Abstract
The L-type Ca2+ channel (LTCC) is the major mediator of Ca2+ influx in cardiomyocytes, leading to both mechanical contraction and activation of signaling cascades. Among these Ca2+-activated cascades is calcineurin, a protein phosphatase that promotes hypertrophic growth of the heart. Coimmunoprecipitations from heart extracts and pulldowns using heterologously expressed proteins provided evidence for direct binding of calcineurin at both the N and C termini of α11.2. At the C terminus, calcineurin bound specifically at amino acids 1943 to 1971, adjacent to a well-characterized protein kinase (PK)A/PKC/PKG phospho-acceptor site Ser1928. In vitro assays demonstrated that calcineurin can dephosphorylate α11.2. Channel function was increased in voltage-clamp recordings of ICa,L from cultured cardiomyocytes expressing constitutively active calcineurin, consistent with previous observations in cardiac hypertrophy in vivo. Conversely, acute suppression of calcineurin pharmacologically or with specific peptides decreased ICa,L. These data reveal direct physical interaction between the LTCC and calcineurin in heart. Furthermore, they demonstrate that calcineurin induces robust increases in ICa,L and highlight calcineurin as a key modulator of pathological electrical remodeling in cardiac hypertrophy.
Keywords: action potential remodeling, Ca2+ channels, calcineurin, electrophysiology, hypertrophy
Perturbation of intracellular Ca2+ signaling and prolongation of the myocyte action potential accompany many forms of heart disease and contribute to the pathogenesis of cardiac hypertrophy and failure.1 The voltage-gated L-type Ca2+ channel (LTCC) (CaV1.2) is the major pathway of Ca2+ influx into cardiomyocytes and an important determinant of action potential morphology. Indeed, the LTCC transduces membrane potential changes into local intracellular calcium transients that initiate a host of physiological events. We and others have reported an increase in LTCC activity in various models of cardiac hypertrophy.2,3 However, molecular mechanisms responsible for the observed increase in channel function in hypertrophy are unknown, although evidence suggests that it involves an increase in channel availability and open probability.4
L-type channel function is governed by an array of reversible phosphorylation and dephosphorylation events. Among the best characterized reactions is a robust, transient activation of L-type Ca2+ current (ICa,L) by protein kinase (PK)A.5,6 More complex regulation by PKC has been reported, with both channel activation and inhibition observed.5,6 Other work has demonstrated ICa,L inhibition by PKG.7 However, despite a growing understanding of the role of phosphorylation in the regulation of ICa,L, less is known regarding the role of protein phosphatases in these reactions. Some reports have described PP2A8,9 and PP1-mediated10 channel regulation.
Calcineurin (PP2B) is a cytoplasmic Ca2+/calmodulin-dependent protein phosphatase that contributes to hypertrophic signaling in many models of cardiac hypertrophy including that induced by elevated afterload.1 Calcineurin links cytoplasmic Ca2+ to transcriptional regulation of multiple genes involved in the hypertrophic and failure programs, and expression of constitutively active calcineurin in transgenic mice is sufficient to drive robust hypertrophy and failure.11 Calcineurin is activated in human heart failure and participates in hypertrophic signal transduction in models of cardiac1 and skeletal muscle,12 biomechanical stress, and fiber type–specific gene expression in skeletal muscle.12
We reported previously that the activity and abundance of the LTCC are altered in pressure-overload cardiac hypertrophy through a calcineurin-dependent pathway.2 Based on these findings, we hypothesized that calcineurin is a component of the LTCC macromolecular complex in heart and participates in stress-dependent regulation of channel function.
Materials and Methods
Coimmunoprecipitation
Rat left ventricle was homogenized on ice using a Dounce homogenizer in buffer containing (mmol/L) 10 Tris (pH 7.4), 10 EDTA, 10 EGTA, 1% sucrose, supplemented with Mini-Complete protease inhibitor (Roche). Following gentle centrifugation (5000 rpm, 3 minutes) the supernatant was subjected to high-speed ultracentrifugation (200 000g, 15 minutes) to collect the cytosolic fraction. The pellet was rehomogenized in membrane extraction buffer containing (mmol/L) 10 Tris-HCL (pH 7.4), 20 EDTA, 10 EGTA, 150 NaCl, 1% Triton X-100, and protease inhibitors and centrifuged as before to collect the membrane fraction (supernatant).
Statistical Methods
Averaged data are reported as means±SEM. Statistical significance was analyzed using a Student’s unpaired t test or 1-way ANOVA followed by Bonferroni’s method for post hoc pair-wise multiple comparisons.
All protocols were approved by the Institution’s Animal Care and Use Committee. An expanded Materials and Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Results
Calcineurin Colocalizes With the LTCC
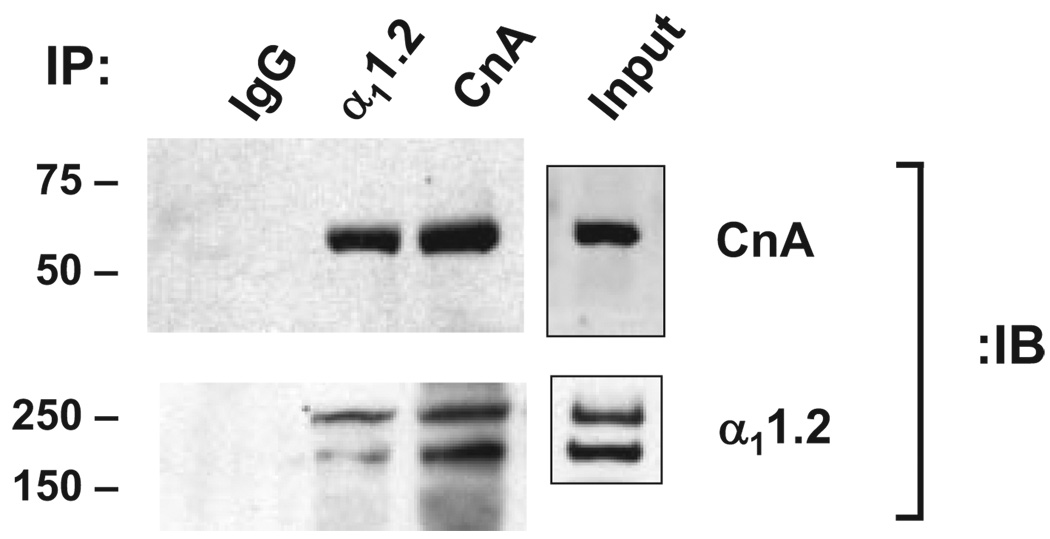
Calcineurin is a heterodimer of catalytic A (CnA) and Ca2+-binding regulatory B subunits.13 To determine whether calcineurin is a component of the LTCC macromolecular complex, we immunoprecipitated α11.2 from rat ventricular lysates and probed for CnA. A strong band migrating at ≈61 kDa was readily identified (Figure 1). As a positive control, we immunoblotted for α11.2, which revealed bands at 240 and 190 kDa, consistent with the full-length and processed isoforms prevalent in heart. Conversely, immunoprecipitation with anti-CnA antibody pulled down α11.2. Immunocytochemical studies revealed colocalization of CnA and α11.2 at murine myocyte Z bands (data not shown).
Figure 1.
