Abstract
Research into the biology of the endogenous gaseous mediators nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) has significantly expanded over the last decade. A number of drugs (already in clinical use) and drug candidates (in preclinical or clinical trials) exert their effects via donation of these mediators and/or via modulation of their intracellular second messenger pathways. Due to their important biological roles, gaseotransmitters offer many therapeutic opportunities. However, their unique chemical and pharmacological properties can also represent unusual challenges for translational science.
Nitric oxide (NO), for many decades, was only known as a toxic gas: familiar only to scientists interested in the physics of lighting and related atmospheric events or in the toxicology of car engine exhausts and smog. The discovery in the late 80’s that NO is produced by mammalian cells (1,2) started a true revolution in the field of gaseotransmitters. It has been established that NO is generated by a family of enzymes (NO synthases) from the amino acid L-arginine: many fundamental regulatory functions of NO have been discovered in the cardiovascular system, immune system and the central and peripheral nervous system (3). A decade later, carbon monoxide (CO) (another toxic gas: previously known in toxicological and medical sciences as a dangerous byproduct of engines and furnaces) emerged as a neurotransmitter and cardiovascular and immune regulator. CO is produced as a result of heme metabolism via a family of enzymes (heme oxygenases) (5,6). Even more recently a third gas, hydrogen sulfide (H2S) (previously mainly recognized as a toxic gas and environmental hazard produced in volcanic emissions, swamps and certain industries) is rapidly emerging as an endogenous biological mediator, produced by cysteine by several enzymes (CBS, CSE and 3-MPST), with important regulatory roles in the nervous and cardiovascular system and in the regulation of cell and whole-body metabolism (7,8).
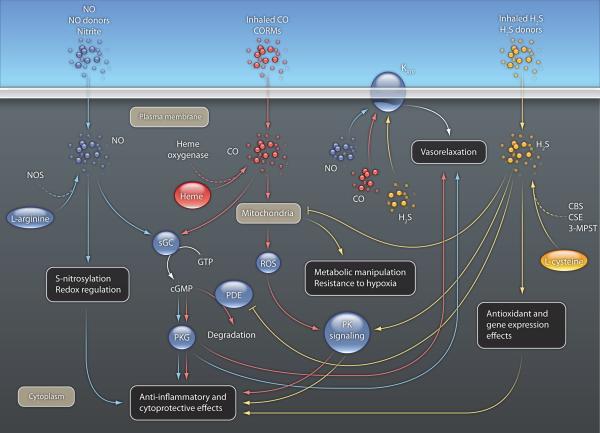
There are many significant similarities between the biology and pharmacology of these three molecule (Table 1, Fig. 1). At physiological concentrations, all three gases act as vasodilators, as well as broad-spectrum anti-inflammatory and cytoprotective agents. As reviewed recently (9), many therapeutic approaches (in clinical or preclinical stages) are based on various aspects of gaseotransmitter pharmacology. These approaches, among others, include the therapeutic administration of these molecules (in inhaled gaseous form or as parenteral or oral prodrugs); modulators of intracellular pathways affected by these molecules; administration of the biological precursors of these gases (Table 1).
Table 1.
Biological and therapeutic aspects of the three gasotransmitters: nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S).
