Abstract
Previous studies showed that chronic estrogen treatment increases tryptophan hydroxylase-2 (TpH2) mRNA in the caudal dorsal raphe nucleus (DRN), and this increase was associated with decreased anxiety. The present study explored the interaction of estrogen and targeted, bidirectional manipulation of TpH2 expression in the caudal DRN by knockdown or viral overexpression, to decrease or increase tryptophan hydroxylase expression respectively, on anxiety behavior. Rats were ovariectomized and replaced with empty or estradiol capsules (OVX, OVX/E, respectively). Animals received microinfusions of either antisense TpH2 or control morpholino oligonucleotides into caudal DRN and were later tested in the open field test. A separate group of animals were microinfused with TpH2-GFP or GFP-only herpes simplex viral vectors into caudal DRN and tested in the open field. The bidirectional impact of manipulations on TpH2 expression was confirmed using a combination of quantitative protein and mRNA measurements; TpH2 expression changes were limited to discrete subregions of DRN that were targeted by the manipulations. Estradiol decreased anxiety in all behavioral measures. In the OVX/E group, TpH2 knockdown significantly decreased time spent in the center of the open field, but not in the OVX group, suggesting that TpH2 knockdown reduced the anxiolytic effects of estrogen. Conversely, TpH2 overexpression in the OVX group mimicked the effects of estrogen, as measured by increased time spent in the center of the open field. These results suggest that estrogen and TpH2 in the caudal DRN have a critical interaction in regulating anxiety-like behavior.
Keywords: Serotonin, raphe, Herpes virus vector, Gene transfer, mRNA, estradiol
1
Anxiety disorders are the most prevalent form of psychiatric disorder (Ramos et al., 2002) and are twice as prevalent in women as men (Pigott, 2003). Several lines of evidence suggest that anxiety disorders are most frequent during times when estrogen levels are low and may be relieved by estrogen treatment (Best et al., 1992, Sichel et al., 1995, Arpels, 1996, Gregoire et al., 1996). Furthermore, estrogen replacement sometimes augments the effects of selective serotonin reuptake inhibitors in women with anxiety or depression (Rasgon et al., 2002), suggesting an interaction between estrogen and serotonin in this regard.
Estrogen modulates binding and transcript levels of serotonin transporter and receptors in the dorsal raphe nucleus (DRN); this leads to enhanced serotonergic activity (Rubinow et al., 1998, Bethea et al., 2002, Bethea et al., 2009). The DRN, a major source of 5-HT innervation in the forebrain, is composed of multiple, functionally distinct subregions that receive anatomically distinct inputs; these subregions vary in their expression of estrogen receptors (Shughrue et al., 1997, Alves et al., 1998, Lu et al., 2001), and project to different areas in the forebrain (Lowry et al., 2008). Therefore, it is not surprising that different subsets of DRN neurons respond to diverse stimuli and affect 5-HT neurotransmission in a region-specific manner (Kirby et al., 1995, Adell et al., 1997, Kirby et al., 1997, Kirby and Lucki, 1998). Estrogen effects on serotonergic function also reflect this anatomical organization. Chronic estrogen treatment has been shown to increase mRNA coding for tryptophan hydroxylase-2 (TpH2), the rate-limiting enzyme for 5-HT biosynthesis, in the DRN (Sanchez et al., 2005, Hiroi et al., 2006b), and this increase is region specific (Hiroi et al., 2006b). Estrogen also decreases 5-HT1B mRNA in select subregions of DRN (Hiroi and Neumaier, 2009), but does not alter 5-HT1A mRNA (Birzniece et al., 2001, Hiroi and Neumaier, 2009). Together these results suggest a permissive effect of estrogen on serotonin synthesis and release. Because we also found that TpH2 expression in caudal dorsal raphe nucleus (caudal DRN) correlated inversely with anxiety-like behavior (Hiroi et al., 2006b), we hypothesize that increased TpH2 in caudal DRN is mechanistically involved in estrogen’s anxiolytic effects. In order to investigate this idea, we examined the impact of anatomically targeted, bidirectional manipulation of TpH2 expression in caudal DRN followed by testing in the open field, a strategy that we have previously found to be very sensitive to both increases and decreases in anxiety-like behavior. We used either morpholino oligonucleotides to knockdown TpH2 expression or an HSV viral vector expressing epitope tagged TpH2 in caudal DRN and then examined the interaction of these manipulations with estrogen’s anxiolytic effects. The results suggest that TpH2 in caudal DRN neurons may have important interactions with estrogen-induced anxiolysis, as TpH2 knockdown interferes with, and TpH2 overexpression mimics the effects of estrogen on these behaviors.
2. EXPERIMENTAL PROCEDURES
2.1 Subjects
A total of 114 female Sprague-Dawley rats between the ages of 60 and 90 days were used for behavioral experiments and 55 male Sprague-Dawley rats around the age of 60 to 77 days were used for biochemical verification of morpholino antisense oligonucleotide knockdown and virus-mediated gene transfer. Rats were group-housed with three animals per cage on a 12-hour light/dark cycle (lights on at 6 a.m.), and all behavioral measures were performed during the light period. Animals were acclimated to the colony rooms for about one week before any behavioral manipulation.
2.2 TpH2 viral vector construction
To prepare a full-length Tph2 cDNA containing hemagglutinin (HA) epitope at the N-terminus, PCR with the proofreading Platinum Pfx polymerase (Invitrogen, Carlsbad CA) was employed. Template consisted of 25ng of whole brain total RNA reverse transcribed using oligo-dT primers and the Omniscript RT as recommended by the manufacturer (Qiagen, Valencia CA). The upstream primer (5′-AACCGGTGCCGCCACCATGTACCCATACGATGTTCCAGATTACGCTTACCCATACGA TGTTCCAGATTACGCTCAGCCCGCAATGATGATGTTTTC-3′) contained an Age I site and consensus Kozak sequence preceding the start codon, followed by two tandem copies of the HA epitope (followed by bases 121 to 143 of the full length Tph2 cDNA (Walther and Bader, 2003). The downstream primer (5′-GCCTAGGCAAGTTTCTGTTTATTGAG-3′) contained bases 2578 to 2557 from the full length Tph2 cDNA (Walther and Bader, 2003) flanked by a single Avr II site. The 2.5kB PCR product was gel purified, manually isolated under long wave UV light, digested with β-agarase (New England Biolabs, Ipswich MA), followed by ethanol precipitation and resuspension in 10mM Tris pH8. This TpH2 cDNA was cloned into pPCR-Blunt II-TOPO using the Zero Blunt TOPO PCR cloning kit as described by the manufacturer (Invitrogen, Carlsbad CA). Positive clones were confirmed by PCR screening and the inserts were sequenced in their entirety to confirm the correct sequence. A clone with no coding errors except a single silent C → T mutation in the codon for histadine 179 codon (Walther and Bader, 2003), was selected for construction of the TpH2 herpes simplex virus type 1 (HSV) amplicon.