Calcineurin associates with L-type Ca2+ channel. Immunoprecipitations (IP) of α11.2 and CnA from rat ventricular lysate and immunoblots (IB) for both. IgG used as a negative control.
Direct Interaction Between α11.2 and Calcineurin
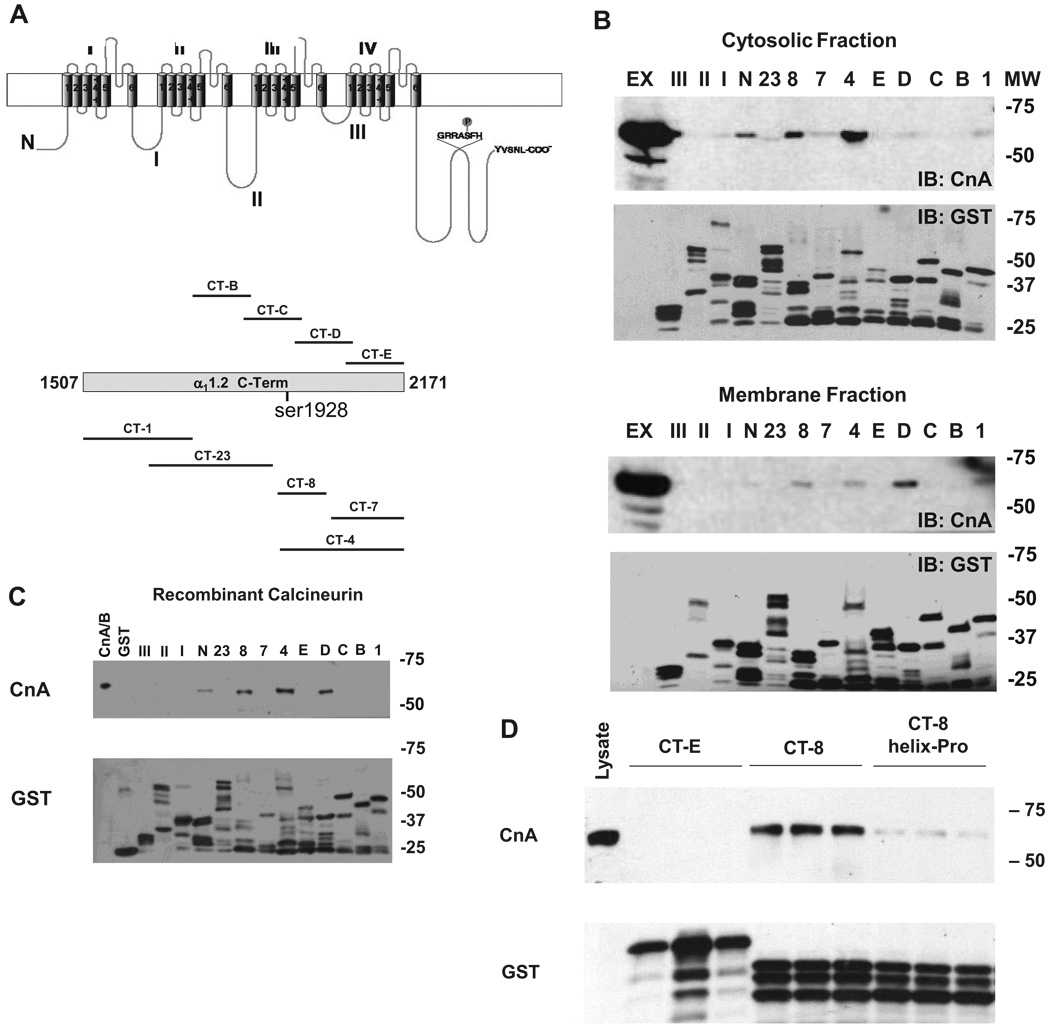
To map the CnA binding site, we engineered overlapping glutathione S-transferase (GST) fusion proteins spanning the intracellular domains of α11.2. The C terminus (CT) was further subdivided into numbered CT fragments (Figure 2A; Online Table I). Equal quantities of α11.2 GST fusion proteins were used to pull down CnA from ventricular (Figure 2B) or brain tissue (data not shown) lysates. A consistent subset of GST fusion proteins (CT-4, CT-8, CT-D) corresponding to overlapping regions of α11.2 pulled down CnA from both cytosolic and membrane fractions (Figure 2B). Each of these peptides overlaps considerably, suggesting the presence of a specific calcineurin-binding site within the α11.2 sequence. The N terminus of α11.2 also pulled down calcineurin, suggesting that calcineurin and α11.2 may have multiple sites of interaction.
Figure 2.
Calcineurin binds directly to CT and N terminus of α11.2. A, Schematic diagram of α11.2. The CT was further subdivided into overlapping regions. GRRASFH contains Ser1928. B, Representative GST-pulldown experiments from rat ventricular lysate probed for CnA reveal binding to CT-4 and CT-8, and variable binding to CT-D and N terminus. Ex indicates tissue-extract input. C, GST pulldown with recombinant calcineurin A and B subunits (CnA/B). CnA/B indicates input. D, Pulldown experiments with mutant CT-8 containing a single proline insertion in the α-helix.
To test whether the interaction between calcineurin and α11.2 is direct or mediated by intervening proteins, GST-α11.2 fusion proteins were incubated with purified recombinant calcineurin (CnA/B, expressed as a complex of catalytic A and regulatory B subunits). Immunoblots probed for CnA revealed an identical pattern of GST fusion protein interactions as was seen with native lysates from heart or brain (CT-4, CT-8, CT-D, and N terminus) (Figure 2C). These data, then, demonstrate a specific and direct interaction between CnA and α11.2 without intermediary scaffolding elements.
The minimal interaction domain (amino acids 1909 to 2029) contains several potential PxIxIT motifs, a calcineurin-binding domain found in many calcineurin-interacting partners (Online Figure I, A). We tested whether one such potential motif was responsible for calcineurin binding to α11.2 by mutating residues PSI to AAA (amino acids 2018 to 2020) on CT-4. In GST-pulldown experiments, CnA bound wild-type CT-4 and CT-4 PSI-AAA with similar efficiency, suggesting this region is not the binding site. As a second test, we performed competition-binding assays using overlapping peptides spanning amino acids 1976 to 2029 of CT-8, the region containing the potential PxIxIT motifs (Online Figure I, C). Despite the presence of several-fold molar excess of peptide relative to both CnA and CT-8, CnA bound avidly to CT-8 (Online Figure I, B).
Robson–Garnier algorithms14 applied to the primary sequence of CT-8 suggested the presence of 2 distinct domains: an N-terminal domain (amino acids 1909 to 1968) containing an α-helix, and CT domain (amino acids 1969 to 2029), containing the potential PxIxIT motifs and manifesting no definitive structure (Online Figure I, D). Because our findings (above) suggested that the unstructured region is not responsible for CnA binding, we analyzed the N-terminal α-helix. To do this, we engineered serial truncations of CT-8 (Online Figure I, D). GST pulldowns revealed binding of CnA to amino acids 1909 to 1971 and 1943 to 2029 within CT-8, implicating the α-helix as necessary to bind CnA (Online Figure I, E). Furthermore, disruption of helical structure by introduction of a proline rendered CT-8 incapable of binding CnA (Figure 2D). Together, these data establish amino acids 1943 to 1971 as the minimal region required for interaction between CnA and the CT of α11.2.
