Abstract
Genital human papillomavirus (HPV) infection is one of the most commonly diagnosed sexually transmitted infections world-wide. Over the last two decades, research has established a strong causal link between specific types of HPV infection and cancer, particularly cervical, anal, vulvar/vaginal, penile, and oropharyngeal cancer. Limited epidemiological studies of anogenital HPV infection have been conducted in Hispanic populations (including Puerto Rico), and population-based incidence and prevalence estimates of HPV infection among Hispanics are limited. Studies that evaluate knowledge and awareness of HPV among men are also scarce. With the world-wide introduction of two new prophylactic vaccines against high-risk HPVs causing cervical cancer, and the recent FDA approval of the quadrivalent vaccine in preventing genital warts in men, there is an urgency to determine the burden of HPV in Hispanic populations before vaccine programs are implemented on a widespread basis. Knowledge and acceptability of the vaccine prior to implementation of these programs are also necessary to allow a targeted assessment. This review article summarizes existing research on HPV infection and HPV-related morbidities in men, with a particular emphasis on Hispanic men in the United States and Puerto Rico. Three major areas are discussed: (1) genital warts, (2) HPV and related cancers and (3) biobehavioral and psychosocial factors related to HPV infection and vaccination. Specific recommendations for advancing HPV research and knowledge among Hispanic populations also are suggested.
Keywords: HPV infection, Men, Hispanics, Puerto Rico, Anal cancer, Penile cancer
Infectious agents are among the few known causes of cancer and contribute to a variety of malignancies (1). One of these infectious agents is human papillomavirus (HPV), one of the most commonly diagnosed sexually transmitted infections (STIs) world-wide (2) and a key causal precursor for cervical cancer. HPV infection is considered the second most important infectious agent related to cancer, just below Helicobacter pylori. Overall, 5.2% of all cancers worldwide can be attributed to HPV infection (3). The World Health Organization (WHO) estimates that the prevalence of HPV infection is between nine and thirteen percent or about 630 million people (2). An estimated 20 million are infected with HPV in the United States (US), with 6.2 million new diagnoses annually (4–5). While a majority of infections are asymptomatic or self-limited, acquisition of specific types of HPV can result in a significant economic burden with 418 million dollars of estimated direct medical costs for non-cervical HPV types related conditions in the US (6).
More than 100 HPV types have been identified with more than 30 being sexually transmitted (7). Anogenital HPV types have been classified into low-risk types (non-oncogenic), which are associated with anogenital warts (condyloma acuminata), oral and conjunctival papillomas, recurrent respiratory papillomatosis (in infants and young children), and mild dysplasia (8). High-risk types (oncogenic) are associated with high-grade dysplasia and various cancers. Current estimates of the attributable fraction, the proportion of cancer cases preventable by the elimination of HPV, are very high (9): 100% for cervical, 90% for anal, 40% for vulvar and vaginal, 50% for cancer of the penis, and between 33 and 72% of oropharyngeal cancers (10).
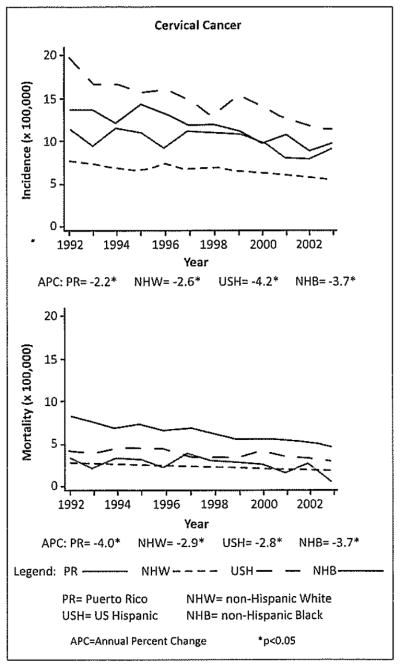
Although there has been increasing interest in understanding the burden of HPV infection and related comorbidities in men (11), studies in this group are still quite limited (12), particularly among Hispanics. Research and surveillance data have indicated that Hispanics have been disproportionately affected by HIV/AIDS (13), Other STIs (14) and may engage disproportionately in high-risk sexual behaviors (15). In addition, a higher incidence of cervical cancer is observed in Puerto Rico and Hispanic women in the US compared with non-Hispanic whites (Figure 1). These factors could reflect a potential higher prevalence of HPV infection in these populations, as well as disparities in screening rates in these groups (16) suggesting that Hispanic and Puerto Rican men might also be at higher risk for HPV infection and related health outcomes. This high incidence might also lead to a significant economic burden. In Puerto Rico for example, it was estimated that the economic impact of HPV related cancers in 2004 was approximately 7.5% (close to 5 million dollars) of the total cancer costs (17).
Figure 1.
Incidence and mortality trends of infection related cancers in Puerto Rico and among selected racial/ethnic groups in the United States.
Source: Modified from: Incidence and mortality rates of selected infection-related cancers in Puerto Rico and in the United States. BMC Cancer, 2010.
Even though HPV infection is highly prevalent in sexually active men, (18), most research studies have focused only in women. Studies in men are of particular relevance because, as with other STIs, men play a key role in the transmission dynamics to both male and female sexual partners. It is also clear that HPV infection in men is a serious clinical issue, given the association of HPV infection with a variety of cancers in men, including anal cancer and a subset of penile and oral cancers (19).
With the world-wide introduction of two new prophylactic vaccines (bivalent vaccine protecting against HPV 16 and 18, and the quadrivalent vaccine protecting against HPV 16, 18, 6 and 11) against oncogenic HPVs causing cervical cancer in women (20), and the recent FDA approval of the quadrivalent vaccine to prevent genital warts also in men, there is an urgency to determine the burden of HPV in Hispanic populations before vaccination programs are implemented on a widespread basis. Knowledge of the burden of the disease and its related morbidities prior to implementation of these programs will allow a better assessment and understanding of the short-term and long-term effectiveness of this primary prevention strategy for cervical neoplasia and genital warts. In addition, it will permit exploring the prevalence of type-specific HPVs in these populations, not currently included in the HPV vaccines available in the market.