We have previously produced HSV amplicons allowing expression of both a gene of interest and enhanced GFP (eGFP) from separate transcriptional cassettes; this viral vector system is notable for neuron-specific transgene expression that lasts for approximately seven days; eGFP expression and epitope tagged gene transcription colocalize because they are expressed from the same amplicon (Clark et al., 2002, Barot et al., 2007). In order to produce an HSV amplicon expressing HA-tagged TpH2 and the eGFP marker; a HA-TpH2 cDNA insert was prepared by cutting the Zero Blunt TOPO vector containing it with AgeI, blunting with Klenow, recutting with AvrII, followed by gel purification of the resulting 2.5kB fragment. This fragment was ligated into the dual expressing HSV amplicon immediately downstream of the HSV promoter using a blunted BglII and unmodified AvrII site. Restriction mapping and sequencing across ligation junctions confirmed the desired pHSV-HA-TpH2 amplicon. This amplicon was packaged into HSV particles as previously described (Clark et al., 2002); typical vector titers were 1 × 108 infective units/mL and were confirmed to be replication deficient with a plaque assay.
2.3 Phosphorodiamidate Morpholino Oligonucleotides (PMOs)
Phosphorodiamidate Morpholino Oligonucleotides modified with the fluorescent dye, lissamine (Gene-Tools, LLC) were the antisense oligonucleotides used to knockdown TpH2 in the caudal DRN. PMOs consist of a modified backbone with the riboside moiety of each subunit converted to a morpholine moiety and uses phosphodiamidate inter-subunit linkages that are non-ionic and nuclease resistant. The combination of strong binding characteristics and resistance to nucleases allows for more efficient antisense knockdowns with lower concentrations as compared to other antisense types, and thereby reduces nonspecific toxicity (Morcos, 2001, Heasman, 2002). The carboxyl-terminal lissamine enables fluorescent detection and also introduces polarity to otherwise non-ionic PMOs.
We designed a 25-mer TpH2 morpholino antisense oligonucleotide (αTpH2) to span the border of exon 6 and intron 6 (5′-CCCGAGAGGTCTTACCTTTCAGAAA), blocking the snRP site and causing deletion of exon 6 from the mature mRNA, resulting in a frame shift and a truncated form of the TpH2 protein. PMOs were injected “naked”, without any delivery agents that may be associated with nonspecific toxicity (Summerton, 1999, Morcos, 2001). PMOs (150μM) were resuspended in saline with warming at 60°C for 5 min. Saline vehicle and scrambled PMOs (SCR) were injected as controls. SCR was a standard control sequence corresponding to a splicing variant in the human erythrocyte b-globulin mRNA (5′-CCTCTTACCTCAGTTACAATTTATA-lissamine); it had no matches in the rat genome when the sequence was searched in the Basic Local Alignment Search Tool, BLAST (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi).
2.4 OVX and hormone capsule implantation
The protocol for ovariectomy and hormone replacement was described in detail previously (Hiroi et al., 2006b, Hiroi and Neumaier, 2006). In brief, a small incision was made on both sides of the abdomen below the ribs. The fallopian tube was clamped and the ovaries were removed. The muscle was sutured, and the skin was closed with wound clips. An incision was made in the nape of the neck and a tunnel was made under the skin for hormone capsule implantation. The skin was closed with wound clips. Hormone capsules were made a day prior to the surgery day using Silastic tubing (Dow Corning, Midland MI) 3mm long, filled with crystallized 17β-estradiol (Steraloid, Wilton NH) or left empty for placebo. The ends were plugged with small pieces of wooden dowel and sealed with silicone glue.
The length of the Silastic tube determined the rate of hormone release (Smith, 1978). According to previous studies using a similar method of hormone administration (Bridges, 1984, Hope et al., 1992, Sell et al., 2000, Zhou et al., 2002), capsules of these lengths produce serum estradiol levels of 30–40 pg/ml, approximating the moderate circulating levels of estradiol found during the normal estrous cycle of the adult female rat (Smith et al., 1975, Freeman, 1994). Using this same procedure, we confirmed that plasma estradiol levels in estradiol-treated OVX rats was significantly higher than OVX rats treated with blank capsules (32.0 ± 2.8 pg/ml vs. 7.4 ± 0.9 pg/ml, respectively; t(12) = 8.34, p < 0.0001) (Hiroi and Neumaier, 2006).
2.5 Vaginal smears and analysis
Vaginal smears were obtained to confirm OVX and hormone replacement following procedures described previously (Hiroi et al., 2006b, Hiroi and Neumaier, 2006). A wet cotton swab was inserted approximately 5 mm into the vagina and smeared on glass slides. Cells were fixed for 5 min in 100% methanol, dried for 10 min, and stained for 15 min in 5% Giemsa stain (Sigma, St. Louis, MO). Stained cells were analyzed via light microscopy. The procedure was done in the testing room every morning for 4 days before the behavioral test day and at least 1 hour before behavioral testing to allow any stress from this procedure to subside. The OVX rats had a predominance of leukocytes with nucleated and cornified cells similar to that found in a rat in diestrus stage of the estrous cycle, indicating a successful removal of the ovaries and hormones; in general, OVX/E rats had a predominance of nucleated and cornified cells with a small number of leukocytes, similar to that found during proestrus or estrus.
2.6 Stereotaxic injections
Sterotaxic surgeries were performed using a similar protocol to that described previously (Clark et al., 2002). Ten days following OVX and hormone replacement, rats were anesthetized with inhalation of 3% isoflurane using a veterinary gas anesthesia machine and placed in a Stoelting stereotaxic device. Scalp fur was shaved and the scalp was cleaned with betadine. Scalp incisions were made, periosteum was scraped, and a hole was drilled at the site of injection. The needle was slowly lowered −7.1 mm deep for females and −7.3 mm deep for male rats at a 25 degree angle toward midline to avoid the third ventricle. Following coordinates were used for caudal DRN: −7.6 mm from bregma and −2.5 mm from midline for females and −8.1 mm from bregma and −2.9 mm for male rats; for midrostral DRN: −7.1 mm from bregma and −2.9 mm for male rats.
In the PMO knockdown study, female rats were divided into two groups: OVX (n=33) or OVX/E (n=33). These groups were further divided into saline (OVX-Sal: n=11; OVX/E-Sal: n=11), scrambled (OVX-SCR: n=12; OVX/E-SCR: n=11) or TpH2 antisense (OVX-αTpH2: n=10; OVX/E-αTpH2: n=11) PMO injected groups. Animals were microinjected with either 0.2 μl of 150 μM αTpH2 or SCR PMO, or saline directly into the caudal DRN. In the overexpression study, female rats were divided into OVX (n=24) or OVX/E (n=24) and further divided into GFP (OVX-GFP: n=12; OVX/E-GFP: n=12) or TpH2-GFP (OVX-TpH2: n=12; OVX/E-TpH2: n=12) injected groups. 2 μl of TpH2-GFP or GFP viral vector was microinjected into the caudal DRN.
