Abstract
Penicillium marneffei is a dimorphic fungus which is endemic in Southeast Asia. It is an opportunistic pathogen which has emerged to become an AIDS-defining illness in the endemic areas. Early diagnosis with prompt initiation of treatment is crucial for its management. Prompt diagnosis can often be established through careful cytological and histological examination of clinical specimens although microbiological culture remains the gold standard for its diagnosis. Standard antifungal treatment for AIDS patients with penicilliosis is well established. Highly active antiretroviral therapy should be started early together with the antifungal treatment. Special attention should be paid to potential drug interaction between antiretroviral and antifungal treatments. Secondary prophylaxis may be discontinued with a low risk of relapse of the infection once the immune dysfunction has improved.
1. Introduction
Penicillium marneffei was first discovered in 1959 by G. Segretain at the Pasteur Institute in Paris. The strain was isolated from bamboo rats dying of disseminated mycosis in Vietnam. The new species was named P. marneffei in honour of Hubert Marneffe, the Director of Pasteur Institute in Indochina [1, 2]. The first report of human infection due to P. marneffei was also reported by G. Segretain who accidentally pricked his finger with a needle containing the yeast cells of P. marneffei. A small nodule appeared at the site of infection followed by lymphangitis 9 days after the accident [3]. The first natural human infection was reported in 1973 from a patient with Hodgkin lymphoma who lived in Southeast Asia [4]. Before the first case was reported in 1988 in a patient infected with the human immunodeficiency virus (HIV) [5], human penicilliosis was uncommon with less than 40 cases reported in the Southeast Asia [6, 7]. However, the incidence of penicilliosis increased rapidly thereafter with the development of HIV pandemic and the infection became one of the commonest acquired immune deficiency syndrome (AIDS)-defining illnesses among HIV-positive patients in endemic areas [8–10].
2. Mycology
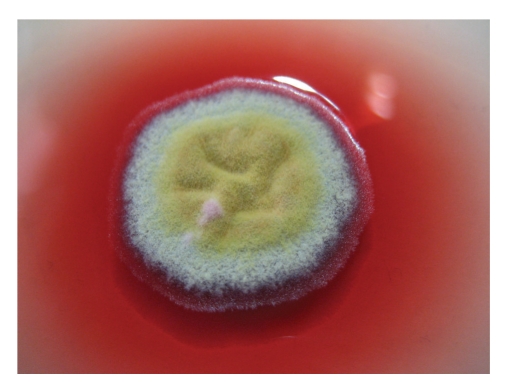
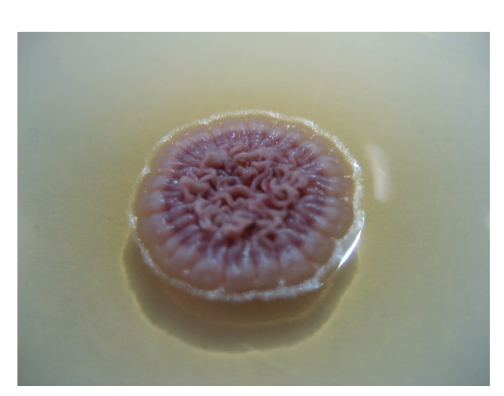
P. marneffei is the only dimorphic fungus in the genus of Penicillium. It exists in mycelial form at 25°C but yeast form at 37°C [1]. It shows a rapid growth rate and matures within 3 days at 25–30°C. Its growth is enhanced in Sabouraud dextrose agar but is inhibited by cycloheximide [11]. At 25°C, the colonies of P. marneffei are granular with shade of greenish-yellow colour and a characteristic red diffusible pigment (Figure 1). Little or no red diffusible pigment is produced at 35 to 37°C (Figure 2). Microscopically, the mold form is typical of other Penicillium species with hyaline septated hyphae and fruiting structures composing of branching metulae and phialides which produce spherical conidia in chains (Figure 3).
Figure 1.
Granular colony of P. marneffei with a characteristic red diffusible pigment on Sabouraud's dextrose agar after 7 days incubation at 25°C.
Figure 2.
Yeast-like colony of P. marneffei without red diffusible pigment on Sabouranud's dextrose agar after 7 days of incubation at 35°C.
Figure 3.
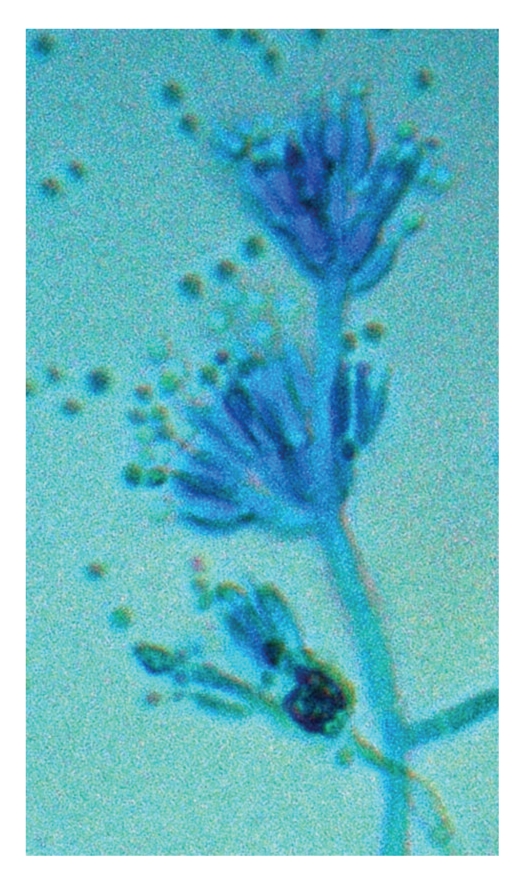
Microscopy of the mold form of P. marneffei showing septated hyaline hypae and fruiting structures composing of branching metulae and philiades with spherical condidia in chains (lactophenol cotton blue ×400).