Finally, as heterologous expression of mammalian proteins in bacteria often leads to early termination events during synthesis (Figure 2B), we expressed α11.2 CT constructs fused to myc-epitope tags in mammalian cells. Immunoprecipitation using anti-myc antibodies in HEK-293 cells that coexpressed calcineurin fused to GFP and probed for GFP-CnA revealed a specific band at ≈75 kDa (the expected mass of CnA plus GFP) only for peptides containing amino acids 1909 to 1969 (Online Figure I, F). These data, then, are consistent with our findings using proteins expressed in bacteria and point to this CT region of α11.2, as required for calcineurin binding.
Calcineurin Binds α11.2 CT With High Affinity
To obtain a semiquantitative estimate of the affinity of interaction between α11.2 and CnA, we performed GST-pulldown experiments using varying amounts of CT-8 while maintaining a constant, low concentration of recombinant calcineurin (15 nmol/L) (Online Figure II). Purified immobilized CT-8 was added in increasing concentrations, and supernatants were collected after each pulldown to assess bound (pellet) versus unbound (supernatant) calcineurin. Approximately equivalent amounts of CnA cosedimented when CT-8 was present at half the concentration of CnA (CT-8:CnA, 0.5), whereas almost all the CnA was bound when more than 3 times as much CT-8 was present (CT-8:CnA, ≥3). These data lend credence to the specificity of the pulldown assays, and they suggest that the interaction between α11.2 and CnA occurs at relatively high (approximately nanomolar) affinity.
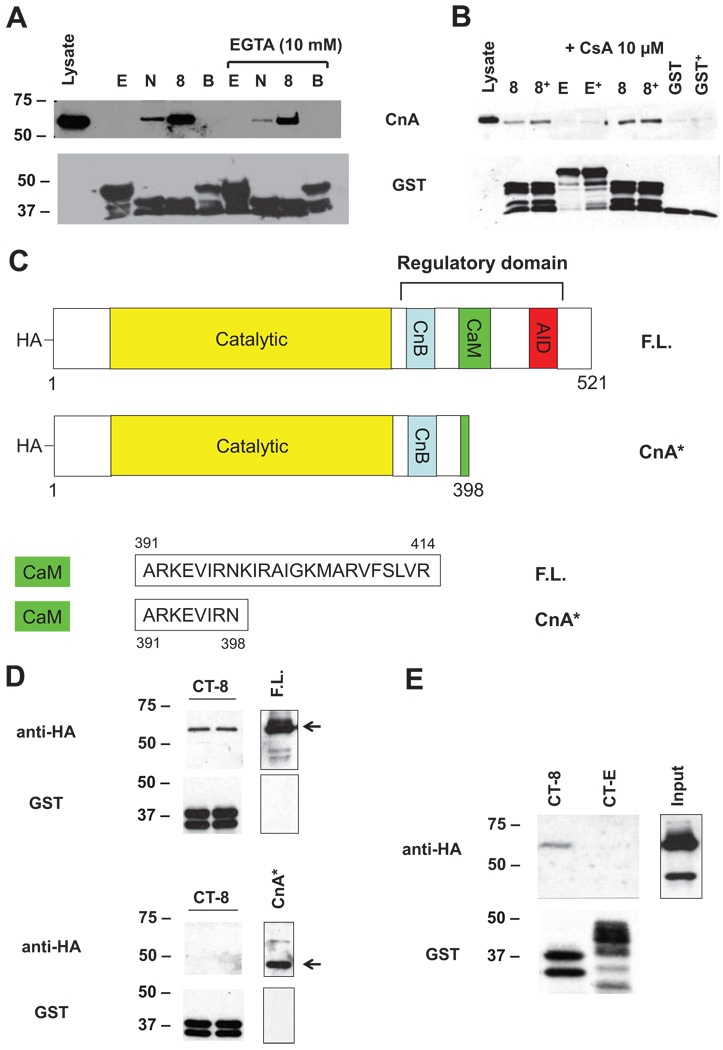
CnA Regulatory Region Is Required for α11.2 Binding
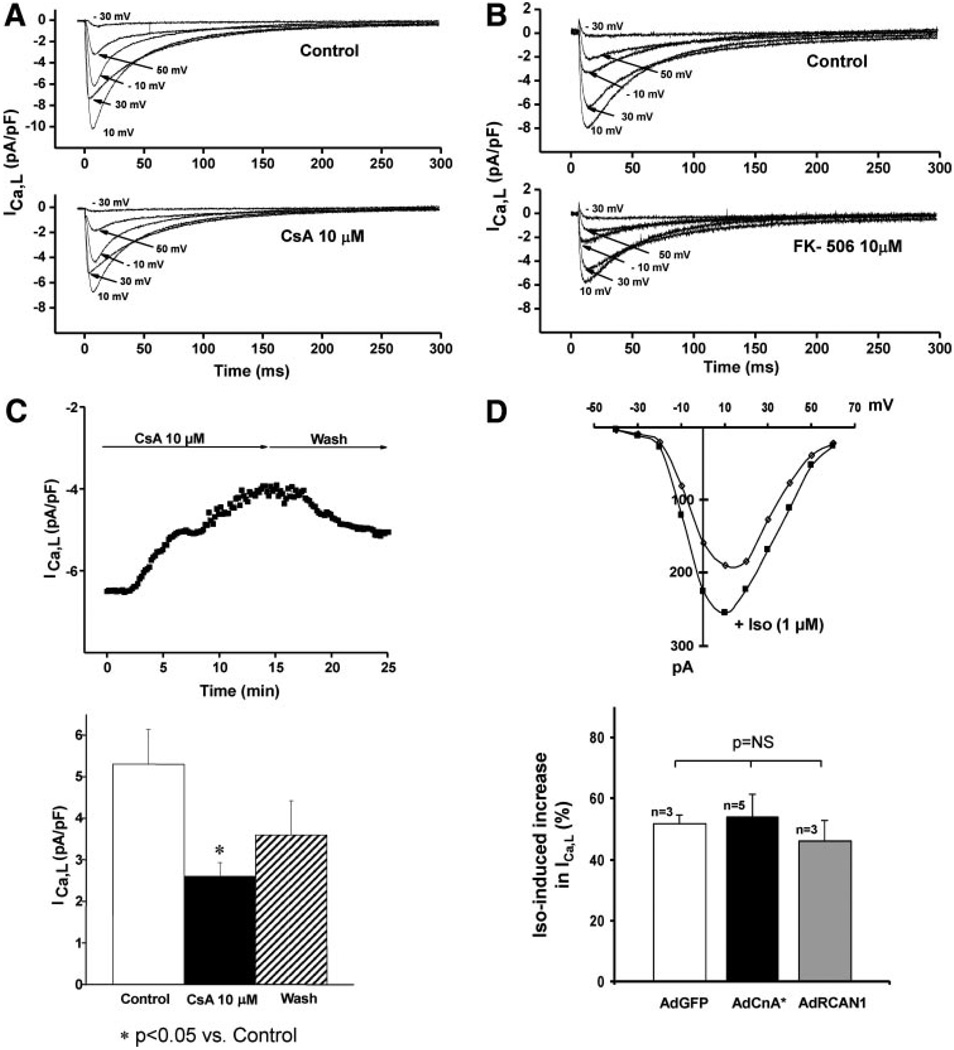
Experiments reported so far were performed in the presence of EGTA, which inactivates calcineurin by chelating Ca2+. We observed similar levels of binding in the absence of EGTA (Figure 3A), a condition where calcineurin is expected to be active. We also observed that CnA was able to bind CT-8 in the presence of cyclosporine A (CsA 10 µmol/L, Figure 3B), an inhibitor that obstructs the calcineurin catalytic domain.13 To test further the role of the catalytic domain, we expressed full-length CnA (F.L.) or a constitutively active mutant (CnA*) lacking the CT autoinhibitory domain that obstructs access to the catalytic site of the enzyme (Figure 3C). In 6 independent experiments, only F.L. (61 kDa) bound CT-8, whereas no signal was detected with lysates from cells expressing CnA* (48 kDa) (Figure 3D) or from cells coexpressing both F.L. and CnA* (Figure 3E). Taken together, these findings suggest that calcineurin A binds the channel via its CT regulatory region.