Infusions were made using a microprocessor-controlled pump (World Precision Instruments, Sarasota FL) connected to a Hamilton syringe (#30 needle) at a rate of 0.1 μl/min for all injections. The needle was left in place for 10 min after the infusion before withdrawal and skin was closed with sterile 3-0 monofilament nylon sutures (Ethicon, Irvine CA). Rats were monitored until awake and allowed to recover for 4 days before behavioral procedures, after which they were sacrificed for histological analysis. For behavioral procedures, injection placement was confirmed on 40 μm tissue sections postfixed with 4% paraformaldehyde. Injection accuracy was assessed blindly at the termination of the experiment; animals were excluded from analysis if >50% of lissamine or GFP-labeled neurons were outside of the margins of caudal DRN as indicated in the Paxinos Atlas (Paxinos and Watson, 1986), if there was no lissamine or GFP was apparent or if there was obvious damage to the tissue at the injection site. Using these criteria, 31 rats were excluded from 114 total rats, with final sample size as follows: In the PMO study, OVX-Sal: n=8, OVX/E-Sal: n=7, OVX-SCR: n=10, OVX/E-SCR: n=9, OVX-αTpH2: n=10, OVX/E-αTpH2: n=11. In the overexpression study, OVX-GFP: n=5, OVX/E-GFP: n=7, OVX-TpH2: n=6, OVX/E-TpH2: n=10.
2.7 Verification of PMO knockdown and HSV overexpression
For the PMO knockdown study, male rats were infused with 0.2 μl of saline (N=10), SCR (N=17) or αTpH2 PMO (N=19; 30 pmol) directly into the midrostral DRN in order to target the largest group of serotonergic cells to verify knockdown. TpH2 knockdown was investigated by immunohistochemistry for qualitative measure and western blot assay for quantitative measure of reduced protein levels. For the HSV overexpression study, male rats were infused with 1 μl of GFP-only (N=4) or TpH-GFP HSV (N=5) into the caudal DRN. TpH mRNA levels were quantified by in-situ hybridization histochemistry (ISHH) as protein samples for western blot were not available.
2.8 Open Field Test
Behavior in the open field was tested 4 days following stereotaxic injections using a start box procedure described previously (Hoplight et al., 2005, Hiroi et al., 2006b, Hiroi and Neumaier, 2006). Under low-illumination red light, animals movements were recorded with an overhead video camera 2m above a 100 cm square black Plexiglas enclosure with 30 cm tall walls. After a pre-test acclimation of at least 30 min in the testing rooms, animals were placed in a closed 9 × 12 cm start box located on one of the walls of the open field for 2 min. Then, the sliding door to the arena was opened remotely and latency to exit the start box was recorded. Behavioral data in the open field were collected for 10 minutes (SMART video tracking software, San Diego Instruments, San Diego CA). In the open field test, time spent in the center of the arena is considered a reliable index of anxiety, responds to anxiolytic agents (Ramos et al., 1997), and is sensitive to stress-induced anxiety states (Pare, 1994, Izumi et al., 1997, Durand et al., 1999). Thus, we quantified percent time spent in the center and corners of the open field test arena for anxiety-like behavior and total distance traveled for overall locomotor activity.
2.9 Immunohistochemistry
Male rats injected with 0.2 μl SCR (N=8) or αTpH2 PMO (N=9) in the midrostral DRN were sacrificed 4 days later then perfused with 4% paraformaldehyde in phosphate-buffer saline with pH 7.4 (PBS) and brains were extracted and postfixed in 4% paraformaldehyde for 4 hours. Brain sections (40μm) were cut on a vibratome in PBS. Sections were permeabilized in PBS-0.5% Triton X-100 (PBS-TX) for 30 min at room temperature, blocked with 0.3% gelatin in PBS-TX for 1 hour at room temperature, and incubated in 1:1000 sheep polyclonal anti-TpH (Chemicon, Temecula CA) at 4°C overnight. Sections were then rinsed with PBS, incubated with secondary antibodies, donkey anti-sheep Alexa-488 or donkey anti-sheep Alexa-568 conjugate (Invitrogen, Carlsbad CA), and rinsed again in PBS.
2.10 Western Blot
Male rats injected with 0.2 μl saline (N=10), SCR (N=9) or αTpH2 PMO (N=10) in the midrostral DRN in the caudal DRN were briefly narcotized with CO2, decapitated, and brains were extracted onto dry ice. Punches of 1mm thick sections of midrostral and caudal DRN tissues were taken with an 18G blunt needle, briefly sonicated and centrifuged at 12,000×g for 15min. The supernatant was assayed for protein concentration by the BCA Protein Assay (Pierce Biochemical, Rockford IL). 15 μg of protein was separated on a polyduramide protein electrophoresis gel at 30mA for 2 hours and transferred onto a nitrocellulose membrane at 350mA for 90 min at 4 C. Blots were blocked with 5% non-fat milk in Tris buffer saline containing 0.1% Tween-20 (TBS-T) for 1 hour, incubated with 1:1000 sheep anti-TpH primary antibody (Chemicon) diluted in TBS-T containing 5% BSA overnight at 4°C. After TBS-T rinse, membrane was incubated in 1:5000 anti-sheep-HRP conjugated secondary antibody in blocking buffer for 1 hour at room temperature and rinsed again with TBS-T. Blots were developed with SuperSignal Wests Pico Chemiluminescent substrate (Pierce Biochemical) for about 1 min and exposed to film. Films were digitized and band intensities were quantified using Image J software (National Institutes of Health).
2.11 ISHH
TpH2 riboprobes were used for in situ hybridization histochemistry as previously described (Clark et al., 2006) using 10 μm tissue sections collected from midbrain. Autoradiography for the 33P-labeled riboprobe was visualized using phosphorscanning (Cyclone, Packard Instruments, Meridien, CT) and two sections (80 μm apart) from midrostral or caudal DRN (−7.8 and −8.3 relative to bregma, respectively) were analyzed blind to group identity using MCID Image Analysis software (InterFocus Imaging Ltd, Cambridge, England) as described previously (Clark et al., 2006).
2.12 Statistical Analysis
Western band intensities were statistically analyzed using the Kruskal-Wallis test with p<0.05 considered significant. ISHH signals were analyzed using Student’s t-test for each region. All other statistical comparisons were made by using two-way ANOVA with 2 × 2 analysis consisting of hormone (OVX vs OVX/E) vs PMO (SCR vs αTpH2) for the PMO portion of the study and hormone vs overexpression groups (GFP-only vs TpH2-GFP virus) for the overexpression study, followed by LSD post-hoc test, with P < 0.05 considered significant.
