Abstract
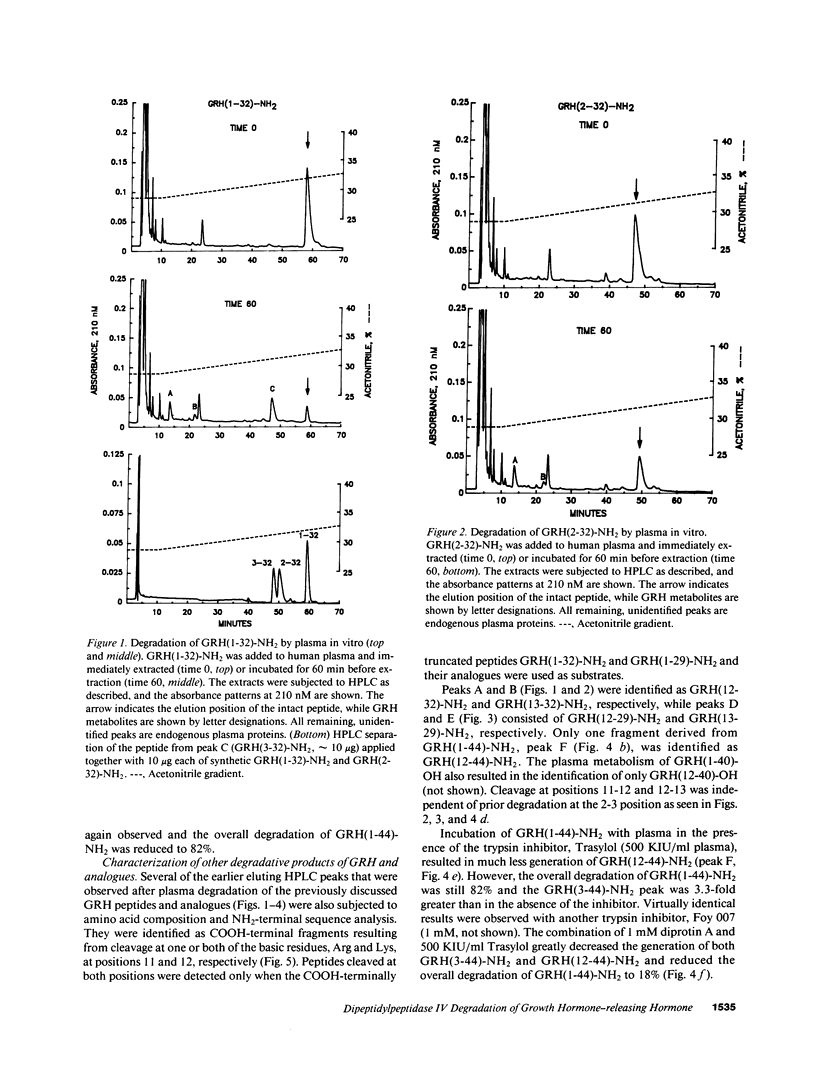
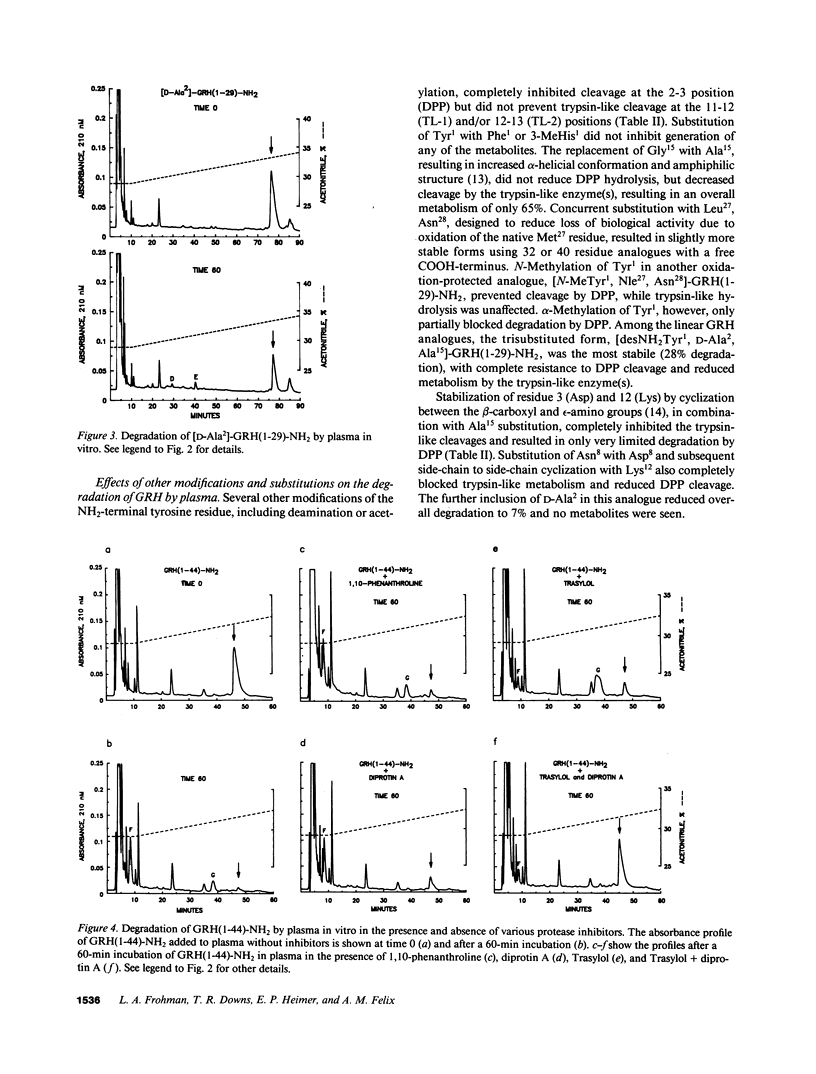
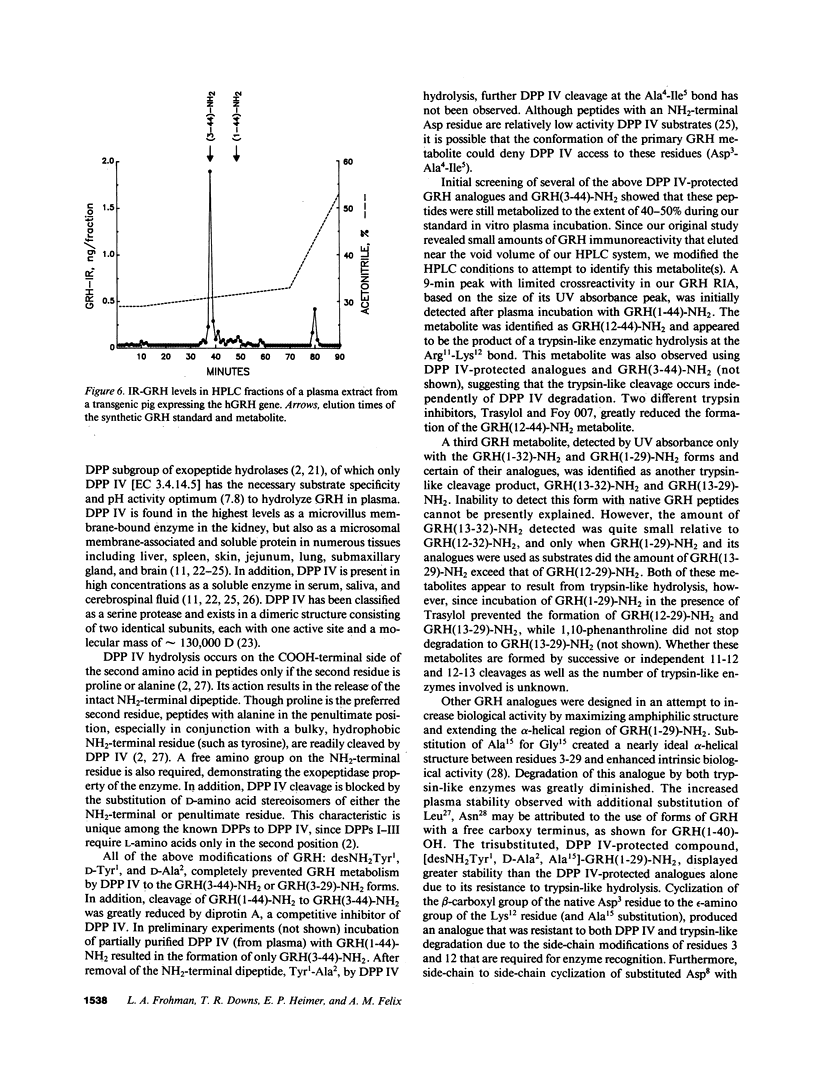
The plasma enzyme responsible for primary proteolytic cleavage of growth hormone-releasing hormone (GRH) at the 2-3 amino acid bond was characterized. Native GRH[GRH(1-44)-NH2 and GRH(1-40)-OH], and COOH-terminally shortened fragments [GRH(1-32)-NH2 and GRH(1-29)-NH2] were rapidly cleaved, while GRH(2-32)-NH2 was not degraded at this site. Moreover, degradation to GRH(3-44)-NH2 was unaffected by an aminopeptidase inhibitor, indicating that this metabolite was generated from a single step cleavage by a dipeptidylpeptidase (DPP) rather than sequential aminopeptidase cleavages. Conversion to GRH(3-44)-NH2 was blocked by diprotin A, a DPP type IV (DPP IV) competitive inhibitor. D-Amino acid substitution at either position 1 or 2 also prevented hydrolysis, characteristic of DPP IV. Analysis of endogenous plasma GRH immunoreactivity from a human GRH transgenic pig revealed that the major peak coeluted with GRH(3-44)-NH2. Native GRH exhibited trypsin-like degradation at the 11-12 position but cleavage at the 12-13 site occurred only with GRH(1-32)-NH2 and GRH(1-29)-NH2. Formation of these metabolites was independent of prior DPP IV hydrolysis but was greatly reduced by trypsin inhibitors. Evaluation of plasma stability of potential GRH super analogues, designed to resist degradation by these enzymes, confirmed that GRH degradation in plasma occurs primarily by DPP IV, and to a lesser extent by trypsin-like enzyme(s).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth A., Schulz H., Neubert K. Untersuchungen zur Reinigung und Charakterisierung der Dipeptidylaminopeptidase IV. Acta Biol Med Ger. 1974;32(2-3):157–174. [PubMed] [Google Scholar]
- Conlon J. M., Sheehan L. Conversion of substance P to C-terminal fragments in human plasma. Regul Pept. 1983 Dec;7(4):335–345. doi: 10.1016/0167-0115(83)90105-2. [DOI] [PubMed] [Google Scholar]