The present article presents a review of the existing research literature on HPV infection and HPV-related morbidities in men, with a particular emphasis on Hispanic men (including Puerto Rico). Three major areas are discussed: (1) genital warts, (2) HPV and related cancers and (3) biobehavioral and psychosocial factors related to HPV infection and vaccination. Specific recommendations for advancing HPV research and knowledge among Hispanic populations are suggested, particularly as HPV vaccination becomes widely available in this ethnic group.
I. Genital Warts
HPV frequently presents clinically as anogenital warts (condyloma acuminata) in both males and females (21). More than 90% of genital warts are associated with HPV types 6 and 11, low-risk types that often are associated with other clinical symptoms such as burning and bleeding (10). Genital warts also can lead to psychosocial consequences such as embarrassment, anger, shame, and can interfere with sexual relationships (22). Genital warts are common in the US, with an estimated one million incident cases each year (11). Estimates from the National Health and Nutrition Examination Survey (NHANES) reported an overall prevalence of genital warts between 1999–2004, of about 5.6% among sexually active men and women aged 18–59 years old (23). The prevalence was higher in women (7.2%) compared with men (4.0%). This disparity might be due to an actual gender differences in the risk for acquiring warts, in their diagnosis or recognition, limited access to health care or due to lack of routine clinical screening of the genitalia among men. Significant racial/ethnic differences were also observed, in which Mexican American men had a self-reported prevalence of 1.7%; significantly lower than non-Hispanic white (4.9%) and non-Hispanic black men (2.4%) (p-value ≤ 0.05).
II. HPV and Cancer
Penile HPV Infection and Cancer
It is estimated that at least 40% of penile cancers are attributable to HPV (24). The prevalence of HPV in penile tumors ranges from 29% to 82% depending on the distribution of histopathologic types in a given report. Oncogenic HPV 16 and/or 18 are the most common genotypes detected in penile squamous cell carcinoma (SCC) (25).
Epidemiological studies have shown that the prevalence of penile HPV infection varies by country (26), varying also in relation to the population studied, and the area of the genitalia sampled (27). In recent cross-sectional studies that have evaluated HPV infection in Hispanic male populations including those in the military (28), asymptomatic men (29), patients at vasectomy clinics (30), university students (31), industry workers, men attending sexually transmitted disease clinics (32) and husbands of women with cervical intraepithelial neoplasia (CIN) grade III or invasive squamous cell carcinoma of the cervix (33–34), prevalence estimates range from 8.7% to 50.9%. The baseline prevalence of HPV reported in a group of healthy military Mexican men was 34.8% for high-risk HPV and 23.9% for low-risk HPV (28). In this study, the most common oncogenic HPV types detected in the penis were HPV-59 (8.8%), followed by HPV-16 (6.0%), and HPV-52 (5.9%), while the most common non-oncogenic types were HPV-84 (7.1%), HPV-6 (4.3%), and HPV-54 (3.9%). Another study among asymptomatic men in the US (29) reported that Hispanic ethnicity was independently associated with oncogenic and nononcogenic HPV types as compared with being non-Hispanic white.
In general, risk factors associated with penile HPV infection include indicators of high-risk sexual behavior (18) such as lifetime number of sex partners (26), number of recent sex partners (35), age at first sexual intercourse (36) and sexual frequency (37). Additionally, patterns of sexual networking in men are believed to be key to understanding HPV transmission dynamics and are also especially important for understanding risk of infection among their female partners (38). The protective effect of condom use has been reported, but transmission may still occur via unprotected areas of genital skin.
Epidemiological studies have identified circumcision as an independent protective factor against genital HPV infection (18, 27, 39). Risk reduction of HPV infection among circumcised men in these studies ranges from 60%–80%. Potentially important for understanding HPV infection among Hispanic men is that Hispanics generally evidence lower rates of circumcision compared to the population at large. For example, NHANES reported that 79% of men in the US were circumcised (40), with prevalence estimates much lower among Mexican American men (42%). A recent population-based study in Puerto Rico estimated that the prevalence of circumcision among adult men was 30.6% (41). Low circumcision rates could leave Puerto Rican men at greater risk for HPV infection, a possibility that highlights the importance of studying the burden of HPV infection in this population. Also, recent studies have evaluated the association between male circumcision and HPV infection clearance and persistence (27,42–43); suggesting that the higher prevalence of HPV among uncircumcised men may be attributed to a longer duration of infection rather than to a greater rate of acquisition.
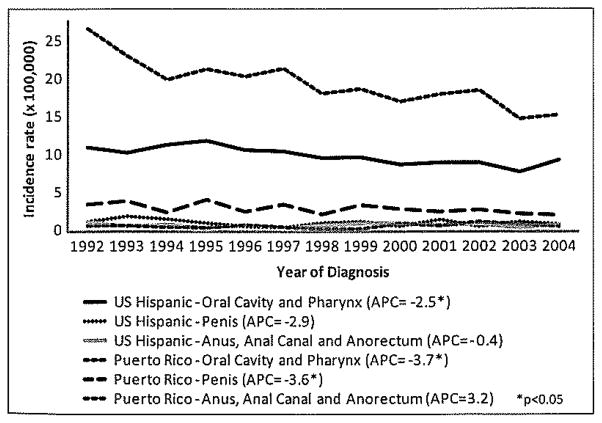
Invasive SCC of the penis is relatively rare in the US and in developed countries, accounting for less than 1% of malignancies among men. An evaluation of cancer registries around the world shows that, although penile cancer is a rare disease, it is particularly prevalent in Uganda, Brazil, and Puerto Rico (44). Between 1998 and 2003, the annual average age-adjusted incidence rate of penile cancer in the US was 0.81 per 100,000 men, but was 72% higher in Hispanics relative to non-Hispanics (45). In Puerto Rico, the rate of penile cancer is considerably higher than in the general US population, at an approximate 4-fold ratio (46). In 2004, the annual age-adjusted (US population) incidence rate of penile cancer in Puerto Rico was 2.21 per 100,000 (Figure 2).