Figure 3.
α11.2 binds calcineurin regulatory region. Representative GST-pulldown experiment using ventricular lysates prepared in the presence vs absence of 10 mmol/L EGTA (A) or 10 µmol/L CsA (B) revealing equivalent interaction of endogenous CnA. C, Schematic representation of F.L. and constitutively active (CnA*) calcineurin constructs. CnA* is truncated at amino acid 398 and lacks the AID and most of the CaM domain. CnB indicates calcineurin B–binding domain; CaM, calmodulin-binding domain. GST pulldowns with lysates from HEK-293 cells expressing either F.L. or CnA* (D) or coexpressing both F.L. and CnA* (E).
Phospho-α11.2 Is a Calcineurin Substrate
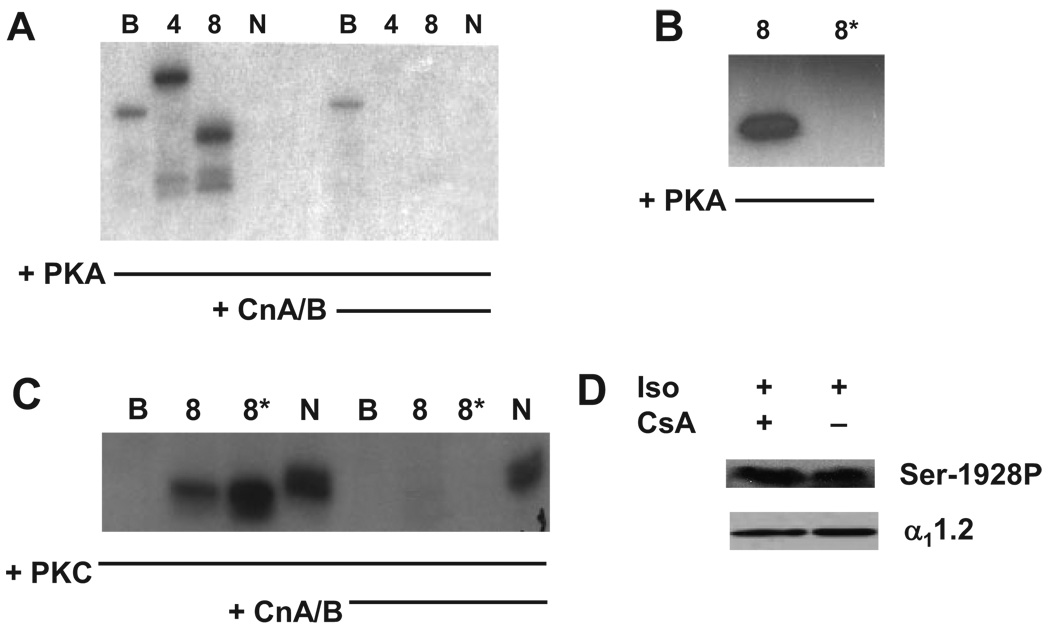
Next, we set out to determine whether calcineurin recognizes α11.2 as a substrate. GST fusion proteins were phosphorylated in vitro by recombinant PKA or PKC and then tested for dephosphorylation by calcineurin. As expected, PKA readily phosphorylated both CT-4 and CT-8 (Figure 4A), proteins that harbor Ser1928. CT-B, which is adjacent to CT-8, was phosphorylated to only a modest extent. As a further control, the N terminus of α11.2, which binds CnA and can be phosphorylated by PKC5 but does not contain consensus sequences for PKA phosphorylation, was not phosphorylated (Figure 4). PKC phosphorylated CT-8 and the N terminus but did not phosphorylate CT-B (Figure 4C).
Figure 4.
Calcineurin recognizes phospho-α11.2 as substrate. Representative autoradiogram of α11.2 GST fusion proteins phosphorylated with recombinant catalytic PKA (A) or PKC (C) and subsequently treated with recombinant calcineurin (CnA/B). B, Representative autoradiogram demonstrating that PKA does not phosphorylate mutant CT-8 containing a S1928A substitution (CT-8*). D, Representative phosphospecific α11.2 Ser1928 Western blot of NRCMs treated with Iso and/or CsA.
We then incubated the 32P-phosphorylated GST fusion proteins with purified recombinant calcineurin (CnA/B). Here, we observed marked decreases in the phosphorylation of α11.2 fragments CT-4 and CT-8 (Figure 4A and 4C), consistent with their serving as substrates for calcineurin phosphatase. Phosphate incorporation in either CT-B by PKA or N terminus by PKC was unchanged in the presence of recombinant calcineurin. To test the specificity of CT-8 phosphorylation, an alanine was substituted for serine at position 1928 (CT-8*). PKA failed to induce phosphorylation of CT-8* (Figure 4B), indicating that PKA-mediated phosphorylation is specific to Ser1928. However, PKC induced phosphorylation of CT-8*, suggesting additional unidentified phosphorylation sites within α11.2, which calcineurin recognizes as substrate (Figure 4C). Together, these data demonstrate that calcineurin is capable of recognizing α11.2 as a substrate in vitro.
To corroborate these findings in a more physiological setting, we tested whether calcineurin targets endogenous α11.2 by studying cultured neonatal rat cardiomyocytes (NRCMs) exposed to isoproterenol (Iso) in the presence of CsA (Figure 4D). As expected, Western blot using anti-Ser1928 phospho-specific antibody demonstrated a time-dependent increase in Ser1928 phosphorylation after Iso treatment (Online Figure III). Importantly, inhibition of calcineurin with CsA elicited no difference in Iso-induced Ser1928 phosphorylation (Figure 4D). Furthermore, overexpression of a constitutively active mutant of calcineurin similarly did not antagonize Iso-induced phosphorylation of Ser1928 (data not shown). Together, these data suggest that endogenous calcineurin does not specifically antagonize PKA-mediated phosphorylation of α11.2 Ser1928 and point to the presence of additional calcineurin target sites on α11.2 that remain to be resolved.