| NO | CO | H2S | |
|---|---|---|---|
| Chemical nature | Reactive free radical, gas | Gas, not a free radical | Reactive gas, not a free radical |
|
Main enzymatic
biosynthetic route(s) |
From L-arginine via nitric oxide synthases. |
From heme via heme oxygenases. |
From L-cysteine, via CSE, CBS and 3-MPST. |
| Metabolism, elimination | The main metabolites are nitrite and nitrate. Major elimination route for nitrite and nitrate: urine; minor elimination route for NO: exhaled air. |
Transiently binds to hemoglobin to form CO-Hb. A small fraction of CO oxidizes to form CO2. Main elimination route: via exhaled air. |
The main metabolites are thiosulfate, sulfite and sulfate. Main elimination route for sulfite, sulfate and thiosulfate: urine; minor elimination route for H2S: exhaled air. |
|
Intracellular effector
pathways |
cGMP-dependent protein kinases; modulation of enzymatic activity by posttranslational protein modifications via nitrosylation; variable effects on enzymes via reactions with heme groups of various enzymes; inhibition of cytochrome c oxidase. |
cGMP-mediated actions with lower potency; KCa channels; intracellular kinase pathways, inhibition of cytochrome c oxidase. |
Activation (opening) of KATP channels; inhibition of cytochrome c oxidase, inhibition of phosphodiesterase, modulation of intracellular calcium, modulation of signal transduction processes. |
|
Therapeutic effects as
inhaled gas |
Inhaled NO is an approved for the therapy of primary pulmonary hypertension of the newborn. Inhaled NO was shown to provide benefit in animal models of ARDS, pulmonary hypertension, sickle cell disease, circulatory shock, transplant rejection, vascular injury, heart failure and other diseases. |
Inhaled CO has been tested in COPD patients and entered into clinical studies for the therapy of graft dysfunction after renal transplantation. Inhaled CO was shown to provide benefit in animal models of ARDS, circulatory shock, transplant rejection, vascular injury and other diseases. |
In preclinical studies, inhaled H2S was shown to provide benefit in acute hypoxia, acute hemorrhagic shock, ventilator-induced lung injury and other diseases. |
|
Therapeutic effects as
liquid formulation |
Not explored. | A CO-containing lavage fluid was found to produce benefit in preclinical models of postoperative ileus. CO- containing preservation fluids improve the function of transplants in preclinical studies. |
Sodium sulfide for injection has entered Phase II clinical trials for cardioprotective effects. In preclinical studies, parenteral sodium sulfide or sodium hydrogen sulfide was shown to exert benefit in myocardial infarction, cardiopulmonary bypass, liver and kidney reperfusion injury, transplant rejection, ARDS and other diseases. |
|
Prodrugs requiring
conversion |
Organic nitrates (nitrovasodilator compounds of various classes) are in routine clinical use in the therapy of cardiac ischemia, cardiac failure, hypertension and other diseases. |
Methylene chloride (which is converted to CO in cells) is a preclinical research tool that provides benefit in animal models of organ failure and transplantation. |
Garlic-derived endogenous polysulfides release H2S and exert vasodilatory and cardioprotective effects in preclinical models. |
|
Spontaneously releasing
chemical donors |
NONOates, nitrosothiols, sydnonimines, and other classes of NO donors are in clinical use or in human trials. Preclinical studies demonstrate their efficacy in cardiac failure, hypertension, pulmonary hypertension, reperfusion injury, stroke, atherosclerosis and wound healing. |
CORMs (Carbon Monoxide Releasing Compounds) demonstrate preclinical efficacy in animal models of reperfusion injury, ARDS, organ transplantation and other diseases. They also exert antimicrobial effects. |
The water-soluble hydrogen sulfide-releasing molecule GYY4137 has been tested in animal models of hypertension and vascular disease. |
| Combined donors | NO-nonsteroidals, NO- steroids, NO-statins, NO- captopril and other combined NO donors exert benefits in various models of inflammation, cardiac and vascular disease; some of them have also been tested in clinical trials including naproxcinod (Phase III trials completed) for arthritis. |
Not explored. | Combined compounds with a sulfide-releasing moiety (e.g. various sulfo- nonsteroidals) demonstrate beneficial effects in preclinical models of inflammation and cardiovascular disease. |
|
Pharmacological
stimulation of the second messenger pathways activated by the gaseotransmitter |
PDE5 inhibitors are in clinical use for male erectile dysfunction, and have demonstrated therapeutic benefit in other cardiovascular diseases in preclinical and clinical studies. Riociguat and cinaciguat (direct activators and stimulators of the soluble guanylyl cyclase enzyme) have shown proof-of-concept efficacy in preclinical and clinical models of pulmonary hypertension and heart failure. |
Not explored. | |
|
Supplementing
substrate(s) or co-factors of the enzyme that produces the mediator |
L-arginine exerts beneficial effects in a variety of cardiovascular diseases in preclinical models and in some of the exploratory clinical studies. L-citrulline has been shown to be effective in preclinical and clinical models of pulmonary hypertension. Supplementation of tetrahydrobiopterin improves endothelium-dependent relaxations in preclinical models of vascular disease. |
Not explored. | Cysteine, the precursor of H2S in some cellular assays leads to H2S formation. Parenteral cysteine injection leads to an increase in the amount of exhaled H2S after parenteral administration in a rodent study. |
Figure 1.