Figure 2.
Incidence trends of HPV related cancers in Hispanic men in Puerto Rico and the US: 1992–2004.
Data Source: Puerto Rico Central Cancer Registry. Puerto Rico Comprehensive Cancer Center, University of Puerto Rico, April 2010.
Anal HPV infection and cancer
Anal HPV infection with oncogenic HPV genotypes is a key causal precursor for anal cancer (47) and its precursor lesion known as anal intraepithelial neoplasia (AIN). As with penile HPV infection, previous estimates of the prevalence of anal HPV infection in men have varied widely. A study among 1,200 men from Brazil, Mexico and the US reported that anal HPV infection was common among men who acknowledge no lifetime sex with other men (13.4%), while the prevalence was three times higher among men who acknowledged sex with other men (43.2%). Subpopulations such as men having sex with men (MSM) (48) and HIV-positive individuals are at an event higher risk of HPV infection and anal cancer (49).
HIV status increases the risk for anal HPV infection and other malignancies among MSM and bisexual men and women. Epidemiological studies have documented that anal HPV infection is nearly universal amongst HIV-positive MSM (50). Studies performed by Palefsky among MSM in San Francisco showed that the prevalence of anal HPV infection was 93% among HIV-positive men and that 73% of these participants showed more than one HPV type (51). In this study, a significant increasing trend in the prevalence of HPV infection was observed as levels of CD4 decreased.
Even though Hispanics are disproportionally affected by HIV/AIDS (52) and represent one of the largest groups of MSM living with HIV/AIDS in the US (53), few studies have examined the impact of HPV infection among Hispanic MSM. In 2006, surveillance data indicated that the overall HIV incidence rate in Puerto Rico was twice the estimated US rate and 1.5 times higher than the estimated rate for Hispanics in the US (54). Between 2004 and 2008, the prevalence of HIV increased from 9.13% to 11.10% among MSM in Puerto Rico, suggesting that behavioral risks among this group might be a contributor to the continuing HIV epidemic (55). A descriptive profile of MSM in Puerto Rico indicated that a significant proportion (62.2%) of MSM reported vaginal sexual practices in the last 12 months, suggesting that men who engage with both male and female sexual partners can serve as bridge populations (55). This is particularly important when considered in the context of observations indicating that anal cancer incidence among HIV positive men will not decline in the antiretroviral therapy (ART) era (56). As an example, studies in San Francisco have demonstrated that not only has the incidence of anal cancer among HIV-positive MSM failed to decline since the introduction of ART, but that the incidence has continued to increase.
The annual incidence rate for anal cancer has increased for both men and women during the past 3 decades (57), with men showing the highest increase. In the US, the incidence of anal cancer among men is relatively low in the general population, with an incidence rate of 1.4 per 100,000 men. Hispanics overall have a lower incidence of anal cancer (0.8 per 100,000) compared with non-Hispanics whites (1.4 per 100,000) and non-Hispanic blacks (1.6 per 100,000) (58). However, incidence is highest among MSM. According to recent data from the US and Europe, the incidence of anal cancer among HIV-positive individuals ranges from 42 to 137 cases per 100,000 person-years, a rate that is 30 to 100 times higher than that of the general population (59–60). In HIV-seronegative MSM, the estimated incidence of anal cancer is 35 cases per 100,000, estimates similar to the incidence of cervical cancer in the US before the introduction of cervical cytology testing (61). From 1987–2004, incidence trends of anal cancer increased in Puerto Rican men (46), with an average annual increase of 4% per year (APC 3.2). In 2004, the age-adjusted (US population) incidence of anal cancer in this population was 0.73 per 100,000 (Figure 2).
Oral HPV infection and cancer
HPV is frequently associated with benign warts (oral papillomas), as well as benign and malignant neoplasms in the oral cavity. HPV 6 and 11 are most associated with oral papillomas (62) whereas; HPV16 is the most frequently detected HPV type in oral SCC, followed by HPV18 (63). The prevalence of oral papillomas/warts in the US estimated in the Third National Health and Nutrition examination survey (NHANES III) 1988–1994, was 1.9% in non-Hispanic whites and 2.2% in both non-Hispanic blacks and Mexican Americans (64). Meanwhile, a recent review of 18 international studies and 4,581 healthy cancer-free individuals determined a pooled prevalence of HPV infection of the oral cavity of 4.5% (95% CI=3.9%–5.1%). In this study, oral HPV-16 accounted for 28% of all HPV detected in the oral cavity, with similar prevalence of oral infection in both men (4.6%) and women (4.4%) (65). To date, studies of oral HPV infection specifically for Hispanics in the US are scarce. In general, risk factors such as oral sex and open-mouthed kissing are associated with oral HPV infection (66). Sexual behaviors are also associated with cancer risk at the oropharyngeal sites most strongly associated with HPV infection (67).