3. Epidemiology
3.1. Endemicity
P. marneffei infection is endemic among HIV-positive patients in many areas in Southeast Asia, including Thailand, Vietnam, Hong Kong, Southern China, Taiwan, India, and Laos [6, 8, 9, 12–20]. So far, all reported cases of P. marneffei infections in AIDS patients have showed epidemiological link with the endemic areas except for one case reported in an African from Ghana who had never been to Southeast Asia [21]. Among the endemic areas, the greatest number of cases was reported in Northern Thailand, where penicilliosis is the third commonest AIDS-indicating disease among HIV-positive patients [8, 22, 23].
3.2. Natural Reservoir and Mode of Transmission
A lot is still unknown about the natural reservoir and route of transmission of P. marneffei. Human and bamboo rats are the only known animal hosts of P. marneffei. Four species of bamboo rats, Rhizomys sinensis, Rhizomys pruinosus, Rhizomys sumatrensis and Cannomys badius, are known to be enzootic reservoirs. The distribution of these bamboo rat species generally follows the distribution of endemicity of P. marneffei [6, 24–28].
It is not certain whether human infection is a result of exposure to infected animals or both bamboo rats and human get infected because of exposure to a common environmental source. The available information seems to suggest the latter. A case-control study in Northern Thailand comparing 80 cases of penicilliosis in patients with AIDS and 160 control patients with AIDS but without penicilliosis showed that exposure or consumption of bamboo rats was not a risk factor for P. marneffei infection. On the other hand, a recent history of occupational or other exposure to soil especially during rainy season was found to be a risk factor [29]. An airborne route of transmission through inhalation of conidia from an environmental source with subsequent dissemination to other body sites during immunosuppression has been postulated [13, 30, 31]. However, soil samples obtained from bamboo rat burrows and residential area of patients with penicilliosis were rarely positive for P. marneffei [25].
Penicilliosis was reported as a cause of laboratory-associated infection. As demonstrated by G. Segretain, localized infection was possible through direct inoculation of the fungus into the skin [1]. Another laboratory-acquired infection was reported in an undiagnosed HIV-positive physician who visited a laboratory where students were handling P. marneffei cultures on the open bench. He developed disseminated infection shortly after the exposure and the presumptive route of acquisition was inhalation [32]. The CDC has recommended Biosafety Level-2 (BSL-2) practices with containment equipment and facilities for propagating and manipulating P. marneffei cultures [33].
3.3. Incubation Period
The incubation period of P. marneffei infection has not been well defined. A report of a patient who lived in an nonendemic area but developed penicilliosis 11 years after visiting Hong Kong has suggested the possibility of a long latency with subsequent reactivation [34]. There is also evidence that primary infection might occur as P. marneffei infection can present early in young children who had acquired HIV perinatally [35].
3.4. Seasonality
A seasonal pattern of P. marneffei infection has been observed in Northern Thailand with increased incidence during the rainy seasons [29, 36]. As there should not be any seasonal variation in the degree of immunosuppression in HIV, the marked seasonality suggests that many of the infections are primary infection and that the heavy rainfall provides a favorable condition for the growth of the fungus, thus increasing the chance of exposure to susceptible host [36].
4. Pathology
The pathology of penicilliosis in different organs varies depending on the host immunity. Anergic and necrotizing tissue reaction are often observed in AIDS patients. Granuloma formation will help localize the infection and prevent further dissemination. Failure of this response in AIDS patients may explain the higher rate of disseminated disease [7–9, 13, 37].
The most frequent sites of involvement are liver and lungs but lymph node, bone marrow, skin and intestines are also affected. In the liver, histiocyte infiltration of the sinusoids and parenchyma is seen, and epithelioid granuloma may be found. Of interest, no correlation of the liver function test results with the histological changes has been observed [38]. In the lymph node, there is often lymphoid depletion with histiocytic proliferation and focal necrosis [39]. In the bone marrow, histiocytic proliferation can be prominent or subtle, with or without granuloma formation. Rarely, a histiocytic response is lacking [40]. Haemophagocytic syndrome has also been reported [41].
