargo proteins involved.
Recycling in myoblast fusion
Another important developmental process that requires efficient endosomal recycling function is myoblast fusion, which is required during growth, regeneration and repair of skeletal muscle. Rac and its activator myoblast city (Dock180) are the best-known components that are required for this cell–cell fusion event, a requirement conserved from D. melanogaster to mammals 102, 103. Rac is enriched at cell–cell contact sites prior to myoblast fusion and likely remodels the local actin cytoskeleton, thereby in some way promoting cell–cell fusion. Rac is also known to activate certain types of clathrin-independent endocytic uptake, and so local Rac activation may also remodel the lipid and integral membrane composition of the contact sites 104. Over the past few years, several additional proteins that are important for endocytic recycling have been shown to function in myoblast fusion. This includes the Arf6 activator Loner (also known as Arf-GEP100) and Arf6 itself 105. The most likely role for these proteins in this process is in directing important cargo from recycling endosomes to the contact membranes. In particular, Rac activation and localization appears impaired after mutation or perturbation of Loner and Arf6 105. Recent data in migrating cells indicate that Rac activation occurs on endosomal membranes, and activated Rac may be transported to specific leading-edge membrane sites by Arf6-mediated recycling 106. In light of these data, it is tempting to speculate that, during myoblast fusion, activated Rac must also be recycled from endosomal activation sites to plasma membrane domains involved in cell–cell fusion. Recent work suggests a role for actin-enriched foci in targeting the fusion of intracellular vesicles to the contact membrane. A requirement for myoferlin and EHD2 in endocytic recycling, and in cell–cell fusion events has also been shown 107. It remains to be determined whether myoferlin and EHD2 are also important for the accumulation of activated Rac and/or other actin regulators at cell contact sites.
Recycling in learning and memory
Experience-dependent modification of synaptic strength is thought to be a key process in learning and memory, and one well-studied example of such a change is the remodelling of dendritic spines in the mammalian hippocampus. Spines increase in membrane area and the number of surface-resident AMPA receptors in response to NMDA receptor activation during long-term potentiation (LTP), and both of these acute changes to the spines depend on Rab11- and EHD1/RME-1-positive recycling endosome function 108, 109. Recent work points to myosin Vb as a key protein in this process (Fig 3) 110. At low calcium levels myosin Vb is found in an inactive conformation, lacking association with recycling endosomes. However, transient increases in calcium levels within dendritic spines after NMDA receptor activation alters myosin Vb conformation, allowing it to associate with recycling endosomes through Rab11-FIP2, leading to locally increased recycling of membrane, AMPA receptors and other endosomal cargo 110. EHD1/RME-1-dependent recycling of G protein-activated inwardly rectifying (GIRK) potassium channels was also increased after NMDA receptor activation, suggesting a similar regulatory mechanism (Fig 3) 111.
Figure 3. Endocytic recycling and long-term potentiation.
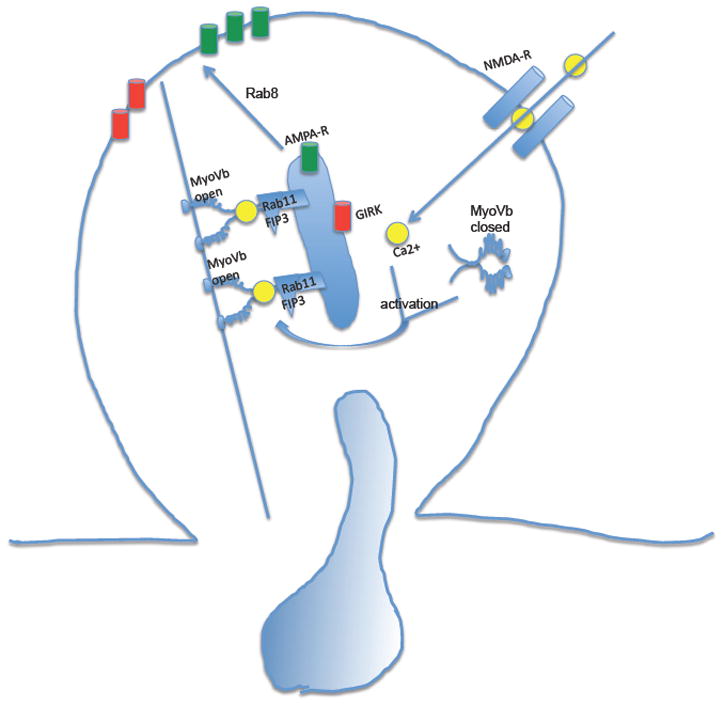
In postsynaptic membranes, NMDA receptor (NMDA-R)-mediated long-term potentiation requires myosin Vb, a conformation-dependent binding partner of Rab11-FIP2. NMDA-R activation results in calcium influx, triggering activation of myosin Vb, and translocation of recycling endosomes and their cargo into the spine. This series of events increases spine membrane area and increases AMPA-R density on the spine surface. GIRK channel density on the spine surface is also increased, likely through the same or similar mechanisms. Final transport from the recycling endosome to the spine surface is mediated by Rab8.