Calcineurin Activation Increases ICa,L
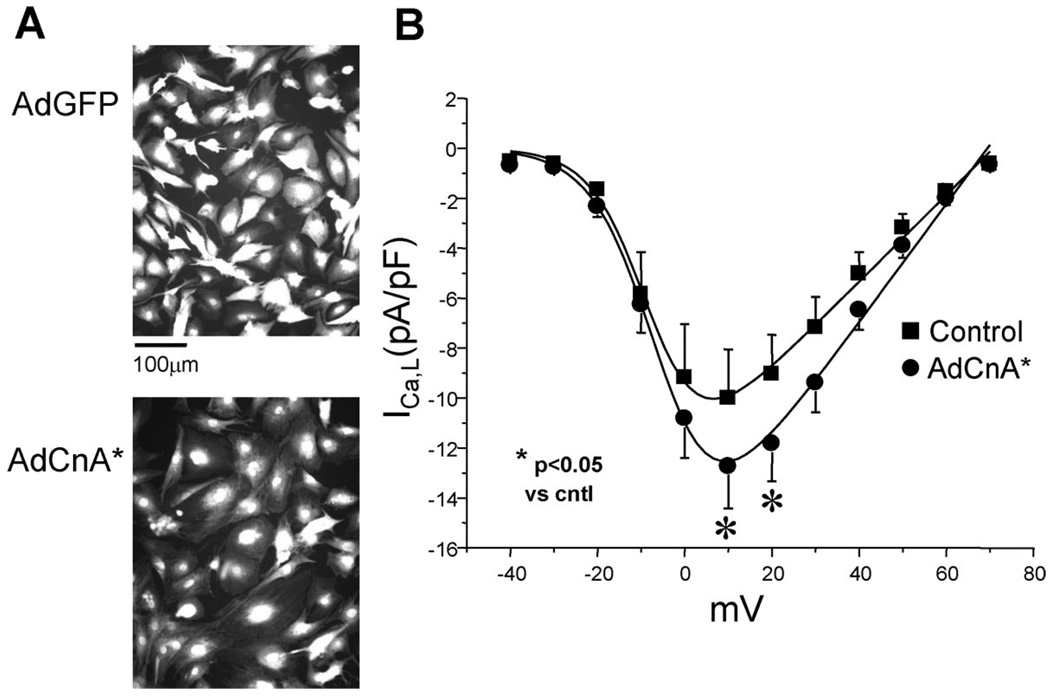
To test whether calcineurin regulates L-type channel function, we infected NRCMs with adenovirus expressing constitutively active calcineurin (AdCnA*). Overexpression of activated calcineurin provoked hypertrophic cell growth, as evidenced by an 84% (±9%, P<0.05; n=100 AdCnA*, n=100 AdGFP) increase in cell cross-sectional area (Figure 5A), increased sarcomeric organization, and upregulated ANF expression (data not shown). Recordings of ICa,L in AdCnA*-infected myocytes revealed a 26% (±4%, P<0.05; n=10 AdCnA*, n=6 AdGFP) increase in ICa,L density with no significant shift in the steady-state current–voltage (I–V) relationship (Figure 5B). Hypertrophy-associated increases in ICa,L exceeded that expected from simple increases in cell size, because current density was significantly increased.
Figure 5.
Activated calcineurin increases ICa,L. A, Neonatal rat cardiomyocytes were infected with adenovirus expressing constitutively active calcineurin (AdCnA*) or GFP (AdGFP) and visualized with fluorescent tracer (CellTracker). AdCnA* led to the expected increase in cell size and associated myocyte hypertrophy. B, Voltage-clamp recordings of ICa,L at increasing membrane potentials. AdCnA* was associated with an increase in peak ICa,L density (AdCnA* [12 pA/pF at +10 mV, n=10] [●] vs control [10 pA/pF at +10 mV, n=6] [■]; *P<0.05), with no shift in I–V relationship.
Inhibition of Calcineurin Diminishes ICa,L
Calcineurin-dependent increases in ICa,L do not exclude the possibility that changes in channel activity are secondary to the hypertrophic phenotype. To address this, we inhibited calcineurin acutely with CsA and evaluated changes in ICa,L in voltage-clamp recordings from dissociated ventricular myocytes. To minimize current rundown or dialysis of intracellular contents, we used an amphotericin B perforated patch technique. Cells were held at −80 mV, stepped transiently to −50 mV to inactivate sodium currents, and then ICa,L was measured (0.1 Hz) at test potentials between −30 and +50 mV. Under these conditions, addition of 10 µmol/L CsA significantly inhibited ICa,L at all potentials without altering the potential at which peak current was observed (Figure 6A). Similar results were observed using tacrolimus (FK-506), a structurally distinct calcineurin inhibitor (Figure 6B). Suppression of peak (+10 mV) ICa,L occurred over a time course of several minutes and was partially reversible following drug washout (Figure 6C). Together, these findings suggest that the effects of CsA or FK-506 on ICa,L are mediated by calcineurin suppression rather than by nonspecific actions of either compound.
Figure 6.
Acute suppression of calcineurin diminishes ICa,L. Representative whole-cell voltage-clamp measurements of ICa,L in dissociated ventricular myocytes before and after calcineurin suppression by 2 structurally distinct inhibitors, CsA (10 µmol/L) (A) and tacrolimus (FK-506; 10 µmol/L) (B). C, Representative recording depicting time course of CsA-induced ICa,L inhibition and partial reversibility on drug washout. Mean data from 4 experiments are shown. D, Representative I–V curve and cumulative (bar graph) data showing equivalent increases in ICa,L after exposure to Iso (1 µmol/L).
CsA inhibited ICa,L without inducing a shift in the steady-state I–V relationship (Online Figure IV, A). Moreover, the kinetics of ICa,L inactivation were not altered (Online Figure IV, B), suggesting that the effects of calcineurin do not involve antagonism of PKA-dependent phosphorylation of the channel. To test this further, we investigated the effects of Iso in the presence of calcineurin activation (Figure 6D). Iso (1 µmol/L) induced similar (P=NS) increases in ICa,L density in cells infected with AdGFP (51.9±3%, n=3), AdCnA* (54.1±7.4%, n=5), and AdRCAN1 (regulator of calcineurin protein 1) (a protein inhibitor of calcineurin15) (46.1±7%, n=3) (Figure 6D). These data, then, provide strong evidence that PKA and calcineurin activate the channel independently.