Some of the intracellular effector pathways of NO, CO and H2S. All of these pathways are necessarily present in a single cell type at the same time.
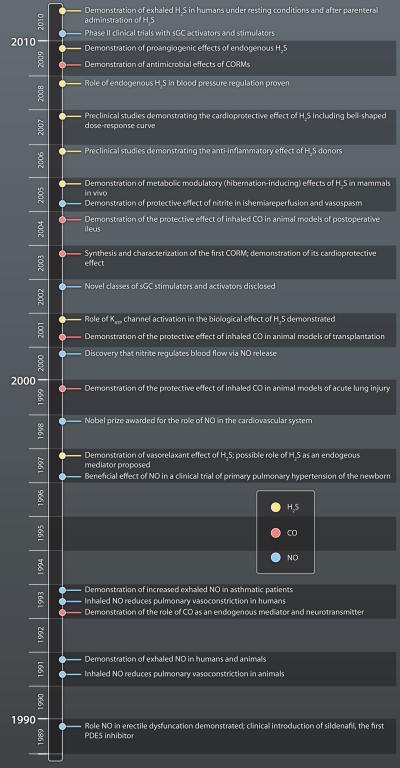
It is interesting to note that the first clinical introduction of a donor of a gaseotransmitter preceded the rational understanding of the underlying biological processes by many years. Nitroglycerin and organic nitrates have been used as vasodilator and anti-angina compounds for over a century, while the discovery that they release NO, which then activates an endogenous second messenger system (guanylyl cyclase/ cGMP) came about only in the 70’s (10). Furthermore, the realization that these drugs, in effect, are ‘replacement therapy’ for the vasodilator ‘endothelium-derived relaxing factor’ (EDRF), (i.e. nitric oxide) came in the late 80’s (1-3).
In the beginning, the phosphodiesterase 5 (PDE5) inhibitor, sildenafil, was studied in clinical trials aimed at cardiac protection. It was during these trials, that the compound was serendipitously found to elicit an unexpected biological effect: induction of an erectile response (11). The delineation of the compound’s exact cellular mode of action only occurred subsequently. Detailed understanding of this mechanism (which involves the inhibition of PDE5 leading to inhibition of cGMP degradation in the corpus cavernosum smooth muscle), which, in effect, is an enhancement of the biological response to endogenously produced NO) became essential for the rational design and development of next-generation PDE5 inhibitors (12).
A third clinical example is the use of inhaled NO gas for the therapy of pulmonary hypertension. In this case, introduction of this drug into clinical use was the result of a rational understanding-based drug testing / drug development approach. Fundamental laboratory research observations in the mid 90’s suggested that there are insufficient amounts of biologically active NO in the pulmonary vasculature in animals with severe pulmonary hypertension. It was hypothesized, therefore, that therapeutic replacement of this ‘missing’ NO, via the inhalation route, may provide significant benefit in newborn infants with primary pulmonary hypertension. This hypothesis was subsequently confirmed, first in preclinical and later in a series of clinical studies (13).
Many aspects of the pharmacology and experimental therapy of gaseotransmitters are radically different from the characteristics of small molecule therapeutics. These unique aspects provide interesting opportunities and challenges for basic research and translational science and are overviewed in the subsequent sections.