Oral cavity and pharyngeal cancer is the 10th most common cancer among Hispanic men in the US (68) and the 4th most common cancer in men in Puerto Rico (69). This cancer type is of particular relevance in Puerto Rico as its incidence in men is among the highest in the American region (70) and among various racial/ethnic groups in the US (18.5 per 100,000 from 1998–2002). Oral cavity cancer includes cancer of the lip, tongue, gum, floor of the mouth, salivary glands and palate; while pharyngeal cancer includes cancers of the oropharynx, nasopharynx and hypopharynx. The incidence of the disease is higher in men than in women, a disparity probably explained by higher risk factors for the disease among men, such as tobacco consumption (71). Although a substantial percentage of oral and pharyngeal cancers is attributed to alcohol and tobacco exposure, epidemiological data also provide evidence for an independent association between HPV infection and a subset of these tumors, particularly with HPV-16 (72–73). While oral cavity cancer seems to be more strongly associated to alcohol and tobacco exposure than to HPV infection, oropharyngeal cancer (base of the tongue, oropharynx, tonsils and soft palate) seems to be more strongly associated to HPV infection, with tonsillar SCC being the sub-site most strongly and consistently associated with HPV.
A recent meta-analysis by IARC has confirmed HPV as an independent risk factor for oral and oropharynx cancers (63). In this meta-analysis, HPV was detected in 3.9% (95% CI, 2.596–5.3%) of oral cavity tumors and 18.3% (95% CI=12.0%–24.7%) of oropharyngeal tumors (63). Meanwhile, a recent study in Mexico showed that oncogenic-HPV was strongly associated with oral and oropharyngeal SCC, supporting that HPV16 and HPV18 are also risk factors for oral cancer in this population (74). In Cuba, a country with an intermediate incidence of oropharyngeal cancer, strong associations of the disease have been found for tobacco, alcohol consumption, low fruit consumption (75), and HPV (63). Nonetheless, many aspects of these associations remain to be investigated, such as the potential interactions between HPV infection and lifestyle practices of individuals that increase oral and pharyngeal cancer risk. All this information is essential to better define the precise contribution of HPV to the etiology of these tumors and for the development of prevention interventions.
Another area of research interest is the fact that recent studies report that molecular and epidemiological profiles suggest that HPV-positive oropharyngeal cancer is a distinctive disease entity that differs from HPV-negative oropharyngeal cancer (76), with different genome-wide expression profiles and clinical outcomes. For example, HPV-positive oropharyngeal cancer patients seem to be more responsive to chemotherapy and radiation than HPV-negative patients (77). Further elucidation of these differences is important for better understanding the etiology of this disease, its prevention and management.
Although Hispanic men overall have lower rates of oral and pharyngeal cancer than non-Hispanic men, men in Puerto Rico have a higher incidence of the disease compared with Hispanics and non-Hispanic whites in the US (71). They also have a higher mortality risk (p<0.05) of the disease compared with Hispanics, non-Hispanic whites and non-Hispanic blacks in the US (71). In 2004, the age-adjusted (US population) incidence of oral and pharyngeal cancer in Puerto Rico was 15.48 per 100,000 (Figure 2). In the US, the annual incidence rates of potentially HPV-associated oropharyngeal and oral cavity cancers, specifically cancers of the tonsil and base of tongue, significantly increased from 1998 through 2003, an average of 3% per year, whereas the incidence rates of cancer sites not previously shown to be associated with HPV, such as cancer of the oral tongue and larynx, generally decreased (73). It has been suggested that increases in rates of cancer in certain sites of the oropharynx, despite the reductions in tobacco consumption in die US in the last decades, could be explained by HPV infections (78). In Puerto Rico, from 1987–2003, incidence trends of cancer of the oral cavity and pharynx decreased, with an average annual decrease of 3.8% for men and 2.9% for women (79). These declines are consistent with declines reported since the 1960’s in Puerto Rico (71, 80), although, like It has been studied in the US, further research should specifically compare cancer trends between HPV-related and non-related sub-types.
Population-based data on oral HPV infection do not yet exist for Puerto Rico. However, various studies suggest that the population of Puerto Rico is at high risk for oral HPV infection and potentially HPV-related malignancies. In a case series (n=118) of patients of head and neck SCC, HPV 16 DNA was present in 44% of the tumors, suggesting a high prevalence of HPV 16 among the head and neck cancer population in Puerto Rico (81). Similar results have been observed in head and neck cancer (82) and oral SCC (83) patients in Mexico, with prevalence estimates of HPV-16 infection in 70% and 67% of tumors, respectively. In Puerto Rico, although a substantial percentage of oral and pharyngeal cancer is also attributed to alcohol and tobacco exposure, about one quarter of oral cancer cases in men and approximately half of those in women in Puerto Rico are not attributed to these exposures (84), highlighting the relevance to explore other potential risk factors in this population.
IIl. Biobehavioral and Psyschosocial Factors Related to Infection and Vaccination
Knowledge of HPV Infection
Although there are increasing studies about HPV awareness and knowledge, these efforts have mainly focused on women. Studies in men, particularly among those of Hispanic origin, are scarce. In those studies that have evaluated gender differences, it is notable that men have lower knowledge about HPV compared with women (85). Their knowledge is also limited in relation to the long-term consequences of HPV and regarding its modes of transmission.
A cross-sectional household survey among men and women 15–74 years in Puerto Rico (86) found that the percentage of people who reported to have ever heard about HPV infection was poor, particularly for men (17.6%). A small percentage of men interviewed in this sample were also unaware of the existence of an HPV vaccine at the time (13.5%). This significant lack of knowledge among Puerto Rican men raises concerns regarding the consequences that unawareness of a highly infectious disease will have on transmission to their female and/or male sexual partners. Thus, results highlight the need to implement primary preventive interventions aimed at integrating HPV screening and education in primary health care settings.
Another study among Hispanic men and women in the Texas-Mexico border addresses the issue of understanding the diagnosis of HPV infection (87). In this qualitative study, men initially interpreted a diagnosis of HPV as an indication of their partner’s infidelity, which reflects not only a lack of knowledge of HPV infection in men but also reflects strong cultural norms within this group. This study supports previous research findings showing that STI-associated stigma can be a powerful barrier to STI-related information seeking, screening, and treatment behaviors, and that these patterns may vary by ethnicity (88–89).