3. RESULTS
3.1 TpH2 antisense PMO infusion decreased TpH protein levels in a discrete subregion of DRN without causing toxicity
PMOs were efficiently taken up by cells without transfection agent as indicated by intense cytoplasmic fluorescence (Figure 1). There was no histological indication of cytotoxicity and no caspase-3 immunoreactivity was detectable in any of the groups studied (data not shown), suggesting that there was no overt toxicity, including apoptosis, associated with the PMO injections. Scrambled control PMO had no apparent effect on TpH protein levels, as demonstrated by colocalization of PMO label with intense TpH immunoreactivity (Figure 2A–C, G). Western blot also showed no significant difference between SCR, saline, or unoperated treatment groups (Figure 3). However, antisense (αTpH2) PMO markedly reduced TpH immunoreactivity in cells labeled with PMO (Figure 2D–F, H) and western blot analysis indicated decreased TpH protein in the midrostral DRN (injection site) compared to each control group (p=0.036, Figure 3A), suggesting knockdown of TpH2 protein. The αTpH2 group showed over 60% knockdown of TpH2 immunoreactivity from tissue punches, but the immunohistochemistry suggests that the extent of knockdown in neurons showing antisense PMO labeling was nearly complete. In contrast, there were no significant differences in tryptophan hydroxylase immunolabeling of neurons in the caudal DRN (about 1 mm caudal from the infused site, Figure 3B) between these groups, indicating that region showing knockdown of TpH2 protein was discrete and restricted to the midrostral DRN in these animals.
Figure 1.
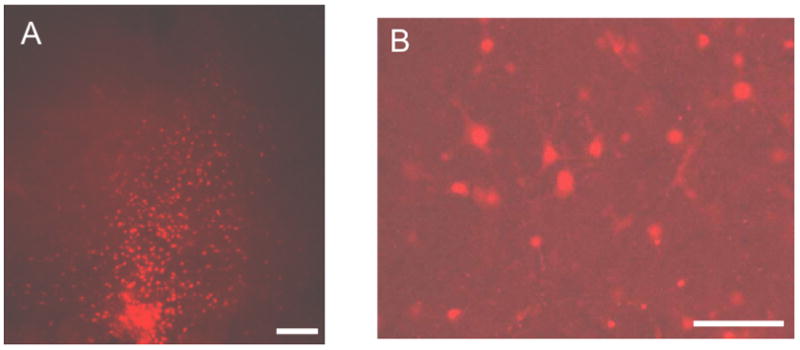
PMOs were successfully taken up by the cells in the DRN. A representative fluorescent image of PMO injection in the DRN at 20X (A) and 40X (B) magnification. Scale bar, 500μm (A), 50μm (B).
Figure 2.
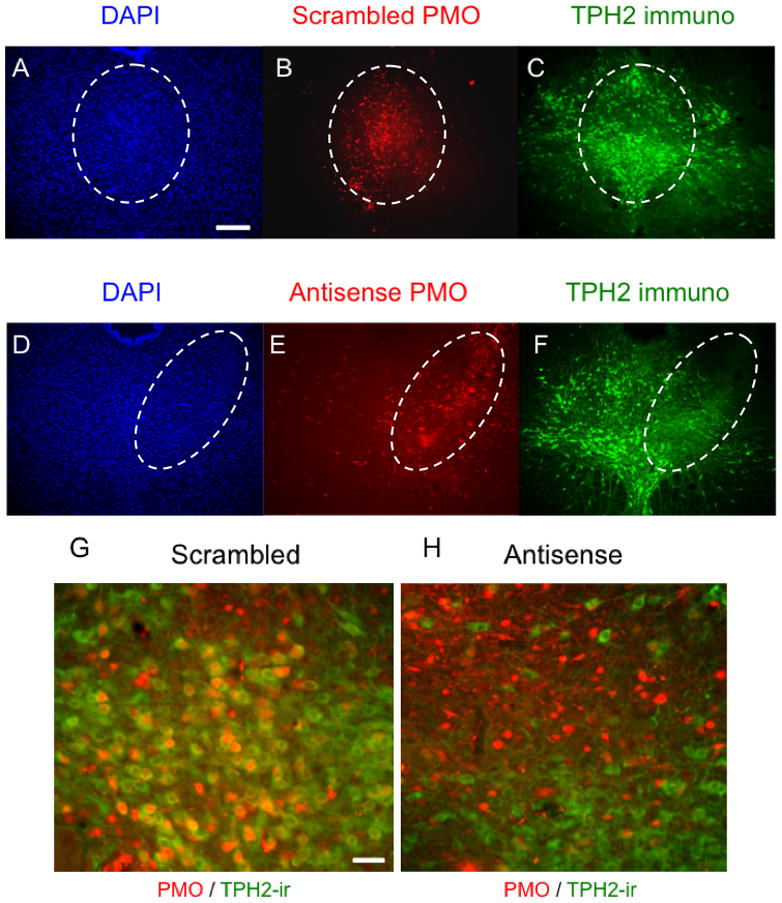
TpH immunoreactivity is reduced by PMO infusions in the midrostral DRN. Injections of scrambled PMO in the midrostral DRN (B) did not change DAPI signals (A) or TpH immunoreactivity (C). On the other hand, injections of αTpH2 PMO (E) markedly reduced TpH immunoreactivity (F) without affecting DAPI signals (D). G and H show magnified view (40X) of the scrambled and αTpH2 PMO injection site, respectively. Dashed ovals encircle the region with lissamine-PMO injection. Scale bar, 500μm (A–F), 20μm (G, H).
Figure 3.
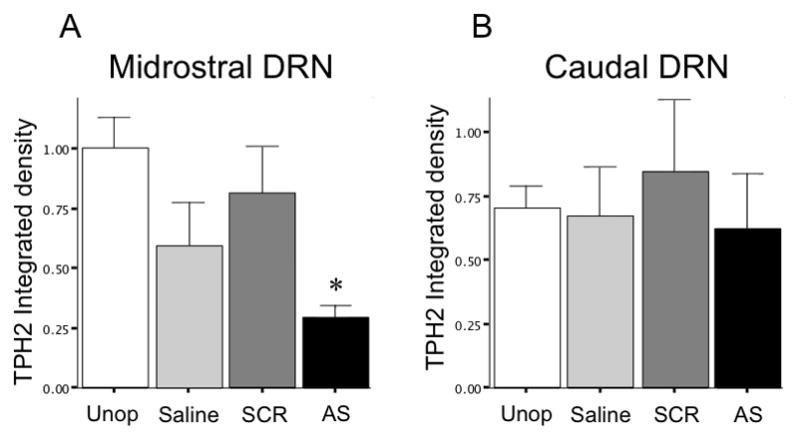
TpH protein expression is reduced by PMO infusions in the midrostral DRN. Injections of αTpH2 PMO in the midrostral significantly reduced TpH immunoblot integrated density compared to the unoperated (Unop), saline or scrambled PMO injected groups (A). However, it had no effect on TpH integrated density in the neighboring caudal DRN (B), which was not targeted by these injections. TpH integrated density shown by mean ± SEM. * p=0.039.
3.2 TpH2 knockdown in the caudal DRN reversed the anxiolytic effects of estrogen
For clarity, we have combined the saline and SCR PMO infused groups in this study, as there was no difference found in the biochemical (see previous segments of results section) or behavioral parameters (see Table 1). These combined control groups will be referred to as OVX-Ctrl and OVX/E-Ctrl.
Table 1.