The small GTPase Rab8 appears to regulate the final insertion of AMPA receptors into the plasma membrane of dendritic spines 112, 113. Interestingly, recent work in C. elegans suggests that a pool of postsynaptic AMPA receptors are endocytosed in a clathrin-independent manner, and that recycling of this pool of receptors requires the RAB-10 GTPase, a close relative of Rab8 114. It remains to be determined whether clathrin-independent endocytosis contributes to similar processes in the mammalian nervous system.
Perspectives
In a number of ways the recycling endosome resembles the TGN in its relationship with the plasma membrane. For instance, the plasma membrane fusion machinery, which was first defined for secretory vesicles, is also used by vesicles derived from the recycling endosome 60. This view is furthered by recent findings that a significant fraction of secretory cargo leaving the Golgi passes through the recycling endosome en route to the plasma membrane 115, 116. The recycling endosome appears to be enriched in PtdIns4P, like the Golgi, with portions enriched in PtdIns(4,5)P2 and cholesterol, like the plasma membrane 15, 67, 117. Like the Golgi, the recycling endosome requires PLD and its lipid product PA for cargo export, and Arf proteins regulate the accumulation of such lipids 55, 118. How the recycling endosome interfaces with the Golgi will be an important question in the coming years.
Much remains to be learned about the mechanisms of recycling from the endosomal pathway. Many of the protein and lipid components that are important for recycling endosome function described here have been identified, but most are not understood in detail. In particular, there is no clear model for how cargo carriers are produced from the recycling endosome. Several technical hurdles must be cleared to better understand recycling endosome budding in detail. Our understanding of the budding mechanism would greatly benefit from an in vitro reconstitution system like those developed for the early secretory pathway. In addition, an in vivo imaging system allowing direct visualization of recycling endosome budding would be a great help. For instance, little is known of the order of recruitment of regulatory proteins during recycling endosome budding. Such technical advances have so far proved elusive for the recycling endosome and are a major challenge for the field.
Likewise, the field is still at an early stage in integrating the information gained so far into an understanding of higher-order physiology within the cell and among interacting cells. One very interesting aspect that deserves greater attention is how endocytosis is coordinated with recycling and degradation, and how clathrin-independent pathways coordinate with the clathrin-dependent pathways. There are many indications from the literature that such pathways are coupled or inversely coupled, but little is known of the mechanism(s) of such coupling. Furthermore, issues of physiology are likely to depend critically on cell type, environment and developmental stage, yet most of our information about these pathways comes from a few cultured cell lines. More work in the context of whole organs and organisms is required to understand how the pathways identified are adapted to such conditions.
Contributor Information
Barth D. Grant, Email: grant@biology.rutgers.edu.
Julie G. Donaldson, Email: jdonalds@helix.nih.gov.