Inhibition of ICa,L by CsA Is Calcineurin-Dependent
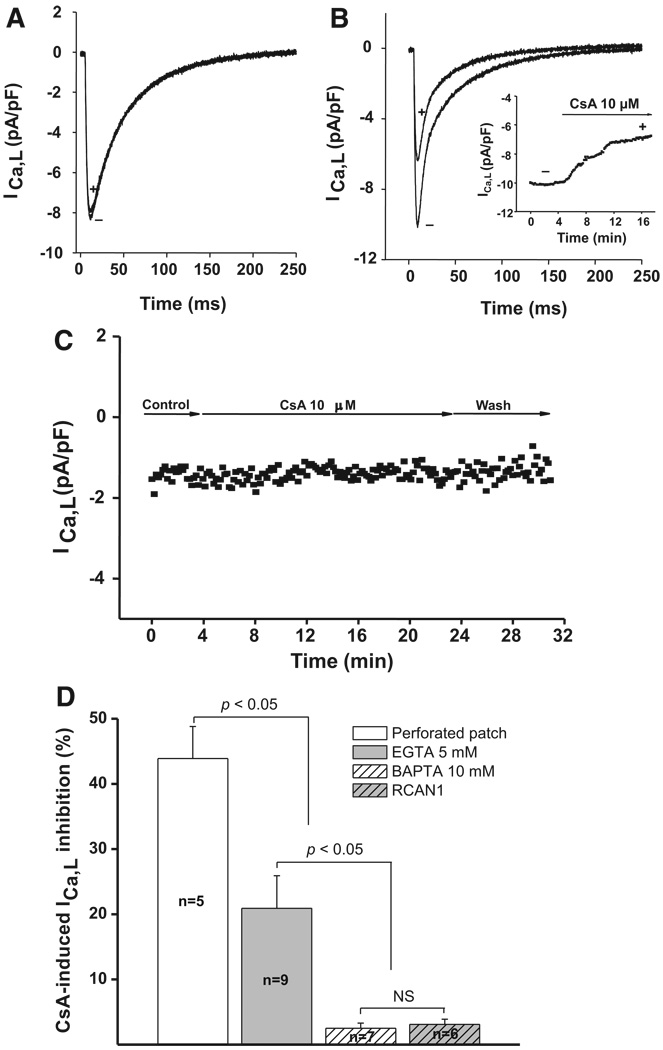
To determine whether the inhibitory effects of CsA on ICa,L are calcineurin-dependent, we studied ICa,L in the context of negligible calcineurin activity. Calcineurin is inactive in the absence of Ca2+, so we hypothesized that pharmacological suppression of calcineurin should have no effect on ICa,L in the presence of BAPTA (because calcineurin is inactivated by elimination of intracellular Ca2+). ICa,L was recorded in dissociated ventricular myocytes using whole-cell methods, where the pipette filling solution included 10 mmol/L BAPTA, a chelator that binds Ca2+ nearly 2 orders of magnitude faster than EGTA.16 Under these conditions, where intracellular Ca2+ is irreversibly chelated, addition of 10 µmol/L CsA did not diminish ICa,L (Figure 7A); ICa,L declined by only 2.5% (±0.8%, n=7, P=NS) in the presence of intracellular BAPTA as compared to 44% (±5%, n=5, P<0.05) without BAPTA (Figure 7D). To determine whether the loss of CsA-induced inhibition in the presence of BAPTA was simply an artifact of the disrupted-membrane recording method, we studied ICa,L under conditions where intracellular Ca2+ was only partially buffered (pipette filled with 5 mmol/L EGTA). Under these conditions, calcineurin could be partially activated owing to incomplete buffering of intracellular Ca2+, and CsA suppressed ICa,L to an intermediate extent (21±5%, n=9, P<0.05) (Figure 7B and 7D).
Figure 7.
CsA inhibits ICa,L in a calcineurin-dependent manner. A, Whole-cell voltage-clamp recordings of ICa,L in dissociated ventricular myocytes treated with 10 mmol/L BAPTA. In the presence of Ca2+ chelation, and consequent calcineurin inactivation, 10 µmol/L CsA had no effect on ICa,L (10 mmol/L BAPTA [+]; control [−]). B, Representative recordings of ICa,L density from dissociated ventricular myocytes treated with 5 mmol/L EGTA. With incomplete Ca2+ buffering, 10 µmol/L CsA induced a partial reduction in ICa,L (5 mmol/L EGTA [+]; control [−]). C, Representative time course of ICa,L recorded from a ventricular myocyte dissociated from cardiomyocyte-overexpressing RCAN1 transgenic mice. D, Mean data of percentage inhibition of ICa,L induced by 10 µmol/L CsA vs control from dissociated adult ventricular myocytes (perforated patch, 44±5%, n=5, P<0.05), when 5 mmol/L EGTA or 10 mmol/L BAPTA is dialyzed through the patch pipette (21±5%, n=9, P<0.05 and 2.5±0.8%, n=7, P<0.05, respectively), or recordings from ventricular myocytes isolated from RCAN1 transgenic hearts (3±0.8%, n=6, P<0.05).
As another test for nonspecific actions of CsA, we measured Ba2+ current in the setting of buffered intracellular Ca2+ (10 mmol/L BAPTA). Under these conditions, CsA had no effect on ICa,L (data not shown). Finally, we evaluated the effects of CsA on ICa,L in myocytes dissociated from transgenic (Tg) mouse hearts overexpressing RCAN1, a calcineurin-inhibitory protein.15 CsA had no effect on ICa,L (3.0±0.8% decline, n=6, P=NS) in myocytes in which calcineurin activity was suppressed by overexpression of RCAN1 (Figure 7C and 7D).
Targeted Inhibition of Calcineurin Diminishes ICa,L
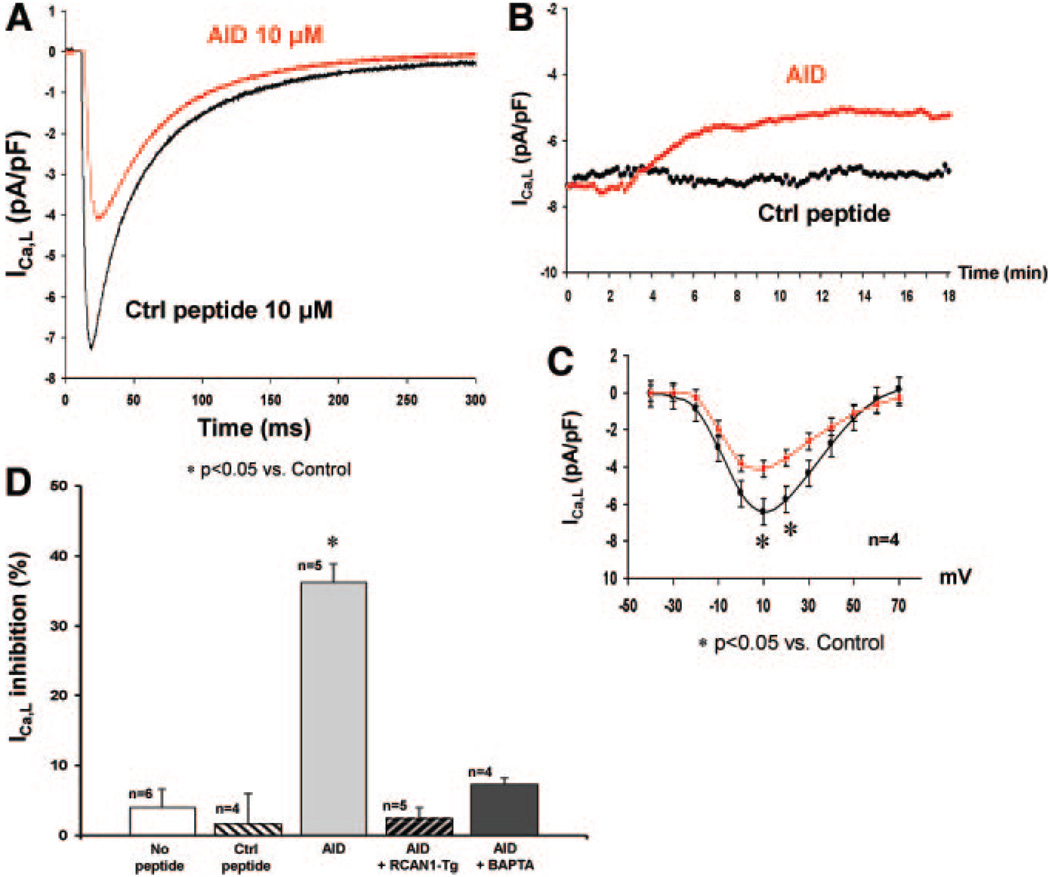
We tested the effects of nonpharmacologic suppression of calcineurin by intracellular dialysis of the calcineurin-inhibitory peptide autoinhibitory domain (AID), derived from the autoinhibitory domain of calcineurin. Calcineurin inhibition by pipette dialysis of 10 µmol/L AID induced a 36% (±3%, n=5, P<0.05) decrease in ICa,L. By contrast, no declines in ICa,L were seen with intracellular dialysis of a control peptide (peptide 2010; 1.7±4%, n=4, P=NS) or no peptide at all (6.1±2.5%, n=6, P<0.05) (Figure 8A, 8B, and 8D). Peptide 2010 was chosen as a control because it did not interfere with calcineurin-binding (Online Figure I, B) and was of a similar molecular mass and pI to AID. Consistent with our findings when calcineurin was suppressed pharmacologically, AID-induced inhibition of ICa,L to a similar extent (36% versus 44%, P=NS) and was not associated with shifts in the steady-state I–V relationship (Figure 8C). Furthermore, AID did not elicit reduction in ICa,L recorded from RCAN1 transgenic myocytes (2.6±1.6%, n=5, P=NS) or with intracellular BAPTA (7.4±0.8%, n=4, P=NS) (Figure 8D), consistent with a specific requirement for calcineurin inhibition.