Vaccine Acceptability
Despite the impact of the HPV vaccine on preventing chronic life-threatening diseases, if men’s knowledge of HPV risk is limited, their level of interest in prophylactic vaccination can also be expected to below. Currently, research on HPV vaccine acceptability is limited to cross-sectional studies, most involving small, convenience samples (90) and with limited participation by Hispanic males. Additionally, existing studies which evaluate the acceptability of the vaccine in men were conducted prior FDA approval of the vaccine, leading to a hypothetical response. In general, although most studies have found a significant association between vaccine acceptance, high-risk behaviors (91) and physician recommendation (92), another study reported moderate interest in the HPV vaccine in young men, even after informing about the benefits of male HPV vaccination in reducing cervical cancer risk (93). Some studies have shown that Hispanic ethnicity is positively correlated with HPV vaccine acceptability (91), but other studies have shown no differences in vaccine acceptability by ethnicity (94).
Few research studies have examined an interaction effect between predictors of HPV acceptability and ethnicity, with a particular evaluation of sexual preferences. Given that acceptability rates might not correlate directly with vaccine uptake, such information will be important when developing sensitive educational information to increase awareness and completion of vaccination. Finally, given that vaccination of HPV-naïve individuals is optimal for the vaccine to be most clinically effective, assessing the attitudes of parents about HPV vaccine in young boys will also be critical in order to develop successful implementation of widespread HPV vaccination in this group (95).
IV. Conclusion
With the world-wide introduction of two new prophylactic vaccines against high-risk HPVs causing cervical cancer and the recent FDA approval of one of them for men, there is an urgency to determine the burden of the HPV epidemic in Hispanic populations before vaccine programs are implemented on a widespread basis. Knowledge of the burden of the disease prior to implementation of these programs will allow a better assessment and understanding of the short-term and long-term effectiveness of this primary prevention strategy for cervical neoplasia and genital warts, and its potential impact in the prevention of other HPV related malignancies, such as penile, anal and oral cancer. Of particular interest is to examine the burden of HPV infection in US Hispanics, and Puerto Rican men, since these are groups with underlying social disparities associated with health status – including poverty, limited access to quality health care, poor health-seeking, low health literacy and high rates of behavioral risks (96). This is of particular relevance in Puerto Rico, given that population-based data on HPV infection do not yet exist for either men or women. In addition, given the changing patterns of HPV-related malignancies in these groups, research should further access the reasons for these changes in patterns of disease occurrence, including changes in patterns of HPV infection, sexual behaviors and/other risk factors and how they influence disease trends. This will also be key to understanding disease epidemiology and future population-based disease control efforts.
Research is particularly warranted among high-risk groups of racial/ethnic minorities, such as: asymptomatic heterosexuals, HIV+ individuals and MSM. Despite the fact that significant differences within Hispanic sub-groups have been reported both for substance use and sexual practices (97), the available data on HPV infection in Hispanics does not permit any assessment of potential sub-group differences within the broad Hispanic/Latino category. Consequently, studies of specific subgroups are needed to disentangle intra-ethnic differences between groups and to better focus both epidemiological research and targeted interventions.
Given that knowledge of HPV infection among Hispanics is likely to be relatively low, it is reasonable to assume that a necessary first step in advancing HPV vaccination in this group will require the development of empirically-tested initiatives that will test the best strategies to foster positive attitudes towards vaccination. Recent studies on other STIs, including chlamydia, syphilis, and HIV, indicate that interventions that are tailored to specific ethnicities are more effective (98). While encouraging, the generalizability of these interventions for HPV knowledge and vaccine uptake in Hispanic and Puerto Rican men remains unknown.
Although the research agenda has to be driven towards understanding the burden of HPV infection in men, these efforts have to be in parallel with interventions to improve knowledge of HPV and acceptability of the HPV vaccine for two groups in particular: high-risk men and Hispanic/Puerto Rican parents. These interventions will have to be sensitive to cultural norms, which vary within the Hispanic/Latino community, and across generations. Targeting these groups within the Hispanic and Puerto Rican population might have multiple clinical and public health benefits such as: l) decreasing HPV infection sequelae in men, 2) decreasing HPV infection sequelae in men’s sexual partners (both men and women), and 3) increasing heard immunity at the population level (99).
Acknowledgments
This work was supported by the following grants from the National Institute of Health: Support Opportunity for Addiction Research (SOAR) (R03), [NIDA RO3 027939-01], National Institute of Allergy and Infectious Diseases Grant 1 SC2 AI090922-01, Training in Computational Genomic Epidemiology of Cancer (National Cancer Institute [NCI] 5R25CA094186-08), the NCI Grant U54CA96297 for the Puerto Rico Cancer Center/The University of Texas M. D. Anderson Cancer Center, Partners for Excellence in Cancer Research, and the RCMI Program Grant G12RR03051 from the University of Puerto Rico. We wish to express our gratitude to Dr. Angela Pattatucci, Director of the Center for Evaluation and Sociomedical Research, University of Puerto Rico, Graduate School of Public Health for her review and suggestions to this manuscript.
References
- 1.Pagano JS, Blaser M, Buendia MA, Damania B, et al. Infectious agents and cancer: Criteria for a causal relation. Semin Cancer Biol. 2004;14:453–471. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Pagliusi S. Vaccines against Human Papillomavirus. World Health Organization; Retrieved January 25, 2009 from the World Wide Web: http://www.who.int/vaccines/en/hpvrd.shtml. [Google Scholar]
- 3.Steben M, Duarte-Franco E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol Oncol. 2007;107:S2–S5. doi: 10.1016/j.ygyno.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 5.Gerberding JL. Center for Disease Control and Prevention; 2004. Report to Congress: Prevention of Genital Human Papillomavirus Infection: Centers for Disease Control and Prevention. Retrieved November 11, 2008 from the World Wide Web: http://www.cdc.gov/std/HPV/2004HPV%20report.pdf. [Google Scholar]
- 6.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;98:500, e1–7. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Human Papillomaviruses and Cancer: Questions and Answer Factsheet. Available at: http://www.cancer.gov/cancer-topics/factsheet/Risk/HPV.