- Enright W. J., Chapin L. T., Moseley W. M., Zinn S. A., Tucker H. A. Growth hormone-releasing factor stimulates milk production and sustains growth hormone release in Holstein cows. J Dairy Sci. 1986 Feb;69(2):344–351. doi: 10.3168/jds.s0022-0302(86)80412-x. [DOI] [PubMed] [Google Scholar]
- Felix A. M., Heimer E. P., Wang C. T., Lambros T. J., Fournier A., Mowles T. F., Maines S., Campbell R. M., Wegrzynski B. B., Toome V. Synthesis, biological activity and conformational analysis of cyclic GRF analogs. Int J Pept Protein Res. 1988 Dec;32(6):441–454. doi: 10.1111/j.1399-3011.1988.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R. Measurement of growth hormone-releasing factor. Methods Enzymol. 1986;124:371–389. doi: 10.1016/0076-6879(86)24029-x. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R., Williams T. C., Heimer E. P., Pan Y. C., Felix A. M. Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus. J Clin Invest. 1986 Oct;78(4):906–913. doi: 10.1172/JCI112679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman L. A., Thominet J. L., Webb C. B., Vance M. L., Uderman H., Rivier J., Vale W., Thorner M. O. Metabolic clearance and plasma disappearance rates of human pancreatic tumor growth hormone releasing factor in man. J Clin Invest. 1984 May;73(5):1304–1311. doi: 10.1172/JCI111333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K. M., Fukasawa K., Sahara N., Harada M., Kondo Y., Nagatsu I. Immunohistochemical localization of dipeptidyl aminopeptidase IV in rat kidney, liver, and salivary glands. J Histochem Cytochem. 1981 Mar;29(3):337–343. doi: 10.1177/29.3.6787113. [DOI] [PubMed] [Google Scholar]
- Griffiths E. C., McDermott J. R. Enzymic inactivation of hypothalamic regulatory hormones. Mol Cell Endocrinol. 1983 Nov;33(1):1–25. doi: 10.1016/0303-7207(83)90053-9. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Brazeau P., Böhlen P., Esch F., Ling N., Wehrenberg W. B. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982 Nov 5;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- Hart I. C., Chadwick P. M., Coert A., James S., Simmonds A. D. Effect of different growth hormone-releasing factors on the concentrations of growth hormone, insulin and metabolites in the plasma of sheep maintained in positive and negative energy balance. J Endocrinol. 1985 Apr;105(1):113–119. doi: 10.1677/joe.0.1050113. [DOI] [PubMed] [Google Scholar]