5. Clinical Feature
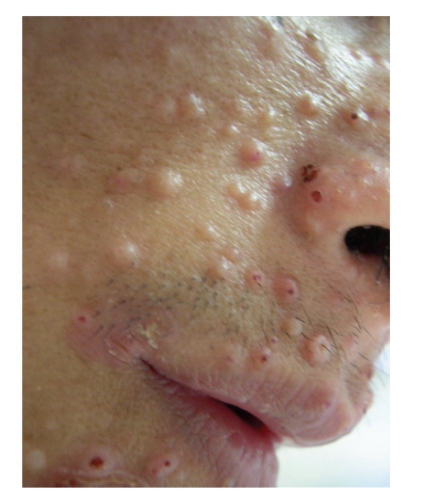
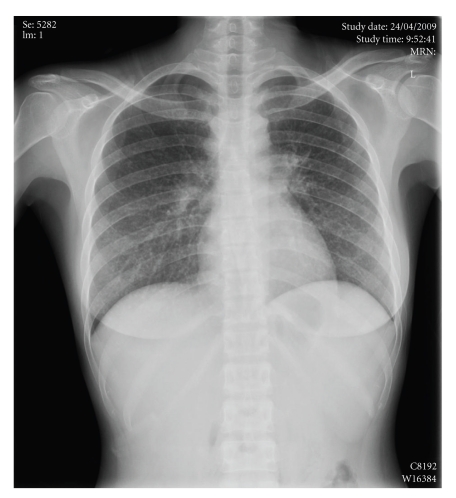
Penicilliosis is mostly seen in late HIV infection with CD4+ count less than 100/uL. Up to 80% or more of the cases have CD4+ count below 50/uL [8, 18, 42]. Table 1 summarizes the clinical features at presentation [8, 17, 18]. Most patients have constitutional symptoms with fever, weight loss and malaise. Skin manifestation such as subcutaneous abscesses and papule-like ulcers may be present [43]. Molluscum-contagiosum-like lesion is not infrequent (Figure 4). It is common to have signs and symptoms reflecting involvement of reticuloendothelial system including anaemia, hepatosplenomegaly and lympadenopathy. Respiratory involvement is often present, with productive cough, dyspnoea and haemoptyisis. Chest X-ray may show diffuse reticular infiltration (Figure 5), localized alveolar infiltrates or cavitary lesion [44]. Diarrhoea is not uncommon and sometimes may be bloody. The infection may rarely present as acute abdomen [45, 46]. Other presenting symptoms include osteoarthritis, genital ulcers and oral lesions [16, 47–51].
Table 1.
Symptomatology of penicilliosis in HIV-positive patients.
| Study location (number of subjects) |
|||
|---|---|---|---|
| Signs/symptoms | Thailand [8] | India [17] | Hong Kong [18] |
| (N = 80) | (N = 36) | (N = 47)* | |
| Fever | 93% | 97% | 96% |
| Skin lesion | 71% | 81% | 28% |
| Anaemia | 78% | 86% | 79% |
| Hepatomegaly | 51% | 39% | 28% |
| Splenomegaly | 16% | 15% | |
| Lymphadenopathy | 58% | 33% | 62% |
| Diarrhoea | 31% | 22% | 15% |
| Cough | 49% | — | 40% |
| Presence of other OIs | 55% | 77% | 57% |
OI: opportunistic infection.
*94% of the 47 subjects are confirmed HIV positive.
Figure 4.
Molluscum-contagiosum-like skin lesions associated with P. marneffei infection.
Figure 5.
Chest X-ray showing diffuse mottling of both lungs simulating military tuberculosis.
Central nervous system involvement is uncommon. A group from Vietnam has, however, reported the development of a syndrome of acute altered mental status with confusion, agitation, or depressed consciousness in the setting of subacute febrile illness [50]. Examination of the cerebrospinal fluid (CSF) could be normal, and abnormal cell count was seen only in one third of the cases. 71% had elevated CSF protein and 24% cases had a CSF glucose/serum glucose ratio <0.5. The disease course was rapidly progressive with a high mortality.
Since penicilliosis is usually seen in advanced stage of HIV infection, 55 to 77% of cases may have other concurrent opportunistic infections such as tuberculosis, disseminated herpes zoster, Pneumocystis jiroveci pneumonia, cryptococcosis, toxoplasmosis and should be watched out for [8, 17, 18].