References
- 1.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 3.Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annu Rev Biochem. 2009 doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 4.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandvig K, Torgersen ML, Raa HA, van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol. 2008;129:267–76. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–49. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 9.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–23. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 10.Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–26. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Woodman PG, Futter CE. Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20:408–14. doi: 10.1016/j.ceb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–87. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–17. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powelka AM, et al. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 17.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–52. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyster CA, et al. Discovery of New Cargo Proteins that Enter Cells through Clathrin-Independent Endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. This study identified endogenous plasma membrane proteins that enter human cells by CIE, thus providing new cargo proteins that can be examined in different cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barral DC, et al. CD1a and MHC Class I Follow a Similar Endocytic Recycling Pathway. Traffic. 2008;9:1446–1457. doi: 10.1111/j.1600-0854.2008.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarselli M, Donaldson JG. Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J Biol Chem. 2009;284:3577–3585. doi: 10.1074/jbc.M806819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann P, et al. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9:377–88. doi: 10.1016/j.devcel.2005.07.011. First report of specific intracellular sorting of CIE cargo protein, syndecan, by phosphinositides, syntenin and Arf6. [DOI] [PubMed] [Google Scholar]
- 22.Walseng E, Bakke O, Roche PA. MHC class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Q, et al. Identification and characterization of a new class of trafficking motifs for controlling clathrin-independent internalization and recycling. J Biol Chem. 2007;282:13087–97. doi: 10.1074/jbc.M700767200. [DOI] [PubMed] [Google Scholar]
- 24.Chen CC, et al. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell. 2006;17:1286–97. doi: 10.1091/mbc.E05-08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi A, et al. A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr Biol. 2007;17:1913–24. doi: 10.1016/j.cub.2007.10.045. Novel role for Alix/Bro1 proteins, previously implicated in MVB formation, in RME-1/EHD-regulated endocytic recycling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 27.Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–52. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daumke O, et al. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 29.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–73. doi: 10.1038/ncb1627. Genome-wide analysis of genes involved in endocytosis and recycling in C. elegans finds role for polarity proteins in CDE and for recycling of CIE cargo. [DOI] [PubMed] [Google Scholar]
- 30.Fares H, Greenwald I. Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics. 2001;159:133–45. doi: 10.1093/genetics/159.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–26. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw JD, Cummings KB, Huyer G, Michaelis S, Wendland B. Yeast as a model system for studying endocytosis. Exp Cell Res. 2001;271:1–9. doi: 10.1006/excr.2001.5373. [DOI] [PubMed] [Google Scholar]
- 33.Choudhury A, Sharma DK, Marks DL, Pagano RE. Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling. Mol Biol Cell. 2004;15:4500–11. doi: 10.1091/mbc.E04-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Sluijs P, et al. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–40. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- 35.Deneka M, et al. Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. Embo J. 2003;22:2645–57. doi: 10.1093/emboj/cdg257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated Regulation of a Rapid Rab4-dependent Recycling Pathway. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–25. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, et al. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. Embo J. 2008;27:1183–96. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allaire PD, et al. Connecdenn, a novel DENN domain-containing protein of neuronal clathrin-coated vesicles functioning in synaptic vesicle endocytosis. J Neurosci. 2006;26:13202–12. doi: 10.1523/JNEUROSCI.4608-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patino-Lopez G, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–30. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5 and Rab11. Journal of Cell Biology. 2000;149:901–913. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma M, Naslavsky N, Caplan S. A role for EHD4 in the regulation of early endosomal transport. Traffic. 2008;9:995–1018. doi: 10.1111/j.1600-0854.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell. 2006;17:163–77. doi: 10.1091/mbc.E05-05-0466. Reports on the interaction of EHD1 and EHD3 proteins with the same Rab11 effector, FIP2, leading to spatially distinct functions of these scaffolding proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonteich E, et al. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–33. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horgan CP, et al. Rab11-FIP3 is critical for the structural integrity of the endosomal recycling compartment. Traffic. 2007;8:414–30. doi: 10.1111/j.1600-0854.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 46.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell. 2008;19:4224–37. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magadan JG, Barbieri MA, Mesa R, Stahl PD, Mayorga LS. Rab22a regulates the sorting of transferrin to recycling endosomes. Mol Cell Biol. 2006;26:2595–614. doi: 10.1128/MCB.26.7.2595-2614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babbey CM, et al. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–75. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelham HR. Insights from yeast endosomes. Curr Opin Cell Biol. 2002;14:454–62. doi: 10.1016/s0955-0674(02)00352-6. [DOI] [PubMed] [Google Scholar]