Size matters
The three gaseotransmitters represent some of the smallest, biologically active molecules. They diffuse easily through cell membranes, reaching their multiple intracellular targets. Inhalation of these gases can produce significant local (intrapulmonary) actions, and, in some cases, systemic effects as well. In the case of inhaled NO, the original hypothesis was that majority of the biological effects will occur inside the lung tissue (producing selective pulmonary vasodilatation), because the reaction of NO with hemoglobin after reaching the blood was thought to inactivate the molecule (13). However, subsequent work demonstrated that the oxidation products of NO (nitrite, nitrate) as well as some of its other reaction products (e.g. S-nitrosothiols) can exert remote biological effects. Thus, inhaled NO has recently been shown to exert beneficial effects in exploratory trials in patients with liver transplantation or limb ischemia (14,15). In the case of CO, the inhaled gas binds to hemoglobin in the red blood cells, which may serve as a CO-carrier, delivering CO to the tissues (16,17). In the case of inhaled H2S, a recent study compared the levels of biologically active reactive sulfide species in the blood in response to inhaled H2S vs. infusion of a H2S donating formulation, thereby establishing a de facto bioequivalency between the two modes of delivery (18).
Endogenous mediators
In theory, endogenous molecules may have a distinct advantage when applied as therapeutics, because the body ‘already knows’ how to ‘deal with them’: the cellular responses are predictable, and specific elimination pathways are present. In some way, gaseotransmitter therapy may be viewed as ‘hormone replacement therapy’ of an exceptional kind. Nevertheless, as with all therapeutic agents (including hormone therapy, and especially with gases that can be both viewed as toxic agents and as biological regulators) it is important to determine, at which point do these elimination pathways become overwhelmed, and when does the therapeutic response turn into a toxicological effect. Since all three gaseotransmitters are produced endogenously (as part of the human body’s normal physiology), there are significant basal levels of these gases in many cells and tissues. This is obviously quite different from the scenario encountered with most synthetic small molecule drugs. An added complexity is that endogenous levels of all three gaseotransmitters may increase or decrease in various disease conditions. One one hand, these changes may be interesting for diagnostic purposes. On the other hand, the possibility exists that such changes may affect the overall biological response to the exogenously administered gaseotransmitter. For instance, it is well known that the vasodilator response to NO donors is potentiated in blood vessels in response to pharmacological inhibition of endogenously produced NO (19).
Taking the fork in the road
“When you come to a fork in the road, take it” is a piece of convoluted advice attributed to baseball legend Yogi Berra. Typically, one of the key requirements for small molecule therapeutic agents is to have a clearly defined, specific mode of action (e.g. blockade of a single receptor subtype, or selective inhibition of a particular intracellular enzyme isoform). In contrast, gaseotransmitters are natural multitaskers: they have evolved to act on multiple cellular targets, in order to induce a complex and coordinated biological response. The multiple cellular targets and pathways affected by the gaseotransmitters (in many cases, in a concentration-dependent and cell/tissue-dependent fashion) can create unique challenges for translating the laboratory observations into therapeutic applications.
Key checkpoints in the biological effects of gaseotransmitters are amenable to therapeutic intervention. For NO, the principal pathway of smooth muscle relaxation (and hence, increase of blood flow, lowering of blood pressure or penile erection, depending on the type of smooth muscle involved) involves the activation of the soluble guanylyl cyclase (sGC) enzyme by interacting with an iron molecule on its heme prostethic group (followed by the elevation of intracellular levels of cGMP, resulting in the activation of cGMP-dependent protein kinases, or S-nitrosylation of proteins) (1-3). In addition to measuring the physiological responses, various parameters (biomarkers) related to these pathways can be monitored (for instance, circulating cGMP levels) (20).