6. Laboratory Diagnosis
6.1. Cytological and Histological Examination
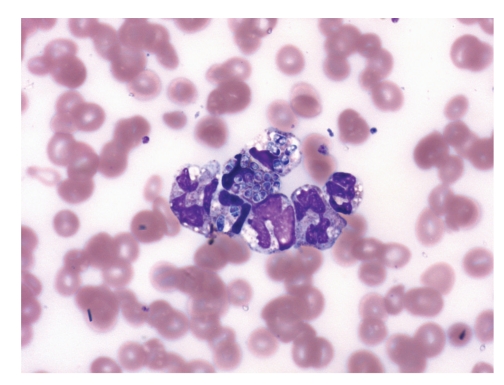
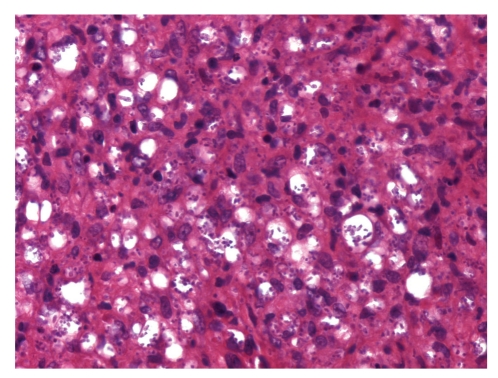
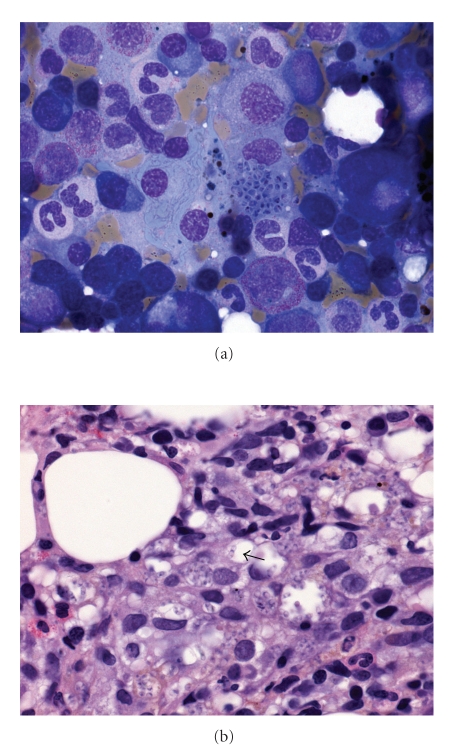
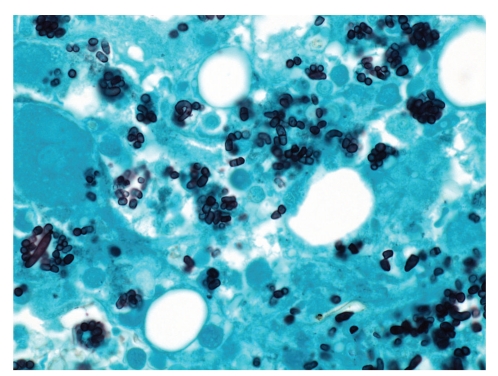
The diagnosis of penicilliosis may be suspected or made through examination of cytology or biopsy specimens. Cytology specimens are more readily obtained by less invasive procedures such as fine-needle aspiration of lymph nodes, sputum cytology and touch smear of skin [8, 37, 52–54]. For high grade fungaemia, yeast cells may be seen inside monocytes in peripheral blood smear (Figure 6) [40]. The yeast cells may be sparse or abundantly found in histiocytes or extracellularly (Figures 7 and 8), and are most readily demonstrated by fungal stains such as periodic acid-Schiff and silver methenamine stains (Figure 9). Detection of nonbudding yeast cells with characteristic central transverse septum would give a presumptive diagnosis which should be confirmed by microbiological culture.
Figure 6.
Peripheral blood monocytes with ingested yeast cells (May Grünwald Giemsa ×1000).
Figure 7.
Lymph node biopsy showing histiocytic proliferation with numerous round to oblong yeast cells (haematoxylin and eosin ×400).
Figure 8.
(a) Marrow aspirate showing a histiocyte engorged with yeast cells with reddish pink inclusions (May Grünwald Giemsa ×1000). (b) Trephine biopsy showing histiocytic proliferation with vague granuloma formation and ingested yeast cells. Some yeast cells have transverse septum (arrow) (haematoxylin and eosin ×400).
Figure 9.
Silver methenamine stain showing colonies of P. marneffei. Some yeast cells are oval to oblong in shape with a transverse septum (silver methenamine ×1000).
P. marneffei infection can sometimes be histologically occult, and the yeast cells may resemble cellular debris because of their size and staining pattern. Furthermore, granuloma formation may be absent because of anergic response in AIDS. Therefore, fungal stains such as silver methenamine stain should be performed on trephine biopsies in febrile AIDS patients from endemic area even in the absence of marrow granuloma [40].
A number of microorganisms have to be differentiated from P. marneffei on cytologic preparation or tissue section. Their distinguishing features are shown in Table 2. Histoplasma capsulatum is the commonest microorganism that may be confused with P. marneffei due to their similar size and staining properties. Distinction between them relies on the detection of central transverse septum which is characteristic of P. marneffei as it reproduced by binary fission or the demonstration of budding yeast cells which are typical of Histoplasma species. Epidemiologic link to area of endemicity of the two fungi can also aid in the diagnosis [40, 52, 53, 55].
Table 2.
Distinguishing features of microorganisms which may be confused with Penicillium marneffei in tissue examination [40, 56, 57].
| Similarities | Differences | |
|---|---|---|
| Histoplasma capsulatum | Yeast cells with similar staining properties and size Found within histiocytes and similar inflammatory response |
Budding instead of septated yeast cells |
|
| ||
| Pneumocystis jiroveci | Cyst form similar size stained positive silver methenamine stain | Round cysts containing single or paired comma shaped argyrophilic foci in walls |
|
| ||
| Leishmania spp. | Amastigotes within histiocytes in H&E section | Presence of bar shaped kinetoplasts within amastigotes seen under oil immersion, PAS stain negative |
|
| ||
| Toxoplasma gondii | May appear as intracellular organisms in H&E section or Giemsa stain | Size smaller, can be found within in other somatic cell types, not stained with silver methenamine |
H&E: haematoxylin and eosin; PAS: periodic acid-Schiff.