- Hartrodt B., Neubert K., Fischer G., Demuth U., Yoshimoto T., Barth A. Degradation of beta-casomorphin-5 by proline-specific-endopeptidase (PSE) and post-proline- cleaving-enzyme (PPCE). Comparative studies of the beta-casomorphin-5 cleavage by dipeptidyl-peptidase IV. Pharmazie. 1982 Jan;37(1):72–73. [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Heiman M. L., Nekola M. V., Murphy W. A., Lance V. A., Coy D. H. An extremely sensitive in vitro model for elucidating structure-activity relationships of growth hormone-releasing factor analogs. Endocrinology. 1985 Jan;116(1):410–415. doi: 10.1210/endo-116-1-410. [DOI] [PubMed] [Google Scholar]
- Hui K. S. A novel dipeptidyl aminopeptidase in rat brain membranes. Its isolation, purification, and characterization. J Biol Chem. 1988 May 15;263(14):6613–6618. [PubMed] [Google Scholar]
- Kato T., Nagatsu T., Fukasawa K., Harada M., Nagatsu I., Sakakibara S. Successive cleavage of N-terminal Arg1--Pro2 and Lys3-Pro4 from substance P but no release of Arg1-Pro2 from bradykinin, by X-Pro dipeptidyl-aminopeptidase. Biochim Biophys Acta. 1978 Aug 7;525(2):417–422. doi: 10.1016/0005-2744(78)90237-1. [DOI] [PubMed] [Google Scholar]
- Kato T., Nagatsu T., Kimura T., Sakakibara S. Fluorescence assay of x-prolyl dipeptidyl-aminopeptidase activity with a new fluorogenic substrate. Biochem Med. 1978 Jun;19(3):351–359. doi: 10.1016/0006-2944(78)90035-2. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., George S. G., Ingram J., Kershaw D., Wood E. J., Young A. R. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J. 1976 Jul 1;157(1):169–182. doi: 10.1042/bj1570169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Umbach M., Brantl V., Teschemacher H. Studies on the enzymatic degradation of beta-casomorphins. Life Sci. 1983;33 (Suppl 1):137–140. doi: 10.1016/0024-3205(83)90463-0. [DOI] [PubMed] [Google Scholar]