The cytoprotective/anti-inflammatory effects of NO (as well as of CO and H2S) involve multiple more complex pathways. This underlines the importance of appropriate biomarker identification. At high local concentrations, the cytotoxic effects of NO, as well as CO and H2S involve several interrelated pathways, including a direct inhibition of mitochondrial respiration (3-8). In the case of NO, an additional pathway of cytotoxicity involves the formation of peroxynitrite (a highly reactive species formed by its rapid reaction with superoxide) (21). Conceptualization of the therapeutic effect/toxic side effect concept may become particularly challenging when the therapeutic mode of action (e.g. interaction with cytoprotective/anti-inflammatory kinase pathways) and the toxicity (such as the inhibition of mitochondrial respiration) stems from the very same molecular target. For instance, in the case of CO, it has been demonstrated that the inhibition of cytochrome c oxidase (followed by inhibition of mitochondrial respiration, and generation of reactive oxygen species) is responsible for both the toxic effects of this gas and for the therapeutic modulation of anti-inflammatory signal transduction (22). In such cases, the direction of the net biological effect (protection or toxicity) may be a function of the degree of cytochrome c inhibition.
Remember Paracelsus!
A characteristic aspect of gaseotransmitter pharmacology is the bell-shaped dose-response relationship curve. The beneficial (e.g. cytoprotective, anti-inflammatory) effects of NO, CO or H2S typically occur at low (near-physiological) concentrations, while at higher concentrations the effects diminish, and toxicity may ensue. For instance, murine studies demonstrated that the reduction of myocardial infarct size by nitrite (a precursor of NO) or by H2S occurs at certain, well-defined doses: upon further elevation of the dose, the protection is lost, or the compound may even worsen the outcome (23,24). Of course, we know since the times of Paracelsus (1493-1541) that “all drugs are poisons; the benefit depends on the dosage”. But it seems that the exact definition of the therapeutic dose range, and careful monitoring for signs of potential toxicity at higher exposures is especially relevant for gaseotransmitters like NO, CO and H2S.
It has been already mentioned that the toxicology of all three gases has been extensively studied for many decades (in the context of environmental and/or medical sciences). Nevertheless, the very chemical nature of NO, CO or H2S may lead to reservations against the general idea of using such ‘well-known toxic gases’ (or compounds that contain/release them) for therapeutic purposes.
“It’s a gas, man!”
The gaseous nature of NO, CO and H2S lends a convenient mode of delivery via inhalation. In fact, currently the only FDA-approved use of an inhaled therapeutic gaseous drug is inhaled NO, which is the first line of therapy for primary pulmonary hypertension of the newborn (13). The lung, however, represents a ‘two-way street’ for gaseotransmitters. In all three cases (NO, CO and H2S), healthy humans exhale significant amounts of these gases (5,6,25,26); this can be used either as a potential diagnostic tool (25), or a way to monitor exposure to infusion of prodrugs of the gaseous transmitters, as recently shown in the case of intravenously administered H2S (27).
During gaseotransmitter therapy, the therapeutic ‘agent’ is the gas itself. Thus, the delivery of the gaseous molecule (or a prodrug that releases it) may occur via inhalation or via oral or parenteral administration, while the actual biological effect is mediated by a soluble gas, which diffuses into the various compartments of the body. The unique characteristics of gaseotransmitters require a complex integration of pharmacology and biology, when thinking about dose-responses and biological exposures.
In certain cases, targeting certain downstream effector pathways of gaseotransmitters with ‘classical’ small molecule drug candidates represents a viable alternative approach to the direct administration of the gaseotransmitters or their prodrugs. For instance, inhibitors of PDE5 enhance cGMP-mediated responses (11,12). The fact that a specific PDE isoform is present in the corpus cavernosum tissue provides the opportunity for tissue selectivity (thus avoiding overt systemic effects, such as lowering of blood pressure).
An emerging class of clinical-stage compounds (stimulators or activators of soluble guanylyl cyclase) (28-30) are being developed to either bind to the NO-responsive heme group of the sGC enzyme (or, in case of the oxidized form of the enzyme, which misses the heme group, to ‘mimic’ the conformation of a missing heme group on the enzyme, thereby re-activating the enzyme).
Stick it on!