6.2. Microbiological Culture
Isolation of P. marneffei remains the gold standard for diagnosis. Among all the clinical specimens studied, the bone marrow gives the highest yield for culture, approaching 100%. This is followed by skin biopsy (90%) and blood culture (76%) [8]. HIV-positive patients with penicilliosis have a higher incidence of fungaemia when compared with HIV-negative patients [58, 59]. Both automated blood culture system and blood culture medium for mycobacterium tuberculosis are able to support the growth of P. marneffei [60]. The time to positivity for automated blood culture is around 4 days (range: 1.5–7 days) (personal observation). Although P. marneffei exists in yeast form at 37°C, septated hyphae-like structures but not yeast cells are detected in the initial gram smear taken from the positive blood culture (Figure 10). The hyphae structures will break down into arthroconidia-like yeast cells with time.
Figure 10.
Septated hyphae-like structures but not yeast forms are demonstrated in the initial gram smear taken from a positive blood culture (Gram ×1000).
6.3. Serology and Antigen Testing
Various types of antigen and antibody testing specific to P. marneffei have been described but they are not widely available [58, 61–67]. It is noted that HIV-positive patients with penicilliosis have a lower level of antibody and a higher level of antigen of P. marneffei when compared with HIV-negative patients penicilliosis [58]. Galactomannan assays for Aspergillus species is also known to detect the galactomannan of Penicillium species and can aid in the diagnosis of penicilliosis. Among 15 cases of penicilliosis in HIV patient, 73.3% was found to be positive with Platelia Aspergillus enzyme immunoassay kit (Bio-rad) with a median OD index of 4.419 [68]. In another series, almost 80% of penicilliosis patient was also found to be galactomannan positive by Pastorex Aspergillus testing (Bio-rad) with a median titre of 1 : 8 [18]. It is now our routine practice to screen all newly diagnosed HIV-patients with galactomannan testing for early detection of potential cases of penicilliosis.
6.4. Molecular Testing
PCR assay specific for P. marneffei has been developed in research setting but is not available for routine clinical use [69–73].
7. Treatment
7.1. Antifungal Susceptibility
There is no standardized technique or interpretation criteria for antifungal susceptibility testing for dimorphic fungus. The result of susceptibility testing in dimorphic fungus is influenced by the method, incubation duration, incubation condition and medium used. The inhibitory level of the same drug can be different against the yeast or the mycelial form of the same fungal isolate and the correlation between in vitro testing and in vivo efficacy is largely unknown [74–76].
P. marneffei is susceptible to 5-flucytosine and the azole group of antifungal agents including miconazole, ketoconazole and itraconazole. Fluconazole is the least active among the azoles in in vitro setting. Treatment response of the azoles appears to correlate well with in vitro result, being high with itraconazole but poor with fluconazole. Amphotericin B is clinically effective although in vitro susceptibility test often shows variable results [4, 74, 77].
For the newer antifungal agents, voriconazole has been shown to have activity comparable with that of itraconazole and the preliminary clinical data is encouraging [78, 79]. For the echinocandins, both anidulafungin and micafungin have some degree of activity against P. marneffei [76, 80]. In in vitro testing, micafungin was found to have synergistic effect with itraconazole and to a lesser degree with amphotericin B against P. marneffei [81]. However, it is still uncertain whether this can be translated to clinical management of human infection.
7.2. Antifungal Treatment
The mortality rate of untreated penicilliosis is 100% [77]. Any delay in the initiation of antifungal therapy is associated with poor outcome whereas the therapeutic response is good with early institution of treatment [8, 52, 77]. The recommended initial treatment for penicilliosis in HIV-positive patients is intravenous amphotericin B (0.6 mg/kg) for 2 weeks followed by oral itraconazole 400 mg per day for 10 weeks [82]. Treatment with itraconazole alone has also been shown to be effective but is associated with higher relapse rate [83]. It has been recommended that itraconazole alone 400 mg/day for 8 weeks could be considered for mild disease, followed by maintenance therapy with 200 mg per day to prevent relapse [82]. Oral itraconazole is available in capsule and solution form. Oral absorption of capsule is dependent on a low gastric pH and is enhanced by food or cola beverage [84, 85]. It can be erratically absorbed in patients with AIDS patients who may have a low gastric pH and therefore serum levels should be performed if available [86]. On the other hand, itraconazole solution had a more reliable absorption with an enhanced bioavailability but has to be taken on an empty stomach [87, 88].
Two other important issues on the clinical management of penicilliosis in HIV-positive patients require special attention. The first is drug interaction between antifungal and antiretroviral agents. A lot of antiretrovirals are known to interact with itraconazole. Itraconazole is a substrate of CYP3A4 but can also inhibit metabolism of many CYP3A4 substrates and increased their concentration. It is known to interact with protease inhibitors, and may increase the plasma concentration of indinavir, ritonavir and saquinavir. On the other hand, indinavir and ritonavir may also increase the plasma concentration of itraconazole [89]. Nonnucleoside reverse transcriptase inhibitors (NNRTIs) significantly reduce itraconazole concentration by promoting its metabolism [90]. Maraviroc, a CCR5 antagonist, is metabolized by CYP3A4 and therefore itraconazole may increase its concentration [91]. Most nucleoside reverse transcriptase inhibitors (NRTIs) and raltegravir, an integrase inhibitor, do not have significant interactions with itraconazole. It is important to check for drug interaction before starting the antifungal or antiretroviral agents.