- 8.Trottier H, Burchell AN. Epidemiology of mucosal human papillomavirus infection and associated diseases. Public Health Genomics. 2009;12:291–307. doi: 10.1159/000214920. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;19:K17–K28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano AR, Anic G, Nyitray AG. Epidemiology and Pathology of HPV disease in males. Gyn Oncology. 2010;117:S15–S19. doi: 10.1016/j.ygyno.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palefsky JM. HPV infection in men. Dis Markers. 2007;23:261–272. doi: 10.1155/2007/159137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. HIV/AIDS Among Hispanics/Latinos: Factsheet. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. http://www.cdc.gov/hiv/hispanics/resources/factsheets/hispanic.htm. [Google Scholar]
- 14.Aral SO. Sexually transmitted diseases: Magnitude, determinants and consequences. Int J STD AIDS. 2001;12:211–215. doi: 10.1258/0956462011922814. [DOI] [PubMed] [Google Scholar]
- 15.Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Schootman M, et al. Age of sexual debut among US adolescents. Contraception. 2009;80:158–162. doi: 10.1016/j.contraception.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz AP, Soto-Salgado M, Calo WA, Tortolero-Luna G, et al. Incidence and mortality rates of selected infection-related cancers in Puerto Rico and in the United States of America. Infect Agent Cancer. 2010;14:5–10. doi: 10.1186/1750-9378-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz Ortiz KJ, Irizarry P, Marín Centeno H, Ortiz AP, et al. Productivity Loss in Puerto Rico’s Labor Market due to Cancer Mortality. PRHSJ. 2010;3:241–249. [PubMed] [Google Scholar]
- 18.Dunne EF, Nielson CM, Stone KM, Markowitz LE, et al. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 19.Palefsky JM. Human papillomavirus-related disease in men: not just a women’s issue. J Adolesc Health. 2010;46:S12–S19. doi: 10.1016/j.jadohealth.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiller JT, Castellsagué X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus LI virus-like particle vaccine clinical trial results. Vaccine. 2008;26S:K53–K61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giuliano AR. Human papillomavirus vaccination in males. Gynecol Oncol. 2007;107:S24–S26. doi: 10.1016/j.ygyno.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 22.Ireland JA, Reid M, Powell R, Petrie KJ. The role of illness perceptions: Psychological distress and treatment-seeking delay in patients with genital warts. Int J STD AIDS. 2005;16:667–670. doi: 10.1258/095646205774357334. [DOI] [PubMed] [Google Scholar]
- 23.Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, national health and nutrition examination survey, 1999–2004. Sex Transm Dis. 2008;35:357–360. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez BY, Barnholtz-Sloan J, German RR, Giuliano A, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer. 2008;113:2883–2891. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschi S, Castellsagué X, Dal Maso L, Smith JS, et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer. 2002;86:705–711. doi: 10.1038/sj.bjc.6600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliano AR, Lazcano E, Villa LL, Flores R, et al. Circumcision and sexual behavior: Factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–1257. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lajous M, Mueller N, Cruz-Valdéz A, Aguilar LV, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–1716. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 29.Nielson CM, Harris RB, Flores R, Abrahamsen M, et al. Multiple-type human papillomavirus infection in male anogenital sites: Prevalence and associated factors. Cancer Epidemiol Biomarkers Prev. 2009;18:1077–1083. doi: 10.1158/1055-9965.EPI-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaccarella S, Lazcano-Ponce E, Castro-Garduño JA, Cruz-Valdez A, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- 31.Lazcano-Ponce E, Herrero R, Muñoz N, Hernandez-Avila M, et al. High Prevalence of Human Papillomavirus Infection in Mexican Males. Comparative Study of Penile Urethral Swabs and Urine Samples. Sexually Transmitted Diseases. 2001;28:277–280. doi: 10.1097/00007435-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, et al. Human papillomavirus infection in men attending a sexually transmitted disease clinic. J Infect Dis. 2003;187:1064–1070. doi: 10.1086/368220. [DOI] [PubMed] [Google Scholar]
- 33.Castellsagué X, Bosch FX, Muñoz N, Meijer CJ, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 34.Castellsagué X, Ghaffari A, Daniel RW, Bosch FX, et al. Prevalence of penile human papillomavirus DNA in husbands of women with and without cervical neoplasia: a study in Spain and Colombia. J Infect Dis. 1997;176:353–361. doi: 10.1086/514052. [DOI] [PubMed] [Google Scholar]
- 35.Svare EI, Kjaer SK, Worm AM, Osterlind A, et al. Risk factors for genital HPV DNA in men resemble those found in women: A study of male attendees at a Danish STD clinic. Sex Transm Infect. 2002;78:215–218. doi: 10.1136/sti.78.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellsague X, Ghaffari A, Daniel RW, Bosch FX, et al. Prevalence of penile human papillomavirus DNA in husbands of women with and without cervical neoplasia: A study in Spain and Colombia. J Infect Dis. 1997;176:353–361. doi: 10.1086/514052. [DOI] [PubMed] [Google Scholar]
- 37.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, et al. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004;31:601–607. doi: 10.1097/01.olq.0000140012.02703.10. [DOI] [PubMed] [Google Scholar]
- 38.Doherty IA, Padian NS, Marlow C, Aral SO. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. J Infect Dis. 2005;191:S42–54. doi: 10.1086/425277. [DOI] [PubMed] [Google Scholar]
- 39.Giuliano AR, Lazcano E, Villa LL, Flores R, et al. Circumcision and sexual behavior: Factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–1257. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu F, Markowitz LE, Sternberg MR, Aral SO. Prevalence of circumcision and herpes simplex virus type 2 infection in men in the United States: The National Health and Nutrition Examination Survey (NHANES), 1999–2004. Sex Transm Dis. 2007;34:479–484. doi: 10.1097/01.olq.0000253335.41841.04. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz AP, Pérez CM. Sexual behaviors among a population-based sample of Puerto Rican Adults: Results from the Epidemiology of Hepatitis C (HCV) in the household, adult population of Puerto Rico study. 2009 Unpublished data. [Google Scholar]
- 42.Hernandez BY, Shvetsov YB, Goodman MT, Wilkens LR, et al. Reduced clearance of penile human papillomavirus infection in uncircumcised men. J Infect Dis. 2010;201:1340–1343. doi: 10.1086/651607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu B, Wu Y, Nielson CM, Flores R, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: A prospective study. J Infect Dis. 2009;199:362–371. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 44.Palefsky J. HPV-Related Disease in Men: Anal and Penile Cancer, in Reducing the Global Burden of HPV-Related Disease: Cervical Cancer and Beyond. 2007 Retrieved from World Wide Web: http://www.medscape.com/viewarticle/565225.