There was no significant difference between Saline (Sal) and scrambled oligonucleotide (SCR) groups in anxiety-like behavior.
| OVX-Sal | OVX-SCR | OVX/E-Sal | OVX/E-SCR | |
|---|---|---|---|---|
| Center | 4.3 ± 0.64 | 5.2 ± 0.68 | 5.2 ± 0.82 | 6.2 ± 0.80 |
| Corner | 44.2 ± 1.66 | 42.3 ± 1.48 | 43.5 ± 1.47 | 41.4 ± 1.62 |
| Total distance | 1853 ± 104 | 1721 ± 104 | 1590 ± 57 | 1793 ± 63 |
Center = Percent time in center of open field; Corner = Percent time in corner of open field; Total distance = Total distance traveled. Values are expressed as mean ± SEM. Student’s t-test between Sal vs SCR for each treatment group revealed p > 0.05
In the open field test, we found overall effects of hormone (F=8.910; p=0.004) as well as PMO (F=5.208; p=0.027) on the time spent in the center of the open field (ANOVA: F=5.194; p=0.003). Post-hoc analysis revealed that OVX/E-Ctrl spent significantly more time in the center of the open field compared to all other groups. Specifically, OVX/E-Ctrl spent more time in the center of the open field compared to OVX-Ctrl (p =0.004 OVX vs OVX/E, Figure 4A), suggesting that estrogen treatment decreased anxiety in OVX rats. However, OVX/E-αTpH2 animals spent less time in the center compared to OVX/E-Ctrl (p=0.038, OVX/E-Ctrl vs OVX/E-αTpH2), suggesting that TpH2 knockdown in the caudal DRN reversed the anxiolytic effect of estrogen. OVX/E-αTpH2 center time was not statistically different from either OVX groups. There were no significant differences between OVX-Ctrl and OVX-αTpH2 groups, indicating that TpH2 knockdown in the caudal DRN had no effect in the absence of estrogen. A compatible trend was also observed in the time spent in the corner of the open field, although it did not reach statistical significance (Figure 4B). There were no significant differences in the total distance traveled in the open field.
Figure 4.
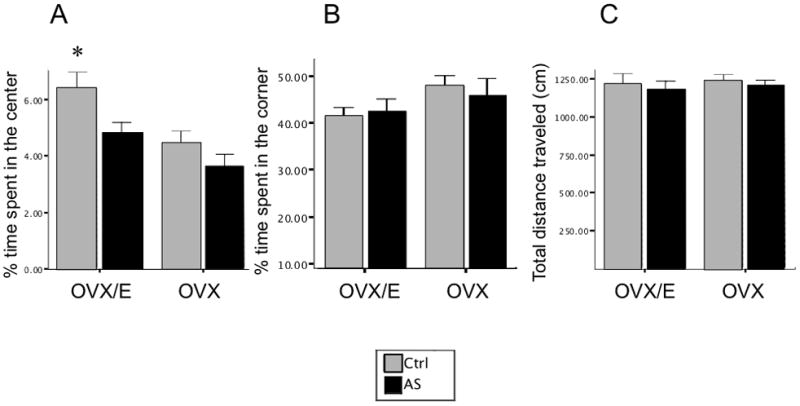
TpH2 knockdown in the caudal DRN reversed the anxiolytic effects of estrogen in the open field test. Estrogen treatment in OVX rats significantly increased time spent in the center of the open field compared to all other groups (A). Percent time spent in the center (A), corner (B), and total distance traveled (C) are shown by mean ± SEM. * p=0.004.
3.3 TpH2-HSV selectively increased TpH2 mRNA in the caudal DRN
Visualization of GFP-labeled cell bodies revealed that HSV injections were restricted to caudal DRN; no fluorescent signal was observed in cell bodies of mid- or rostral DRN while caudal DRN showed clear fluorescent signals (Figure 5A, E). Furthermore, TpH2-GFP HSV injection aimed at caudal DRN increased TpH2 mRNA density compared to GFP-only HSV, as measured by ISHH (t(7)=3.295; p=0.013; Figure 5B). However, there was no difference in TpH2 mRNA density in the neighboring midrostral-DRN (Figure 5F), indicating that increased expression of TpH mRNA was restricted to the injection site, caudal DRN.
Figure 5.
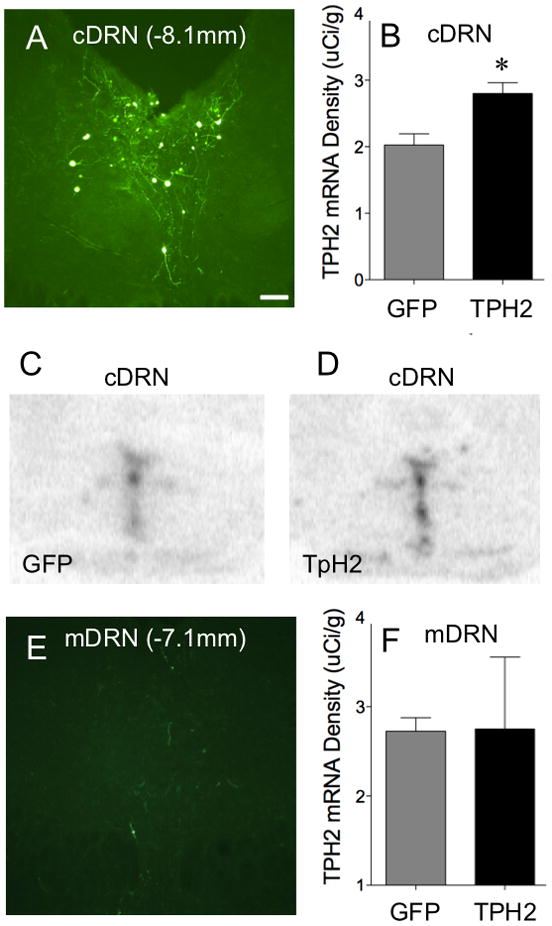
TpH-GFP HSV injection targeting the caudal DRN selectively increased TpH mRNA expression in the caudal DRN. A and E show representative fluorescent image of TpH2-GFP viral vector targeting the caudal DRN at 40X magnification (Scale bar, 20μm). TpH2 viral vector infusions are restricted in the caudal DRN (A), as GFP-labeled cells were rare in the neighboring midrostral DRN (E). Representative phosphorimages of TpH2 in situ hybridization (ISHH) signal in the caudal DRN are compared between treatment groups with GFP-only injections (C) versus TpH-GFP injections (D). Analysis of the ISHH signals shows that TpH-GFP injection increased density of TpH mRNA in the caudal DRN (B), but not in the neighboring area, mid-DRN (F), compared to GFP-only HSV injection. TpH mRNA density expressed as mean ± SEM. *p=0.013.