The second issue is the optimal timing of initiation of HAART and the risk of development of immune restoration inflammatory syndrome (IRIS) after HAART. Penicilliosis is considered an AIDS-defining illness in endemic areas [8–10] and its diagnosis warrants initiation of HAART [92]. IRIS has only been uncommonly reported in patients with penicilliosis and usually occurred a month after the start of HAART [93–95]. Simultaneous initiation of HAART with antifungal or delayed initiation until the end of the 2 weeks of induction therapy of antifungal therapy can be considered [82]. HAART should not be withheld because of concern for possible development of IRIS. In case of severe symptomatic IRIS, a short-course of steroids may be considered [82].
8. Prevention
During the pre-HAART era, over half of patients developed relapse of penicilliosis within 6 months after discontinuation of antifungal treatment [83, 96]. Secondary prophylaxis with itraconazole 200 mg/day was shown to be well tolerated and highly effective with a reduction in relapse rate from 57% to 0% [96]. Therefore, it has been recommended that all patients who have completed treatment for penicilliosis should be put on secondary prophylaxis with itraconazole 200 mg/day [82].
With the introduction of HAART, there is growing data to suggest that secondary prophylaxis can be stopped after immune restoration [97, 98]. It is suggested that secondary prophylaxis can be stopped for patients who are receiving HAART and have a CD4 count >100/uL for over 6 months. However, secondary prophylaxis should be reintroduced if the penicilliosis relapses or the CD4 count falls below 100/uL [82].
Acknowledgment
The authors would like to offer their special thanks to Dr. T. C. Wu (Department of Medicine, Queen Elizabeth Hospital) for the clinical photo (Figure 4).
References
- 1.Segretain G. Penicillium marneffei n.sp., agent of a mycosis of the reticuloendothelial system. Mycopathologia et Mycologia Applicata. 1959;11(4):327–353. doi: 10.1007/BF02089507. [DOI] [PubMed] [Google Scholar]
- 2.Capponi M, Segretain G, Sureau P. Penicillosis from Rhizomys sinensis. Bulletin de la Société de Pathologie Exotique et de ses Filiales. 1956;49(3):418–421. [PubMed] [Google Scholar]
- 3.Segretain G. Penicillium marneffei n. sp., agent d'une mycose du systeme reticuloendothelial. Mycopathologia et Mycologia Applicata. 1959;11(4):327–353. doi: 10.1007/BF02089507. [DOI] [PubMed] [Google Scholar]
- 4.DiSalvo AF, Fickling AM, Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. American Journal of Clinical Pathology. 1973;60(2):259–263. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 5.Piehl MR, Kaplan RL, Haber MH. Disseminated penicilliosis in a patient with acquired immunodeficiency syndrome. Archives of Pathology and Laboratory Medicine. 1988;112(12):1262–1264. [PubMed] [Google Scholar]
- 6.Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clinical Microbiology Reviews. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drouhet E. Penicilliosis due to Penicillium marneffei: a new emerging systemic mycosis in AIDS patients travelling in China and Southeast Asia. Journal de Mycologie Médicale. 1993;4:195–224. [Google Scholar]
- 8.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344(8915):110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 9.Duong TA. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clinical Infectious Diseases. 1996;23(1):125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Wong KH, Lee SS, Lo YC, et al. Profile of opportunistic infections among HIV-1 infected people in Hong Kong. Zhonghua Yi Xue Za Zhi. 1995;55(2):127–136. [PubMed] [Google Scholar]
- 11.Larone DH. Medically Important Fungi. A Guide to Identification. 4th edition. Washington, DC, USA: ASM Press; 2002. [Google Scholar]
- 12.Clyti E, Sayavong K, Monchy D, Chanthavisouk K. Penicilliosis in laos. Presse Medicale. 2006;35(3, part 1):427–429. doi: 10.1016/s0755-4982(06)74610-3. [DOI] [PubMed] [Google Scholar]
- 13.Deng Z, Ribas JL, Gibson DW, Connor DH. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Reviews of Infectious Diseases. 1988;10(3):640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 14.Clezy K, Sirisanthana T, Sirisanthana V, Brew B, Cooper DA. Late manifestations of HIV in Asia and the Pacific. AIDS. 1994;8(2):S35–S43. [PubMed] [Google Scholar]
- 15.Singh PN, Ranjana K, Indiver Singh Y, et al. Indigenous disseminated Penicillium marneffei infection in the state of Manipur, India: report of four autochthonous cases. Journal of Clinical Microbiology. 1999;37(8):2699–2702. doi: 10.1128/jcm.37.8.2699-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louthrenoo W, Thamprasert K, Sirisanthana T. Osteoarticular Penicilliosis marneffei. A report of eight cases and review of the literature. British Journal of Rheumatology. 1994;33(12):1145–1150. doi: 10.1093/rheumatology/33.12.1145. [DOI] [PubMed] [Google Scholar]