- 45.Hernandez BY, Barnholtz-Sban J, German RR, Giuliano A. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer. 2008;113:2883–2891. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puerto Rico Central Cancer Registry. Puerto Rico Comprehensive Cancer Center. University of Puerto Rico. Date release: February 11, 2009.
- 47.de Pokomandy A, Rouleau D, Ghattas G, Vézina S, et al. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: The HIPVIRG cohort study. J Infect Dis. 2009;199:965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 48.Chin-Hong PV, Vittinghoff E, Cranston RD, Browne L, et al. Age-related prevalence of anal cancer precursors in homosexual men: The EXPLORE study. J Natl Cancer Inst. 2005;97:896–905. doi: 10.1093/jnci/dji163. [DOI] [PubMed] [Google Scholar]
- 49.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4:52–56. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin-Hong PV, Palefsky JM. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis. 2002;35:1127–1134. doi: 10.1086/344057. [DOI] [PubMed] [Google Scholar]
- 51.Palefsky JM, Holly EA, Ralston ML, Arthur SP, et al. Anal cytological abnormalities and anal HPV infection in men with Centers for Disease Control group IV HIV disease. Genitourin Med. 1997;73:174–180. doi: 10.1136/sti.73.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. HIV/AIDS among Hispanic/Latinos. 2007 http://wvvw.cdc.gov/hiv/resources/factsheets/PDF/hispanic.pdf.
- 53.Centers for Disease Control and Prevention. HIV and AIDS in the United States: A picture of today’s epidemic. 2008 http://cdc.gov/hiv/topics/surveillance/united_states.htm.
- 54.Centers for Disease Control and Prevention. Incidence and diagnosis of HIV infection – Puerto Rico. MMWR. 2006;58:589–591. [Google Scholar]
- 55.Colón-López V, Rodriguez-Díaz CE, Ortiz AP, Soto-Salgado M, et al. Epidemiological profile of HIV-related risks among a sample of MSM in Puerto Rico: An overview of substance use and sexual practices. Under Review. [PMC free article] [PubMed] [Google Scholar]
- 56.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4:52–56. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joseph DA, Miller JW, Wu X, Chen VW, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113(10 Suppl):2892–2900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horner MJ, Ries LA, Krapcho M, Neyman N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 59.Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, et al. Marked increase in the incidence of invasive anal cancer among HIV infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22:1203–1211. doi: 10.1097/QAD.0b013e3283023f78. [DOI] [PubMed] [Google Scholar]
- 60.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jemal A, Murray T, Samuels A, Ghafoor A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 62.Castro TP, Bussoloti Filho I. Prevalence of human papillomavirus (HPV) in oral cavity and oropharynx. Braz J Otorhinolaryngol. 2006;72:272–282. doi: 10.1016/S1808-8694(15)30068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrero R, Castellsagué X, Pawlita M, Lissowska J, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 64.Oral health U.S. Dental, Oral and Craniofacial Data Resource Center of the National Institute of Dental and Craniofacial Research, the National Institutes of Health and Division of Oral Health, National Center for Chronic Disease Prevention and Health Promotion, the Centers for Disease Control and Prevention. 2002 Available at http://drc.hhs.gov/report/pdfs/section6-oralinfections.pdf.