3.4 TpH2 overexpression mimicked the anxiolytic effects of estrogen
There was an overall effect of hormone treatment (F=4.604; p=0.043) and interaction between hormone and virus (F=8.628; p=0.007) on the time spent in the center of the open field (ANOVA: F=3.745; p=0.025; Figure 6A). There was also an overall effect of hormone treatment (F=5.273; p=0.031) and interaction between hormone and virus (F=6.314; p=0.019) on the time spent in the corner of the open field (ANOVA: F=3.471; p=0.033; Figure 6B). Post hoc analysis revealed a significant increase in time spent in the center (p=0.003) and decreased time spent in the corner (p=0.005) of the open field in OVX/E-GFP compared to OVX-GFP, yet again indicating an anxiolytic effect of estrogen. Increased expression of TpH2 in OVX animals had a striking effect on anxiety, with these animals spending significantly more time in the center of the open field compared to the OVX-GFP (p=0.025, Figure 6A) and a trend towards spending less time in the corner (Figure 6B). We found no significant difference in center time between the OVX-TpH2 and either OVX/E groups (Figure 6A), suggesting that TpH2 overexpression decreased anxiety to the same degree as estrogen treatment, mimicking its anxiolytic effects. However, increased TpH2 expression in the OVX/E group did not reduce anxiety relative to the OVX/E-GFP (p=0.041, Figure 6B); in fact, increased TpH2 expression in the OVX/E animals seemed to increase anxiety, with a trend for reduced center time and significantly increased corner time. There was no significant difference in overall locomotion between any of the groups (Figures 6C).
Figure 6.
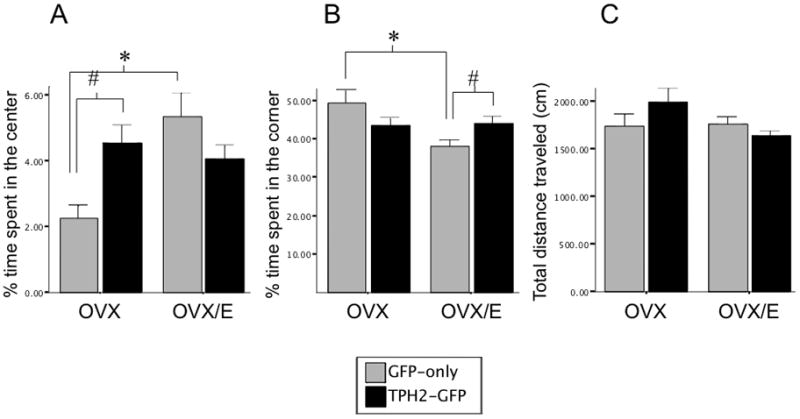
TpH2 overexpression mimicked the anxiolytic effects of estrogen in the OVX rats. A) Infusion of TpH2-GFP viral vector in the caudal DRN of OVX rats increased the time spent in the center of the open field (#p=0.025) to a comparable level as the anxiolytic effects of estrogen treatment in the OVX rats injected with GFP-only viral vector (*p=0.003). B) Estrogen treatment similarly decreased time spent in the corner of the open field (*p=0.005) in GFP-only groups; however, TpH2 overexpression in the OVX/E rats was anxiogenic, as shown by increased time spent in the corner of the open field (#p=0.041). C) There were no significant differences between any of the treatment groups in overall locomotion. Percent time spent in the center (A), corner (B) and total distance traveled in cm (C) are shown by mean ± SEM.
4. DISCUSSION
We previously reported that estrogen increased TpH2 expression selectively in the caudal DRN and that TpH2 expression in caudal DRN was inversely associated with anxiety (Hiroi et al., 2006b). In the present study, we investigated the hypothesis that increased TpH2 in caudal DRN is involved in modulating estrogen’s anxiolytic effects. To this end, we explored the roles of in-vivo TpH2 knockdown and overexpression selectively in the caudal DRN on the anxiolytic effects of estrogen. Our results suggest that estrogen-induced increases in TpH2 mRNA in the caudal DRN are important to its anxiolytic actions, as these manipulations modulated estrogen’s anxiolytic effects.
We used PMOs, steric-block antisense oligonucleotides to selectively knockdown TpH2 mRNA in caudal DRN; this antisense oligonucleotide strategy for in-vivo knockdown did not require a transfection reagent in this application (Fujikawa et al., 2005). It has several advantages over traditional antisense knockdown strategies, including nuclease resistance, high affinity binding, and a coupled fluorescent dye, lissamine, that may enhance cellular uptake by adding polarity to otherwise non-ionic PMOs and allows convenient microscopic localization in tissue (Heasman, 2002). These factors reduce nonspecific toxicity encountered with transfection reagents or high concentrations of less stable antisense oligonucleotide reagents (Summerton, 1999, Morcos, 2001). Knockdown appeared to be very efficient, with over 60% reduction in tryptophan hydroxylase protein as measured by western blot from punches and virtually complete knockdown in neurons exhibiting lissamine fluorescence. The PMO approach is anatomically more precise than chemical inhibitors of tryptophan hydroxylase enzyme activity such as p-chlorophenylalanine because the PMOs are less likely to diffuse away and can be directly detected by fluorescence microscopy. We were able to limit TpH2 knockdown to a select DRN subregion, as shown by decreased tryptophan hydroxylase immunoreactivity restricted to the infused subregion and not in the neighboring areas. Anatomical precision is an advantage because different subregions of DRN provide serotonergic innervation to different forebrain regions that mediate distinct elements of emotional behavior, such as anxiety. Indeed, TpH2 expression is regulated in a region-specific manner by estrogen, and different subregions mediate different effects on emotional behavior (Lowry, 2002, Hiroi et al., 2006a, Clark et al., 2007). Furthermore, this strategy does not lesion the targeted or surrounding non-serotonergic cells as is the case for traditional lesion strategies.
We found that reduction of TpH2 expression selectively in the caudal DRN reversed the anxiolytic effects of estrogen, as measured by decreased time spent in the center of the open field. Ovariectomy increased anxiety, and TpH2 knockdown in these animals did not increase anxiety further. Thus, these results support the hypothesis that induction of TpH2 in the caudal DRN is a key mechanism of how estrogen reduces anxiety. Alternatively, it is also possible that TpH2 knockdown increased the baseline anxiety level, although there was only a slight, non-significant decrease in the time spent in center of the open field with TpH2 knockdown in the OVX group. In any case, this study does not rule out anxiolytic effects of estrogen that are mediated by other brain regions.
In fact, estrogen’s anxiolytic effects are likely mediated by other regions as well, as the two major types of estrogen receptors (ERα and ERβ) have broad distribution throughout the brain. In DRN, ERα is expressed in nonserotonergic neurons, mainly in rostral DRN in rats (Alves et al., 1998) while ERα is not found in macaque DRN (Bethea et al., 2002, VanderHorst et al., 2004, Vanderhorst et al., 2009); whereas ERβ is expressed in serotonergic neurons of rodents (Lu et al., 1999, Lu et al., 2001, Mitra et al., 2003) and macaque DRN (Gundlah et al., 2000, Gundlah et al., 2001, Bethea et al., 2002), and is clearly expressed in mid and caudal rat DRN (Shughrue et al., 1997, Lu et al., 2001). This unique localization of the estrogen receptors may provide a direct mechanism for estrogen-induced increase of TpH2 mRNA in caudal DRN, as ERβ is found on serotonergic neurons in the DRN.