Prodrugs and donors of NO have been used for many decades for the therapy of angina pectoris and hypertension, as well as many other systemic and topical indications (3,7). For CO, several series of boron- or ruthenium-based compounds (CORMs; Carbon Monoxide Releasing Molecules) have been synthesized in the last decade, in order to provide a parenteral or oral way of therapeutic CO administration. CORMs exert potent therapeutic effects in preclinical studies of ischemia/reperfusion injury and inflammation (7, 31).
A distinct approach for the therapeutic exploitation of NO and H2S relates to the synthesis of modified small molecules with NO- or H2S-releasing functional groups (9). This approach, with recently culminated in Phase III studies with Naproxcinod. The rationale for creating this compound was that incorporation of an NO-donor group into the structure of naproxen may (a) produce a local, NO-mediated vasodilatation, the compound would protect the stomach from the ulcerogenic effect of the parent compound and (b) may exert a slight, potentially therapeutic vasorelaxant/hypotensive effect (32). Although the US FDA has recently declined the NDA package for Naproxcinod (33), it is hoped that in the future, similar conceptual approaches may find their way into clinical use. Similar to the combined NO donors, H2S-releasing non-steroidal anti-inflammatories as well as H2S-releasing PDE inhibitors have been synthesized. Some of these compounds show unique pharmacological profiles such as a therapeutic benefit over the parent compound (9,34).
A life full of surprises
There are many examples where hitherto unknown, gaseotransmitter-related modes of action have been identified for various well-established drugs. For instance, part of the therapeutic effect of statins is now attributed to the upregulation and activation of the endothelial isoform of NO synthase, thereby improving cardiovascular function (35). Some of the therapeutic benefit of resveratrol (the cardioprotective component of red wine) is also mediated by a vascular NO-related pathway (36). The PDE5 inhibitor tadalafil is another interesting example: it has recently been shown that some of its biological effects in the heart occur via the stimulation of H2S production in vivo (37).
The rapid progress of basic and applied science in the field of gaseotransmitters is both exciting (for investigators working in the field) and challenging (for those who attempt to integrate the massive amount of rapidly changing information). As a result of intensive efforts from thousands of scientific groups worldwide, many well-established ‘gaseotransmitter truths’ needed substantial revisions. For instance, nitrite (considered an inactive metabolite of NO) has emerged as a biologically active ‘pro-drug’ for NO, opening up a wide range of therapeutic applications (23, 38). In addition, potent antibacterial effects of CORMs have been identified recently, with obvious therapeutic implications (39). A recent study demonstrates that topical administration of H2S promotes angiogenesis, opening potential therapeutic applications for wound healing (40). Another recent study identifies H2S as the ‘endogenous sildenafil’ (an inhibitor of PDE5, and an enhancer of the effects of endogenously produced NO) (41), a finding that will necessitate a certain re-evaluation of the biological roles of both NO and H2S.
The area of gaseotransmitters remains a rapidly progressing field of preclinical and clinical research. Translational work in this area requires a deep understanding of the biology and pharmacology of these gases, as well as an ability to integrate this scientific knowledge with the principles of drug development, including medicinal chemistry and clinical and regulatory aspects. It is safe to predict that continuing research in the area of gaseotransmitters will lead to the identification of additional new biological concepts with therapeutic and diagnostic potential.