- 65.Kreimer AR, Bhatia RK, Messeguer AL, González P, et al. Oral Human Papillomavirus in Healthy Individuals: A Systematic Review of the Literature. Sex Transm Dis. 2010;37:386–391. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 66.D’Souza G, Agrawal Y, Halpern J, Bodison S, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heck JE, Berthiller J, Vaccarella S, Winn DM, et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:166–181. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cancer Facts & Figures for Hispanics/Latinos 2006–2008. American Cancer Society; 2006. Available at: http://www.cancer.org/downloads/STT/CAFF2006HispPWSecured.pdf. [Google Scholar]
- 69.Puerto Rico Central Cancer Registry, Puerto Rico Cancer Incidence File. Division of Epidemiology, Puerto Rico Department of Health; May, 2007. Accessed: http://www.salud.gov.pr/RCancer/Reports/Documents/Incidencia,%202003.pdf. [Google Scholar]
- 70.International Agency for Cancer Research. GLOBOCAN 2008 database: Table by populations. Lyon, France: Accessed at: http://globocan.iarc.fr/summary_table_site.aspselection=13010&selection=21030&title=Lip%2C+oral+cavity%2C+Other+pharynx&sex=1&type=0&window=1&america=2&sort=0&submit=%&A0Execute%A0. [Google Scholar]
- 71.Suárez E, Calo W, Hernandez Y, Diaz-Toro E, et al. Age-standardized incidence and mortality rates of oral and pharyngeal cancer in Puerto Rico and among Non-Hispanics Whites, Non-Hispanics blacks, and Hispanics and in the USA. BMC Cancer. 2009;9:129. doi: 10.1186/1471-2407-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hennessey PT, Westra WH, Califano JA. Human papillomavirus and head and neck squamous cell carcinoma: Recent evidence and clinical implications. J Dent Res. 2009;88:300–306. doi: 10.1177/0022034509333371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryerson AB, Peters ES, Coughlin SS, Chen VW, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113:2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 74.Anaya-Saavedra G, Ramírez-Amador V, Irigoyen-Camacho ME, García-Cuellar CM, et al. High association of human papillomavirus infection with oral cancer: A case-control study. Arch Med Res. 2008;39:189–197. doi: 10.1016/j.arcmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Garrote LF, Herrero R, Reyes RM, Vaccarella S, et al. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lohavanichbutr P, Houck J, Fan W, Yueh B, et al. Genomewide gene expression profiles of HPV-positive and HPV-negative oropharyngeal cancer: potential implications for treatment choices. Arch Otolaryngol Head Neck Surg. 2009;135:180–188. doi: 10.1001/archoto.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010 doi: 10.1016/S1470-2045(10)70017-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence; An emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 79.Figueroa NR, De la Torre T, Ortiz KJ, Pérez J, et al., editors. Puerto Rico Central Cancer Registry. San Juan, PR; Cancer of the Oral Cavity and Pharynx Stat Fact Sheet. http://www.salud.gov.pr/RCancer/Reports/Pages/default.aspx, based on May 2007 Puerto Rico Central Cancer Registry data submission. [Google Scholar]
- 80.Franceschi S, Bidoli E, Herrero R, Muñoz N. Comparison of cancers of the oral cavity and pharynx worldwide: Etiological clues. Oral Oncology. 2000;36:106–115. doi: 10.1016/s1368-8375(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 81.Báez A, Almodóvar JI, Cantor A, Celestin F, et al. High frequency of HPV16-associated head and neck squamous cell carcinoma in the Puerto Rican population. Head Neck. 2004;26:778–784. doi: 10.1002/hed.20046. [DOI] [PubMed] [Google Scholar]
- 82.Gallegos-Hernández JF, Paredes-Hernández E, Flores-Díaz R, Minauro-Muñoz G, et al. Human papillomavirus: association with head and neck cancer. Cir Cir. 2007;75:151–155. [PubMed] [Google Scholar]
- 83.Ibieta BR, Lizano M, Fras-Mendivil M, Barrera JL, et al. Human papilloma virus in oral squamous cell carcinoma in a Mexican population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:311–315. doi: 10.1016/j.tripleo.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Hayes RB, Bravo-Otero E, Kleinman DV, Brown LM, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10:27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 85.Baer H, Allen S, Braun L. Knowledge of human papillomavirus infection among young adult men and women: Implications for health education and research. J Commun Health. 2000;25:67–78. doi: 10.1023/a:1005192902137. [DOI] [PubMed] [Google Scholar]
- 86.Reyes JC. Association between HPV knowledge and high-risk behaviors in Puerto Rico. Poster session presented at the 25th International Papillomavirus Conference; Malvo Sweden. 2009. [Google Scholar]
- 87.Fernandez ME, McCurdy SA, Arvey SR, Tyson SK, et al. HPV knowledge, attitudes, and cultural beliefs among Hispanic men and women living on the Texas-Mexico border. Ethn Health. 2009;14:607–624. doi: 10.1080/13557850903248621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortenberry JD. The effects of stigma on genital herpes care-seeking behaviours. Herpes. 2004;11:8–11. [PubMed] [Google Scholar]
- 89.Fortenberry JD, McFarlane M, Bleakley A, Bull S, et al. Relationships of stigma and shame to gonorrhea and HIV screening. Am J Public Health. 2002;92:378–381. doi: 10.2105/ajph.92.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev Med. 2007;45:107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 91.Ferris DG, Waller JL, Miller J, Patel P, et al. Variables associated with human papillomavirus (HPV) vaccine acceptance by men. J Am Board Fam Med. 2009;22:34–42. doi: 10.3122/jabfm.2009.01.080008. [DOI] [PubMed] [Google Scholar]
- 92.Reiter PL, Brewer NT, Smith JS. HPV Knowledge and HPV Vaccine Acceptability among a National Sample of Heterosexual Males. Sex Transm Infect. 2010;86:241–246. doi: 10.1136/sti.2009.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerend MA, Barley J. Human papillomavirus vaccine acceptability among young adult men. Sex Transm Dis. 2009;36:58–62. doi: 10.1097/OLQ.0b013e31818606fc. [DOI] [PubMed] [Google Scholar]
- 94.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev Med. 2007;45:107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 95.Podolsky R, Cremer M, Atrio J, Hochman T, Arslan AA. HPV vaccine acceptability by Latino parents: A comparison of U.S. and Salvadoran populations. J Pediatr Adolesc Gynecol. 2009;22:205–215. doi: 10.1016/j.jpag.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol Rev. 2004;26:22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- 97.De La Rosa MR, Khalsa JH, Rouse BA. Hispanics and illicit drug use: A review of recent findings. International Journal of Addiction. 1990;25:665–691. doi: 10.3109/10826089009061327. [DOI] [PubMed] [Google Scholar]
- 98.DiClemente RJ, Wingood GM, Harrington KF, Lang DL, et al. Efficacy of an HIV prevention intervention for African American adolescent girls: A randomized controlled trial. JAMA. 2004;292:171–179. doi: 10.1001/jama.292.2.171. [DOI] [PubMed] [Google Scholar]
- 99.Palefsky JM. HPV infection in men. Dis Markers. 2007;23:261–72. doi: 10.1155/2007/159137. [DOI] [PMC free article] [PubMed] [Google Scholar]