- 17.Ranjana KH, Priyokumar K, Singh TJ, et al. Disseminated Penicillium marneffecei infection among HIV-infected patients in Manipur state, India. Journal of Infection. 2002;45(4):268–271. doi: 10.1053/jinf.2002.1062. [DOI] [PubMed] [Google Scholar]
- 18.Wu TC, Chan JWM, Ng CK, Tsang DNC, Lee MP, Li PCK. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Medical Journal. 2008;14(2):103–109. [PubMed] [Google Scholar]
- 19.vu Hai V, Ngo AT, Ngo VH, et al. Penicilliosis in Vietnam: a series of 94 patients. Revue de Medecine Interne. 2010;31(12):812–818. doi: 10.1016/j.revmed.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Sar B, Boy S, Keo C, et al. In vitro antifungal-drug susceptibilities of mycelial and yeast forms of Penicillium marneffei isolates in Cambodia. Journal of Clinical Microbiology. 2006;44(11):4208–4210. doi: 10.1128/JCM.00902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Y, Tintelnot K, Lippert U, Hoppe T. Disseminated Penicillium marneffei infection in an African AIDS patient. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94(2):p. 187. doi: 10.1016/s0035-9203(00)90271-2. [DOI] [PubMed] [Google Scholar]
- 22.Subsai K, Kanoksri S, Siwaporn C, Helen L. Neurological complications in AIDS patients: the 1-year retrospective study in Chiang Mai University, Thailand. European Journal of Neurology. 2004;11(11):755–759. doi: 10.1111/j.1468-1331.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 23.Sirisanthana T, Supparatpinyo K. Epidemiology and management of penicilliosis in human immunodeficiency virus-infected patients. International Journal of Infectious Diseases. 1998;3(1):48–53. doi: 10.1016/s1201-9712(98)90095-9. [DOI] [PubMed] [Google Scholar]
- 24.Ajello L, Padhye AA, Sukroongreung S, Nilakul CH, Tantimavanic S. Occurrence of Penicillium marneffei infections among wild bamboo rats in Thailand. Mycopathologia. 1995;131(1):1–8. doi: 10.1007/BF01103897. [DOI] [PubMed] [Google Scholar]
- 25.Chariyalertsak S, Vanittanakom P, Nelson KE, Sirisanthana T, Vanittanakom N. Rhizomys sumotrensis and Connomys bodius, new natural animal hosts of Penicillium marneffei. Journal of Medical and Veterinary Mycology. 1996;34(2):105–110. [PubMed] [Google Scholar]
- 26.Deng ZL, Yun M, Ajello L. Human penicilliosis marneffei and its relation to the bamboo rat (Rhizomys pruinosus) Journal of Medical and Veterinary Mycology. 1986;24(5):383–389. doi: 10.1080/02681218680000581. [DOI] [PubMed] [Google Scholar]
- 27.Gugnani H, Fisher MC, Paliwal-Johsi A, Vanittanakom N, Singh I, Yadav PS. Role of Cannomys badius as a natural animal host of Penicillium marneffei in India. Journal of Clinical Microbiology. 2004;42(11):5070–5075. doi: 10.1128/JCM.42.11.5070-5075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Pan L, Wu S. Mycologic investigation on Rhizomys pruinous senex in Guangxi as natural carrier with Penicillium marneffei. Chinese Medical Journal. 1989;102(6):477–485. [PubMed] [Google Scholar]
- 29.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in Northern Thailand. Clinical Infectious Diseases. 1997;24(6):1080–1086. doi: 10.1086/513649. [DOI] [PubMed] [Google Scholar]
- 30.Ungpakorn R. Cutaneous manifestations of Penicillium marneffei infection. Current Opinion in Infectious Diseases. 2000;13(2):129–134. doi: 10.1097/00001432-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Jayanetra P, Nitiyanant P, Ajello L. Penicilliosis marneffei in Thailand: report of five human cases. American Journal of Tropical Medicine and Hygiene. 1984;33(4):637–644. doi: 10.4269/ajtmh.1984.33.637. [DOI] [PubMed] [Google Scholar]
- 32.Hilmarsdottir I, Coutellier A, Elbaz J, et al. A French case of laboratory-acquired disseminated Penicillium marneffei infection in a patient with AIDS. Clinical Infectious Diseases. 1994;19(2):357–358. doi: 10.1093/clinids/19.2.357. [DOI] [PubMed] [Google Scholar]
- 33.CDC. Biosafety in Microbiological and Biomedical Laboratories (BMBL) Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 34.Jones PD, See J. Penicillium marneffei infection in patients infected with human immunodeficiency virus: late presentation in an area of nonendemicity. Clinical Infectious Diseases. 1992;15(4):p. 744. doi: 10.1093/clind/15.4.744. [DOI] [PubMed] [Google Scholar]
- 35.Sirisanthana V, Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pediatric Infectious Disease Journal. 1995;14(11):935–940. doi: 10.1097/00006454-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Nelson KE. Seasonal variation of disseminated Penicillium marneffei infections in northern Thailand: a clue to the reservoir? Journal of Infectious Diseases. 1996;173(6):1490–1493. doi: 10.1093/infdis/173.6.1490. [DOI] [PubMed] [Google Scholar]