- 50.Traer CJ, et al. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol. 2007;9:1370–80. doi: 10.1038/ncb1656. Reports that sorting nexin 4 promotes dynein minus end-directed movement of early endosomes towards the ERC. [DOI] [PubMed] [Google Scholar]
- 51.Lau AW, Chou MM. The adaptor complex AP-2 regulates post-endocytic trafficking through the non-clathrin Arf6-dependent endocytic pathway. J Cell Sci. 2008;121:4008–17. doi: 10.1242/jcs.033522. [DOI] [PubMed] [Google Scholar]
- 52.Naslavsky N, Weigert R, Donaldson JG. Convergence of Non-clathrin- and Clathrin-derived Endosomes Involves Arf6 Inactivation and Changes in Phosphoinositides. Mol Biol Cell. 2003;14:417–31. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–70. doi: 10.1091/mbc.E04-04-0342. Demonstrates that recycling of CIE cargo back to the plasma membrane requires Rab11, Rab22 and actin, distinguishing it from TfR recycling processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol. 2007;9:1381–91. doi: 10.1038/ncb1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jovanovic OA, Brown FD, Donaldson JG. An effector domain mutant of Arf6 implicates phospholipase D in endosomal membrane recycling. Mol Biol Cell. 2006;17:327–35. doi: 10.1091/mbc.E05-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson SE, et al. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2006;17:645–57. doi: 10.1091/mbc.E05-07-0662. Provides evidence that Erk signalling stimulates recycling of CIE cargo back to the plasma membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karacsonyi C, Miguel AS, Puertollano R. Mucolipin-2 Localizes to the Arf6-Associated Pathway and Regulates Recycling of GPI-APs. Traffic. 2007;8:1404–14. doi: 10.1111/j.1600-0854.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 58.Li J, et al. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol. 2007;178:453–64. doi: 10.1083/jcb.200608033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montagnac G, et al. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19:184–95. doi: 10.1016/j.cub.2008.12.043. Evidence that Arf6 can control the direction of endosomal movement by binding to the JIP scaffolding proteins, which bind to either dynein or kinesin. [DOI] [PubMed] [Google Scholar]
- 60.Prigent M, et al. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163:1111–1121. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hattula K, et al. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–77. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- 62.Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–62. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson A, et al. Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell. 2007;18:2687–97. doi: 10.1091/mbc.E05-09-0873. Provides evidence for segregation of recycling cargo into separate subdomains within the recycling endosome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caplan S, et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. Embo J. 2002;21:2557–67. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grant B, et al. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol. 2001;3:573–9. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- 66.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–72. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 67.Jovic M, Kieken F, Naslavsky N, Sorgen PL, Caplan S. EHD1-associated Tubules Contain Phosphatidylinositol-4-Phosphate and Phosphatidylinositol-(4,5)-Bisphosphate and Are Required for Efficient Recycling. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-11-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braun A, et al. EHD proteins associate with syndapin I and II and such interactions play a crucial role in endosomal recycling. Mol Biol Cell. 2005;16:3642–58. doi: 10.1091/mbc.E05-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Welsch S, et al. Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular-vesicular membranes in ESCRT function. Traffic. 2006;7:1551–66. doi: 10.1111/j.1600-0854.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 70.Yan Q, et al. mVps24p functions in EGF receptor sorting/trafficking from the early endosome. Exp Cell Res. 2005;304:265–73. doi: 10.1016/j.yexcr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci U S A. 2007;104:7939–44. doi: 10.1073/pnas.0702511104. Shows reduced recycling during mitosis, which contributes to reduced plasma membrane area and the ‘rounding up’ of dividing cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Damme D, Inze D, Russinova E. Vesicle trafficking during somatic cytokinesis. Plant Physiol. 2008;147:1544–1552. doi: 10.1104/pp.108.120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jantsch-Plunger V, Glotzer M. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr Biol. 1999;9:738–45. doi: 10.1016/s0960-9822(99)80333-9. [DOI] [PubMed] [Google Scholar]
- 74.Xu H, et al. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev Biol. 2002;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- 75.Low SH, et al. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–9. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 76.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–9. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 77.Montagnac G, Echard A, Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr Opin Cell Biol. 2008;20:454–61. doi: 10.1016/j.ceb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–46. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riggs B, et al. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol. 2003;163:143–54. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fielding AB, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. Embo J. 2005;24:3389–99. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schweitzer JK, D’Souza-Schorey C. Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J Biol Chem. 2002;277:27210–6. doi: 10.1074/jbc.M201569200. [DOI] [PubMed] [Google Scholar]