A recent report showing that the ERβ agonist, DPN, is anxiolytic and increases TpH2 mRNA in the caudal DRN (Donner and Handa, 2009) corroborates the idea that estrogen-induced induction of TpH2 may be mediated via ERβ. However, the authors of this study also showed that while a systemic treatment with DPN decreased anxiety-like behaviors, direct application of DPN in the DRN did not alter anxiety-like behaviors in either the open field test or elevated plus maze, albeit the increased expression of TpH2 in the caudal DRN. There are procedural differences between this previous study and our present report that may shed light into understanding these seemingly disparate results. First, the duration of the hormone treatment at the time of behavioral testing in the previous study was notably shorter than the present study (5 days vs 14 days, respectively). As dose and duration of hormone treatment has been shown to be a critical factor in determining the direction of change in serotonin gene expression (Sumner et al., 1999, Smith et al., 2004), as well as anxiety and other behaviors (Diaz-Veliz et al., 1991, Diaz-Veliz et al., 1999, Estrada-Camarena et al., 2003, Wide et al., 2004), longer hormone treatment may be necessary to effectively induce anxiolytic effects. Moreover, the pellet implantation of the hormones in the previous study was reported to spread over all of rostrocaudal and medial-lateral subregions of the DRN, whereas our present study selectively targeted caudal DRN for TpH2 manipulation. As subdivisions of the DRN may have opposing (Hiroi et al., 2006b) and undoubtedly other complex effects on anxiety, diffusion of estrogens into all DRN subdivisions most likely have other unknown effects on the DRN as a whole that counteract the anxiolytic effects of elevated TpH2 mRNA specifically in caudal DRN. Nevertheless, these results gives credence to the argument that although serotonin activity in the caudal DRN plays a critical role in modulating estrogen’s anxiolytic effects, estrogen also has complex interactions with multiple brain systems to regulate specific aspects of anxiety behavior. Although the present study focused on the bidirectional effects of TpH2 manipulation as a strategy rather than multiple behavioral measures to investigate the role of caudal DRN in modulating the anxiolytic effects of estrogen, careful examination of the effects of estrogen in multiple brain regions and its impact on anxiety behavior is warranted.
Conversely, we found that TpH2 overexpression in the caudal DRN in OVX animals was anxiolytic, similar to estrogen’s anxiolytic effects. This result supports the hypothesis that estrogen-induced increase of TpH2 mRNA in the caudal DRN is important for its anxiolytic effects. However, increasing TpH2 mRNA expression in the caudal DRN in OVX/E group had the opposite effect; in this case TpH2 overexpression may be mildly anxiogenic when estrogen is present. There are at least two possible interpretations of these results. First, there may be a bimodal effect of TpH2 expression in caudal DRN with a U-shaped relationship between TpH2 and anxiety (Figure 7). In other words, increasing TpH2 activity from low to moderate levels is associated with reduced anxiety; but higher levels of TpH2 activity could cause increased anxiety. A bimodal relationship may partly explain the seemingly ambiguous literature surrounding 5-HT and anxiety. At first glance, it may seem inconsistent to find that increased 5-HT tone in the caudal DRN can be anxiolytic, since in male rats, increased 5-HT activity in the caudal DRN is usually thought to be anxiogenic (Lowry et al., 2000, Hammack et al., 2002). However, males appear to have higher serotonin synthetic capacity than females (Nishizawa et al., 1997). Perhaps, OVX/E rats with TpH2 overexpression (who presumably have higher TpH2 levels than OVX rats with TpH2 overexpression) have similar 5-HT levels seen in the male rats, thereby resulting in anxiogenic behavior.
Figure 7.
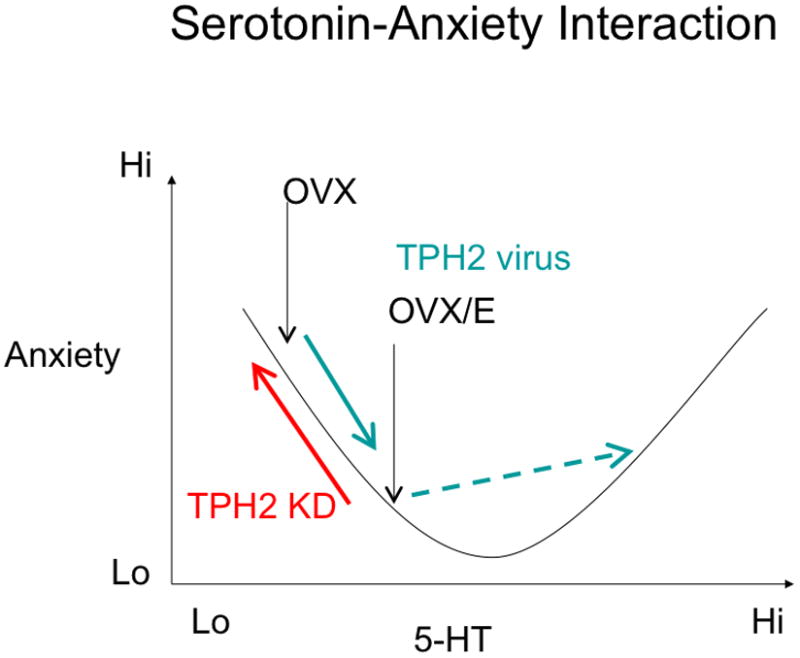
Theoretical serotonin-anxiety interaction. Our data suggest a U-shaped relationship between serotonin and anxiety. Since TpH2 knockdown was anxiogenic in OVX/E rats (red arrow) while TpH2 overexpression was anxiolytic in OVX rats (green arrow), it suggests an inverse linear relationship between anxiety and 5-HT synthetic capacity as shown by the left half of the U-shaped curve. However, we also found that TpH2 overexpression was actually anxiogenic in OVX rats treated with chronic estrogen (dotted green arrow).
These data suggest that increased 5-HT tone is anxiolytic at a lower range of serotonin activity, but is anxiogenic at higher level of serotonergic activity. Because chronic estrogen increases TPH2 mRNA in the caudal DRN, it is tempting to hypothesize that this supraphysiological overexpression TPH2 in OVX/E rats may mimic 5-HT level of male rats whose 5-HT levels are normally higher than females.
Second, these bimodal effects indicate that there may be a differential effect of the level of synthetic capacity of serotonin in low versus high estrogenic states. Although the mechanism underlying TpH2 overexpression is distinct from selective serotonin reuptake inhibitors (SSRIs), a class of antidepressant commonly used to treat anxiety, interesting comparisons are possible. SSRIs successfully treat some symptoms of depression associated with menopause, yet there is evidence that an incomplete response can be augmented by estrogen treatment (Zanardi et al., 2007). It is possible that anxiolytic effects of SSRIs are mediated via increased serotonin activity, and that estrogen may facilitate the antianxiety effects of SSRIs by increasing TpH2 expression and restoring serotonin synthetic capacity.