References and notes
- 1.Furchgott RF. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276:1186–1188. [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ignarro LJ, editor. Nitric oxide - Biology and Pathobiology. Academic Press; 2009. [Google Scholar]
- 5.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 6.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 7.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 8.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–23. doi: 10.1089/ars.2009.2919. 2010. [DOI] [PubMed] [Google Scholar]
- 9.Szabo C. Burgers Medicinal Chemistry. Wiley Interscience; 2010. Medicinal chemistry and therapeutic applications of the gaseotransmitters nitric oxide, carbon monoxide and hydrogen sulfide. [Epub ahead of print] [Google Scholar]
- 10.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 2004;24:452–474. doi: 10.1007/s10540-005-2741-8. [DOI] [PubMed] [Google Scholar]
- 11.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J. Biol. Chem. 1999;274:13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 12.Dorsey P, Keel C, Klavens M, Hellstrom WJ. Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin. Pharmacother. 2010;11:1109–22. doi: 10.1517/14656561003698131. [DOI] [PubMed] [Google Scholar]
- 13.Bloch KD, Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc. Res. 2007;75:339–48. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J. Clin. Invest. 2007;117:2583–91. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathru M, Huda R, Solanki DR, Hays S, Lang JD. Inhaled nitric oxide attenuates reperfusion inflammatory responses in humans. Anesthesiology. 2007;106:275–82. doi: 10.1097/00000542-200702000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Shimazu T, Ikeuchi H, Sugimoto H, Goodwin CW, Mason AD, Jr, Pruitt BA., Jr. Half-life of blood carboxyhemoglobin after short-term and long-term exposure to carbon monoxide. J. Trauma. 2000;49:126–31. doi: 10.1097/00005373-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Owens EO. Endogenous carbon monoxide production in disease. Clin. Biochem. 2010 Jul 22; doi: 10.1016/j.clinbiochem.2010.07.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, Vandenekart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 2010;160:941–57. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncada S, Rees DD, Schulz R, Palmer RM. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2166–70. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conahey GR, Power GG, Hopper AO, Terry MH, Kirby LS, Blood AB. Effect of inhaled nitric oxide on cerebrospinal fluid and blood nitrite concentrations in newborn lambs. Pediatr. Res. 2008;64:375–80. doi: 10.1203/PDR.0b013e318180f08b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 23.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abba AA. Exhaled nitric oxide in diagnosis and management of respiratory diseases. Ann. Thorac. Med. 2009;4:173–81. doi: 10.4103/1817-1737.56009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insko MA, Deckwerth TL, Hill P, Toombs CF, Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br. J. Pharmacol. 2009;157:944–51. doi: 10.1111/j.1476-5381.2009.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toombs CF, Insko MA, Wintner EA, Deckwerth TL, Usansky H, Jamil K, Goldstein B, Cooreman M, Szabo C. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br. J. Clin. Pharmacol. 2010;69:626–36. doi: 10.1111/j.1365-2125.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SAK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Invest. 2006;116:2552–61. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin F, Baskaran P, Ma X, Dunten PW, Schaefer M, Stasch JP, Beuve A, van den Akker F. Structure of cinaciguat (BAY 58-2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase. J. Biol. Chem. 2010;285:22651–7. doi: 10.1074/jbc.M110.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motterlini R. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem. Soc. Trans. 2007;35:1142–6. doi: 10.1042/BST0351142. [DOI] [PubMed] [Google Scholar]
- 32.Geusens P. Naproxcinod, a new cyclooxygenase-inhibiting nitric oxide donator (CINOD) Expert Opin Biol Ther. 2009;9:649–57. doi: 10.1517/14712590902926071. [DOI] [PubMed] [Google Scholar]
- 33. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisDrugsAdvisoryCommittee/UCM211466.pdf.
- 34.Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effect of hydrogen sulfide: development of H2S-releasing drugs as pharmaceuticals. J. Med. Chem. 2010 May 12; doi: 10.1021/jm901638j. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Jacobson JR. Statins in endothelial signaling and activation. Antioxid. Redox Signal. 2009;11:811–21. doi: 10.1089/ars.2008.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson SK, Tucker GA, Brameld JM. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008;67:42–7. doi: 10.1017/S0029665108006009. [DOI] [PubMed] [Google Scholar]
- 37.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120(11 Suppl):S31–6. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 39.Desmard M, Davidge KS, Bouvet O, Morin D, Roux D, Foresti R, Ricard JD, Denamur E, Poole RK, Montravers P, Motterlini R, Boczkowski J. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J. 2009;23:1023–31. doi: 10.1096/fj.08-122804. [DOI] [PubMed] [Google Scholar]
- 40.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21972–7. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen Sulfide Is an Endogenous Inhibitor of Phosphodiesterase Activity. Arterioscler. Thromb. Vasc. Biol. 2010 Jul 15; doi: 10.1161/ATVBAHA.110.209783. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]