Figure 8.
Targeted inhibition of calcineurin decreases ICa,L. A, Representative peak ICa,L densities (+10 mV) and time course (B) recorded from ventricular myocytes after peptide dialysis of 10 µmol/L control peptide (2010, black line) or AID (red line) peptide. C, I–V relationship demonstrating that calcineurin inhibition by AID induced a 36% decrease in peak inward ICa,L density (4.08±1 pA/pF at +10 mV, n=4; P<0.05) vs control peptide (6.42±0.32 pA/pF at +10 mV, n=4), with no shift in I–V relationship. D, Mean data demonstrating that AID induced a 36% (±3%, n=5, P<0.05) decrease in ICa,L density vs control peptide (1.7±4.3%, n=4, P<0.05) or no peptide (4.1±2.5%, n=6, P<0.05). AID had no effect on ICa,L density in RCAN1 transgenic myocytes (2.6±1.6%, n=5, P<0.05) or in the presence of BAPTA (7.4±0.8%, n=4, P<0.05). All experiments were performed on myocytes isolated from at least 3 different mice of the same strain.
Discussion
The LTCC is an element central to multiple processes in the heart, including action potential repolarization, excitation–contraction coupling, and activation of Ca2+-responsive signaling pathways. The L-type channel is also a prominent target of disease-related remodeling, being up- or downregulated in a variety of pathological states. In this study, we demonstrate that (1) calcineurin copurifies with the LTCC in the ventricle; (2) calcineurin interacts directly with N-terminal and CT intracellular domains of the pore-forming subunit α11.2; (3) calcineurin recognizes α11.2 as an enzymatic substrate in vitro; and (4) calcineurin upregulates L-type channel function, because acute inhibition of calcineurin decreases ICa,L in a calcineurin phosphatase-dependent manner. Together, these data uncover a previously uncharacterized regulatory mechanism governing cardiac LTCC function.
The LTCC Macromolecular Complex
An emerging theme in cardiovascular biology emphasizes the importance of specific localization of signaling molecules in proximity to their targets.17 The LTCC comprises a macromolecular complex of various enzymes and anchoring proteins, most of which associate with the long intracellular CT of the α11.2 subunit.6 One study in neurons implicated calcineurin, anchored to the L-type channel by the scaffolding protein AKAP79/150, in antagonizing PKA-mediated activation of ICa,L.18 Here, we demonstrate that calcineurin binds to both the N terminus and CT of α11.2 and that this interaction is direct and not mediated by an intermediary protein. We have identified residues 1943 to 1971 as the minimal region required for specific calcineurin binding. That this interaction occurs with approximately nanomolar affinity is consistent with its being physiologically relevant.
Phosphorylation-Dependent Regulation of Ca2+ Channel Function
The Ca2+ channel is subject to regulation by multiple hormones and neurotransmitters largely through activation of kinases and phosphatases.5,6 Prominent among these is a transient increase in channel activity triggered by PKA phosphorylation. Interestingly, recent evidence suggests that much of PKA-dependent activation of ICa,L in ventricular myocytes does not involve Ser1928.19,20 PKC-dependent mechanisms are similarly complex, acting on 2 threonine residues at the N terminus of α11.2, as well as at Ser1928.5 In the case of N-terminal phosphorylation, an initial transient activation of the channel is followed by sustained inhibition.21 Furthermore, a recent report mapped PKG-mediated inhibition of heterologously expressed L-type channel activity to Ser496 on the β2a subunit.7 Thus, there is considerable variance in the literature regarding the effects and mechanisms of Ca2+ channel phosphorylation.
Whereas a great deal of work has focused on channel regulation by kinases, less is known about the countervailing actions of protein phosphatases. PP2A8,9 and PP110 have been reported to dephosphorylate the channel, but the functional significance of these actions remains poorly characterized. Indeed, we have localized calcineurin binding to a region of α11.2 close to a site where PP2A binds.8 Some studies have suggested that PP2A inhibits Ca2+ channel function,22 possibly by antagonizing the phosphorylation of Ser1928. Here, we show that calcineurin elicits robust upregulation of channel activity. Although still unresolved, one possible mechanism may be interference with PP2A binding and consequent antagonism of PP2A-dependent dephosphorylation.
Calcineurin Inhibition Decreases ICa,L
We have previously shown that targeted inhibition of calcineurin, either pharmacologically with CsA2 or genetically via RCAN1 overexpression,23 attenuated action potential prolongation and eliminated the increase in heart mass associated with pressure-overload hypertrophy. Here, we extend these observations to demonstrate direct effects of calcineurin on the L-type channel. These findings, which are consistent with our overall hypothesis, cannot be explained by simple channel blockade, because 2 entirely divergent means of calcineurin inhibition (pharmacological inhibition with small molecules [CsA, FK-506] of distinct molecular structure; intracellular peptide [AID] exposure) manifested the same response. Because the effect of calcineurin inhibitors was partially reversible, current rundown cannot account for these findings. We hypothesize that calcineurin suppression induces progressive increases in channel phosphorylation because the cognate kinase is no longer antagonized by calcineurin. Accordingly, we are not surprised to observe only partial washout of the inhibitory effects, because a return to steady-state levels of α11.2 phosphorylation would be expected to take time. Intriguingly, CsA and AID induced remarkably similar degrees of ICa,L inhibition (44% and 36%, respectively).
There are reports of potentially nonspecific actions of CsA on LTCC function.24 Here, we report evidence against this. Under conditions where calcineurin is inactive by complete chelation of intracellular Ca2+, CsA had no effect on ICa,L. Also, electrophysiological recordings in ventricular myocytes isolated from RCAN1 transgenic mice demonstrated no effect of acute CsA exposure or peptide-inhibitor dialysis in the context of negligible calcineurin activity. These data, then, indicate that the effects of CsA we observe are mediated by direct inhibition of calcineurin enzymatic activity.