- Lance V. A., Murphy W. A., Sueiras-Diaz J., Coy D. H. Super-active analogs of growth hormone-releasing factor (1-29)-amide. Biochem Biophys Res Commun. 1984 Feb 29;119(1):265–272. doi: 10.1016/0006-291x(84)91647-4. [DOI] [PubMed] [Google Scholar]
- Ling N., Baird A., Wehrenberg W. B., Ueno N., Munegumi T., Chiang T. C., Regno M., Brazeau P. Synthesis and in vitro bioactivity of human growth hormone-releasing factor analogs substituted at position-1. Biochem Biophys Res Commun. 1984 Jul 18;122(1):304–310. doi: 10.1016/0006-291x(84)90475-3. [DOI] [PubMed] [Google Scholar]
- Ling N., Esch F., Böhlen P., Brazeau P., Wehrenberg W. B., Guillemin R. Isolation, primary structure, and synthesis of human hypothalamic somatocrinin: growth hormone-releasing factor. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4302–4306. doi: 10.1073/pnas.81.14.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S., Ziel F. H., Braunstein G. D., Downs T., Frohman L. A. Medical management of acromegaly due to ectopic production of growth hormone-releasing hormone by a carcinoid tumor. J Clin Endocrinol Metab. 1988 Aug;67(2):395–399. doi: 10.1210/jcem-67-2-395. [DOI] [PubMed] [Google Scholar]
- Murphy W. A., Coy D. H. Potent long-acting alkylated analogs of growth hormone-releasing factor. Pept Res. 1988 Sep-Oct;1(1):36–41. [PubMed] [Google Scholar]
- Nagatsu T., Hino M., Fuyamada H., Hayakawa T., Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem. 1976 Aug;74(2):466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Sakai T., Kojima K., Araki E., Sakakibara S., Fukasawa K., Harada M. A sensitive and specific assay for dipeptidyl-aminopeptidase II in serum and tissues by liquid chromatography-fluorometry. Anal Biochem. 1985 May 15;147(1):80–85. doi: 10.1016/0003-2697(85)90011-9. [DOI] [PubMed] [Google Scholar]