TpH2 overexpression in a low estrogenic state may restore higher levels of serotonin synthesis in caudal DRN neurons and their projections to forebrain; however, further increases in TpH2 in the estrogen treated group, who presumably already have higher TpH2 expression in caudal DRN (Hiroi et al., 2006b) has no further benefit for anxiety, or could even be anxiogenic. This excess serotonin activity may lead to a host of other symptoms similar to that seen in “serotonin syndrome” in overdoses of drugs that increases serotonin activity. It would be interesting to examine other physiological characteristics associated with serotonin syndrome, such as increased heart rate, overresponsive reflexes, pupillary dilation and myoclonus in these animals for comparison in future studies. It is also possible that there is a ceiling effect of TpH2 expression, and that estrogen may also have other effects on serotonin neurons, such as modulating enzyme activity or autoreceptor expression (Rubinow et al., 1998, Birzniece et al., 2001, Robichaud and Debonnel, 2005, Hiroi and Neumaier, 2009), as well as on other nonserotonergic systems, as estrogen receptors have a diverse distribution in the brain.
5. CONCLUSIONS
In summary, the present work highlights the role of the DRN serotonergic system in the regulation of anxiety by estrogen. Estrogen differentially activates TpH2 in select subregions of the DRN (Hiroi et al., 2006b), which in turn supplies serotonin innervation to specific brain areas that regulate different aspects of anxiety behavior. The results from our study do not imply that the anxiolytic effect of estrogen is mediated solely through the caudal DRN 5-HT system, thus further studies manipulating estrogen activity directly in the caudal DRN and other brain regions may elucidate the roles of these regions in regulating anxiety. However, we demonstrate a direct role for TpH2 in the caudal DRN in modulating the anxiolytic effects of estrogen indicating that caudal DRN has a more critical role in estrogen mediated anxiolysis than previously acknowledged.
Research Highlights.
We examine the role of TpH2 in caudal DRN in estrogen-induced anxiolysis
Estrogen-induced increase in DRN TpH2 mRNA is associated with decreased anxiety
TpH2 overexpression mimic, while knockdown, reduce anxiolytic effects of estrogen
Acknowledgments
This work was supported by MH63303 and MH75279. The authors would like to thank the following for their technical assistance and expert advice: Dr. Michele Kelly and William Brennan for vector packaging, Dr. Susan Ferguson with western blot, and Dr. Catherine Hagan with RT-PCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41:633–639. [PubMed] [Google Scholar]
- Best NR, Rees MP, Barlow DH, Cowen PJ. Effect of estradiol implant on noradrenergic function and mood in menopausal subjects. Psychoneuroendocrinology. 1992;17:87–93. doi: 10.1016/0306-4530(92)90079-m. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birzniece V, Johansson IM, Wang MD, Seckl JR, Backstrom T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology. 2001;74:135–142. doi: 10.1159/000054679. [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neuroscience. 2007;146:1888–1905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Veliz G, Benavides MS, Butron S, Dussaubat N, Mora S. Behavioral effects of dopamine agonists and antagonists: influence of estrous cycle, ovariectomy, and estrogen replacement in rats. Pharmacol Biochem Behav. 1999;62:21–29. doi: 10.1016/s0091-3057(98)00097-5. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiol Behav. 1991;50:61–65. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormede P, Chaouloff F. Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis axis in SHR and WKY rats. Neuropharmacology. 1999;38:893–907. doi: 10.1016/s0028-3908(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The Neuroendocrine Control of the Ovarian Cycle of the Rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press, Ltd; New York: 1994. pp. 613–648. [Google Scholar]
- Fujikawa T, Tamura K, Kawase T, Mori Y, Sakai RR, Sakuma K, Yamaguch A, Ogata M, Soya H, Nakashima K. Prolactin receptor knockdown in the rat paraventricular nucleus by a morpholino-antisense oligonucleotide causes hypocalcemia and stress gastric erosion. Endocrinology. 2005;146:3471–3480. doi: 10.1210/en.2004-1528. [DOI] [PubMed] [Google Scholar]
- Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347:930–933. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERbeta) mRNA and protein in serotonin neurons of macaques. Brain Res Mol Brain Res. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat dorsal raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006a;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006b;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158:456–464. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope WG, Bruns ME, Thomas ML. Regulation of duodenal insulin-like growth factor I and active calcium transport by ovariectomy in female rats. Proc Soc Exp Biol Med. 1992;200:528–535. doi: 10.3181/00379727-200-43466. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav. 2005;84:707–714. doi: 10.1016/j.physbeh.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Izumi J, Washizuka M, Hayashi-Kuwabara Y, Yoshinaga K, Tanaka Y, Ikeda Y, Kiuchi Y, Oguchi K. Evidence for a depressive-like state induced by repeated saline injections in Fischer 344 rats. Pharmacol Biochem Behav. 1997;57:883–888. doi: 10.1016/s0091-3057(96)00455-8. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- Lowry C, Evans A, Gasser P, Hale M, Staub D, Shekhar A. Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti J, et al., editors. Serotonin and sleep: Molecular, Functional and Clinical Aspects. 001. Birkhauser Verlag AG; Basel, Switzerland: 2008. pp. 25–68. [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Ozawa H, Nishi M, Ito T, Kawata M. Serotonergic neurones in the dorsal raphe nucleus that project into the medial preoptic area contain oestrogen receptor beta. J Neuroendocrinol. 2001;13:839–845. doi: 10.1046/j.1365-2826.2001.00695.x. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Shlaes TA, Gundlah C, Dziennis SE, Lyle RE, Bethea CL. Ovarian steroid action on tryptophan hydroxylase protein and serotonin compared to localization of ovarian steroid receptors in midbrain of guinea pigs. Endocrine. 1999;11:257–267. doi: 10.1385/ENDO:11:3:257. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morcos PA. Achieving efficient delivery of morpholino oligos in cultured cells. Genesis. 2001;30:94–102. doi: 10.1002/gene.1039. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press, INC; San Diego: 1986. [Google Scholar]
- Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621–672. vi–vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- Ramos A, Kangerski AL, Basso PF, Da Silva Santos JE, Assreuy J, Vendruscolo LF, Takahashi RN. Evaluation of Lewis and SHR rat strains as a genetic model for the study of anxiety and pain. Behav Brain Res. 2002;129:113–123. doi: 10.1016/s0166-4328(01)00337-0. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry. 2002;63(Suppl 7):45–48. [PubMed] [Google Scholar]
- Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol. 2005;17:179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sichel DA, Cohen LS, Robertson LM, Ruttenberg A, Rosenbaum JF. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry. 1995;38:814–818. doi: 10.1016/0006-3223(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone administration: peripheral and intracranial implants. In: Meyer RD, editor. Methods of psychobiology. Academy; New York: 1978. pp. 259–279. [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73:119–128. doi: 10.1016/s0169-328x(99)00243-0. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Terasawa E, Ralston HJ., 3rd Projections from estrogen receptor-alpha immunoreactive neurons in the periaqueductal gray to the lateral medulla oblongata in the rhesus monkey. Neuroscience. 2004;125:243–253. doi: 10.1016/j.neuroscience.2003.12.044. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ., 3rd Estrogen receptor-alpha immunoreactive neurons in the brainstem and spinal cord of the female rhesus monkey: species-specific characteristics. Neuroscience. 2009;158:798–810. doi: 10.1016/j.neuroscience.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Rossini D, Magri L, Malaguti A, Colombo C, Smeraldi E. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. Eur Neuropsychopharmacol. 2007;17:400–405. doi: 10.1016/j.euroneuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]