Precedents in Other Systems
In a wide variety of cell types, calcineurin, activated by intracellular Ca2+, feeds back to regulate Ca2+ homeostasis through its interaction with other Ca2+-regulatory proteins. In various tissues including heart, calcineurin directly regulates the inositol triphosphate and ryanodine receptors and the Na+/Ca2+ exchanger.25–27 Calcineurin also regulates voltagegated Ca2+ channels in a variety of systems, although there is disagreement regarding the nature and extent of its regulatory function (Online Table II).
Ca2+ Channel Remodeling and Significance in Cardiac Hypertrophy and Failure
Cardiac hypertrophy is associated with significantly increased risk of both heart failure and arrhythmia and poses a major public health problem.28 There is evidence to suggest that alterations in transmembrane Ca2+ fluxes, and consequent perturbations of Ca2+ homeostasis, contribute to the pathogenesis of hypertrophy by abnormally activating Ca2+-responsive signaling pathways. In several forms of heart disease, increases in LTCC activity are a proximal trigger that activates pathological signaling cascades, including mitogen-activated protein kinases, PKC, and calcineurin.29 Eventually, such abnormal profiles of signal transduction lead to disturbances of gene regulation, which may promote disease progression. Indeed, various transgenic models of L-type channel overexpression reveal the development of hypertrophy and severe cardiomyopathy associated with ventricular fibrosis, myocyte necrosis, and remodeling.30,31
We2 (also this report) and others document relatively modest increases in ICa,L during hypertrophy. In many species, membrane impedance is relatively high during phase 2 of the action potential, so modest changes in ICa,L may have important effects on action potential morphology and duration. Consistent with this, computational analyses32 of ICa,L demonstrated that as little as 22% increase in ICa,L density induced significant depolarization of the phase 2 plateau and delayed phase 3 repolarization (data not shown). Furthermore, entry of a small amount of Ca2+ via L-type channels triggers the release of much larger amounts of Ca2+ from intracellular stores. As a result, modest changes in ICa,L (mediated Ca2+ flux such as those elicited by calcineurin) are amplified within the cell.
Perspective and Limitations
Specific mechanisms underlying the functional regulation of ICa,L by calcineurin remain to be elucidated. One possibility may involve direct protein–protein interaction and conformational changes in α11.2 subunit protein. However, our pulldown experiments revealed equivalent binding of calcineurin to α11.2 in the presence of CsA, suggesting that inhibition of ICa,L by CsA is not attributable to disruption of protein–protein interaction. Also, we report that calcineurin is capable of recognizing α11.2 as a substrate in vitro, although the specific enzymatic substrate remains unknown. Ser1928 is an unlikely target as PKA-dependent phosphorylation of this site is associated with increased channel activity and a hyperpolarizing shift in the steady-state I–V relationship. With that said, both PKC and PKG have been shown to target Ser1928, and PKG-dependent phosphorylation of Ser496 on the β2a subunit inhibits ICa,L. Finally, actions mediated indirectly by downstream targets of calcineurin may exist.33
Supplementary Material
Acknowledgments
We gratefully acknowledge Jun Cheng and Joel Feekes for technical expertise and advice.
Sources of Funding
This work was supported by the Donald W. Reynolds Cardiovascular Clinical Research Center (to J.A.H.); NIH grants HL-075173 (to J.A.H.), HL-006296 (to J.A.H.), HL-080144 (to J.A.H.), HL-072016 (to B.A.R.), and NS035563 (to J.W.H.); and American Heart Association Grants 0640084N (to J.A.H.), 0655202Y (to B.A.R.), and 0535235N (to D.D.H.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Kutschke W, Richardson KE, Karimi M, Hill JA. Electrical remodeling in pressure-overload cardiac hypertrophy: role of calcineurin. Circulation. 2001;104:1657–1663. doi: 10.1161/hc3901.095766. [DOI] [PubMed] [Google Scholar]
- 3.Yatani A, Honda R, Tymitz KM, Lalli MJ, Molkentin JD. Enhanced Ca2+ channel currents in cardiac hypertrophy induced by activation of calcineurin-dependent pathway. J Mol Cell Cardiol. 2001;33:249–259. doi: 10.1006/jmcc.2000.1296. [DOI] [PubMed] [Google Scholar]
- 4.Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RHG, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 5.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 6.Pitt GS, Dun W, Boyden PA. Remodeled cardiac calcium channels. J Mol Cell Cardiol. 2006;41:373–388. doi: 10.1016/j.yjmcc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 8.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 9.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 10.duBell WH, Rogers TB. Protein phosphatase 1 and an opposing protein kinase regulate steady-state L-type Ca2+ current in mouse cardiac myocytes. J Physiol. 2004;556(Pt 1):79–93. doi: 10.1113/jphysiol.2003.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 13.Hemenway CS, Heitman J. Calcineurin. Structure, function, and inhibition. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 14.Garnier J, Gibrat JF, Robson B. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 1996;266:540–553. doi: 10.1016/s0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
- 15.Rothermel BA, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 16.Smith PD, Liesegang GW, Berger RL, Czerlinski G, Podolsky RJ. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N′-tetraacetic acid. Anal Biochem. 1984;143:188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- 17.Marx S. Ion channel macromolecular complexes in the heart. J Mol Cell Cardiol. 2003;35:37–44. doi: 10.1016/s0022-2828(02)00287-0. [DOI] [PubMed] [Google Scholar]
- 18.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334–12338. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Piacentino V, III, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, Rothermel B, Yoo KD, Cabuay B, Demetroulis E, Weiss RM, Kutschke W, Bassel-Duby R, Williams RS. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J Biol Chem. 2002;277:10251–10255. doi: 10.1074/jbc.M110722200. [DOI] [PubMed] [Google Scholar]
- 24.Matthes J, Jager A, Handrock R, Groner F, Mehlhorn U, Schwinger RH, Varadi G, Schwartz A, Herzig S. Ca2+-dependent modulation of single human cardiac L-type calcium channels by the calcineurin inhibitor cyclosporine. J Mol Cell Cardiol. 2004;36:241–255. doi: 10.1016/j.yjmcc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Carafoli E, Genazzani A, Guerini D. Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem Biophys Res Commun. 1999;266:624–632. doi: 10.1006/bbrc.1999.1879. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Nolan B, Kutschke W, Hill JA. Na+/Ca2+ exchanger remodeling in pressure-overload cardiac hypertrophy. J Biol Chem. 2001;276:17706–17711. doi: 10.1074/jbc.M100544200. [DOI] [PubMed] [Google Scholar]
- 27.Katanosaka Y, Iwata Y, Kobayashi Y, Shibasaki F, Wakabayashi S, Shigekawa M. Calcineurin Inhibits Na+/Ca2+ exchange in phenylephrine-treated hypertrophic cardiomyocytes. J Biol Chem. 2005;280:5764–5772. doi: 10.1074/jbc.M410240200. [DOI] [PubMed] [Google Scholar]
- 28.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 29.Vlahos CJ, McDowell SA, Clerk A. Kinases as therapeutic targets for heart failure. Nat Rev Drug Discov. 2003;2:99–113. doi: 10.1038/nrd1009. [DOI] [PubMed] [Google Scholar]
- 30.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca2+-dependent transgenic model of cardiac hypertrophy: a role for protein kinase C{alpha} Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+-and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winslow RL, Rice J, Jafri S, Marban E, O’Rourke B. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure. II: Model studies. Circ Res. 1999;84:571–586. doi: 10.1161/01.res.84.5.571. [DOI] [PubMed] [Google Scholar]
- 33.Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.