- 82.Dyer N, et al. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development. 2007;134:4437–47. doi: 10.1242/dev.010983. [DOI] [PubMed] [Google Scholar]
- 83.Hickson GR, et al. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003;14:2908–20. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grosshans J, et al. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132:1009–20. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- 85.Padash Barmchi M, Rogers S, Hacker U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J Cell Biol. 2005;168:575–85. doi: 10.1083/jcb.200407124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao J, Albertson R, Riggs B, Field CM, Sullivan W. Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J Cell Biol. 2008;182:301–13. doi: 10.1083/jcb.200712036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simon GC, et al. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. Embo J. 2008;27:1791–803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arden SD, Puri C, Au JS, Kendrick-Jones J, Buss F. Myosin VI is required for targeted membrane transport during cytokinesis. Mol Biol Cell. 2007;18:4750–61. doi: 10.1091/mbc.E07-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–31. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 90.Lin SX, Gundersen GG, Maxfield FR. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol Biol Cell. 2002;13:96–109. doi: 10.1091/mbc.01-05-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin Endocytosis in Isolated MCF-7 and Chinese Hamster Ovary Cells: THE INITIAL FATE OF UNBOUND E-CADHERIN. J Biol Chem. 2003;278:21050–7. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- 92.Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. Embo J. 2001;20:4973–86. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–55. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bryant DM, et al. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–28. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- 95.Langevin J, et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:365–76. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 96.Ribeiro C, Ebner A, Affolter M. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell. 2002;2:677–83. doi: 10.1016/s1534-5807(02)00171-5. [DOI] [PubMed] [Google Scholar]
- 97.Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol. 2008;10:964–70. doi: 10.1038/ncb1756. Connects morphological events in development with temporal and spatial regulation of the underlying recycling endosome machinery. [DOI] [PubMed] [Google Scholar]
- 98.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–43. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duncan MC, Peifer M. Regulating polarity by directing traffic: Cdc42 prevents adherens junctions from crumblin’ aPart. J Cell Biol. 2008;183:971–4. doi: 10.1083/jcb.200811057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–8. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 101.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–48. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 102.Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pajcini KV, Pomerantz JH, Alkan O, Doyonnas R, Blau HM. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J Cell Biol. 2008;180:1005–19. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grassart A, Dujeancourt A, Lazarow PB, Dautry-Varsat A, Sauvonnet N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008;9:356–62. doi: 10.1038/embor.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- 106.Palamidessi A, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–47. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 107.Doherty KR, et al. The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J Biol Chem. 2008;283:20252–60. doi: 10.1074/jbc.M802306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 109.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–5. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–48. doi: 10.1016/j.cell.2008.09.057. Elegant study demonstrating how NMDA receptor-stimulated calcium levels allow myosin Vb to associate with Rab11-FIP2 endosomes bearing AMPA receptors, facilitating their delivery to dendritic spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chung HJ, et al. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc Natl Acad Sci U S A. 2009;106:635–40. doi: 10.1073/pnas.0811685106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–5. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem. 2004;279:43870–8. doi: 10.1074/jbc.M404982200. [DOI] [PubMed] [Google Scholar]
- 114.Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell. 2007;18:4387–96. doi: 10.1091/mbc.E07-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ang AL, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–43. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–5. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 117.Hao MM, et al. Vesicular and non-vesicular sterol transport in living cells – The endocytic recycling compartment is a major sterol storage organelle. Journal of Biological Chemistry. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- 118.Padron D, Tall RD, Roth MG. Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors. Mol Biol Cell. 2006;17:598–606. doi: 10.1091/mbc.E05-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–7. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 120.Pfeffer SR. Structural clues to Rab GTPase functional diversity. J Biol Chem. 2005;280:15485–8. doi: 10.1074/jbc.R500003200. [DOI] [PubMed] [Google Scholar]
- 121.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–57. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Cohen LA, et al. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell. 2007;18:2244–53. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chies R, et al. Alterations in the Arf6-regulated plasma membrane endosomal recycling pathway in cells overexpressing the tetraspan protein Gas3/PMP22. J Cell Sci. 2003;116:987–99. doi: 10.1242/jcs.00326. [DOI] [PubMed] [Google Scholar]
- 124.Lavezzari G, Roche KW. Constitutive endocytosis of the metabotropic glutamate receptor mGluR7 is clathrin-independent. Neuropharmacology. 2007;52:100–7. doi: 10.1016/j.neuropharm.2006.07.011. [DOI] [PubMed] [Google Scholar]