- Nakata Y., Kusaka Y., Yajima H., Segawa T. Active uptake of substance P carboxy-terminal heptapeptide (5-11) into rat brain and rabbit spinal cord slices. J Neurochem. 1981 Dec;37(6):1529–1534. doi: 10.1111/j.1471-4159.1981.tb06323.x. [DOI] [PubMed] [Google Scholar]
- Pan Y. C., Wideman J., Blacher R., Chang M., Stein S. Use of high-performance liquid chromatography for preparing samples for microsequencing. J Chromatogr. 1984 Aug 3;297:13–19. doi: 10.1016/s0021-9673(01)89024-5. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., Thorner M., Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982 Nov 18;300(5889):276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- Stein S., Brink L. Amino acid analysis of proteins and peptides at the picomole level: the fluorescamine amino acid analyzer. Methods Enzymol. 1981;79(Pt B):20–25. doi: 10.1016/s0076-6879(81)79008-6. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Blumberg S. Substance P and analogues: biological activity and degradation. Prog Biochem Pharmacol. 1980;16:84–94. [PubMed] [Google Scholar]
- Thorner M. O., Vance M. L., Evans W. S., Blizzard R. M., Rogol A. D., Ho K., Leong D. A., Borges J. L., Cronin M. J., MacLeod R. M. Physiological and clinical studies of GRF and GH. Recent Prog Horm Res. 1986;42:589–640. [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Ogawa K., Naganawa H., Hamada M., Takeuchi T. Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J Antibiot (Tokyo) 1984 Apr;37(4):422–425. doi: 10.7164/antibiotics.37.422. [DOI] [PubMed] [Google Scholar]
- Wehrenberg W. B., Ling N. In vivo biological potency of rat and human growth hormone-releasing factor and fragments of human growth hormone-releasing factor. Biochem Biophys Res Commun. 1983 Sep 15;115(2):525–530. doi: 10.1016/s0006-291x(83)80176-4. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Ogita K., Walter R., Koida M., Tsuru D. Post-proline cleaving enzyme. Synthesis of a new fluorogenic substrate and distribution of the endopeptidase in rat tissues and body fluids of man. Biochim Biophys Acta. 1979 Aug 15;569(2):184–192. doi: 10.1016/0005-2744(79)90053-6. [DOI] [PubMed] [Google Scholar]