- 37.Lim D, Lee YS, Chang AR. Rapid diagnosis of Penicillium marneffei infection by fine needle aspiration cytology. Journal of Clinical Pathology. 2006;59(4):443–444. doi: 10.1136/jcp.2004.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yousukh A, Jutavijittum P, Pisetpongsa P, et al. Clinicopathologic study of hepatic Penicillium marneffei in Northern Thailand. Archives of Pathology and Laboratory Medicine. 2004;128(2):191–194. doi: 10.5858/2004-128-191-CSOHPM. [DOI] [PubMed] [Google Scholar]
- 39.Tsui WMS, Ma KF, Tsang DNC. Disseminated Penicilium marneffei infection in HIV-infected subject. Histopathology. 1992;20(4):287–293. doi: 10.1111/j.1365-2559.1992.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 40.Wong KF. Marrow penicilliosis: a readily missed diagnosis. American Journal of Clinical Pathology. 2010;134(2):214–218. doi: 10.1309/AJCPWVBQCW13DJLO. [DOI] [PubMed] [Google Scholar]
- 41.Pei SN, Lee CH, Liu JW. Clinical case report: hemophagocytic syndrome in a patient with acquired immunodeficiency syndrome and acute disseminated penicilliosis. American Journal of Tropical Medicine and Hygiene. 2008;78(1):11–13. [PubMed] [Google Scholar]
- 42.Antinori S, Gianelli E, Bonaccorso C, et al. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. Journal of Travel Medicine. 2006;13(3):181–188. doi: 10.1111/j.1708-8305.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- 43.Cooper CR, Jr., McGinnis MR. Pathology of Penicillium marneffei: an emerging acquired immunodeficiency syndrome-related pathogen. Archives of Pathology and Laboratory Medicine. 1997;121(8):798–804. [PubMed] [Google Scholar]
- 44.Deesomchok A, Tanprawate S. A 12-case series of Penicillium marneffei pneumonia. Journal of the Medical Association of Thailand. 2006;89(4):441–447. [PubMed] [Google Scholar]
- 45.Ko CI, Hung CC, Chen MY, Hsueh PR, Hsiao CH, Wong JM. Endoscopic diagnosis of intestinal penicilliosis marneffei: report of three cases and review of the literature. Gastrointestinal Endoscopy. 1999;50(1):111–114. doi: 10.1016/s0016-5107(99)70359-7. [DOI] [PubMed] [Google Scholar]
- 46.George I, Sudarsanam T, Pulimood A, Mathews M. Acute abdomen: an unusual presentation of disseminated Penicillium marneffei infection. Indian Journal of Medical Microbiology. 2008;26(2):180–182. doi: 10.4103/0255-0857.40538. [DOI] [PubMed] [Google Scholar]
- 47.Annam V, Inamadar AC, Palit A, Koppad M, Peerapur BV, Yelikar BR. Genital ulcer caused by Penicillium marneffei in an HIV-infected patient. Sexually Transmitted Infections. 2007;83(3):249–250. doi: 10.1136/sti.2006.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiewchanvit S, Mahanupab P, Hirunsri P, Vanittanakom N. Cutaneous manifestations of disseminated Penicillium marneffei mycosis in five HIV-infected patients. Mycoses. 1991;34(5-6):245–249. doi: 10.1111/j.1439-0507.1991.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 49.Tong ACK, Wong MH, Smith NJT. Penicillium marneffei infection presenting as oral ulcerations in a patient infected with human immunodeficiency virus. Journal of Oral and Maxillofacial Surgery. 2001;59(8):953–956. doi: 10.1053/joms.2001.25881. [DOI] [PubMed] [Google Scholar]
- 50.Kantipong P, Panich V, Pongsurachet V, Watt G. Hepatic penicilliosis in patients without skin lesions. Clinical Infectious Diseases. 1998;26(5):1215–1217. doi: 10.1086/520282. [DOI] [PubMed] [Google Scholar]
- 51.Louthrenoo W. Musculoskeletal manifestations of HIV infection in Thailand: an analysis of 100 cases. Journal of Clinical Rheumatology. 1997;3(5):258–268. doi: 10.1097/00124743-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Jan IS, Chung PF, Wang JY, Weng MH, Hung CC, Lee LIN. Cytological diagnosis of Penicillium marneffei infection. Journal of the Formosan Medical Association. 2008;107(6):443–447. doi: 10.1016/S0929-6646(08)60151-5. [DOI] [PubMed] [Google Scholar]
- 53.Chaiwun B, Khunamornpong S, Sirivanichai C, et al. Lymphadenopathy due to Penicillium marneffei infection: diagnosis by fine needle aspiration cytology. Modern Pathology. 2002;15(9):939–943. doi: 10.1097/01.MP.0000027203.44333.95. [DOI] [PubMed] [Google Scholar]
- 54.Ma KF, Tsui MS, Tsang DNC. Fine needle aspiration diagnosis of Penicillium marneffei infection. Acta Cytologica. 1991;35(5):557–559. [PubMed] [Google Scholar]
- 55.Deng Z, Connor DH. Progressive disseminated penicilliosis caused by Penicillium marneffei. Report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. American Journal of Clinical Pathology. 1985;84(3):323–327. doi: 10.1093/ajcp/84.3.323. [DOI] [PubMed] [Google Scholar]
- 56.Connor DH. Pathology of Infectious Diseases. Stamford, Conn, USA: Appleton & Lange; 1997. [Google Scholar]
- 57.Garcia LS. Diagnostic Medical Parasitology. 5th edition. Washington, DC, USA: ASM Press; 2007. [Google Scholar]
- 58.Wong SSY, Wong KH, Hui WT, et al. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. Journal of Clinical Microbiology. 2001;39(12):4535–4540. doi: 10.1128/JCM.39.12.4535-4540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang JQ, Yang ML, Zhong XN, et al. A comparative analysis of the clinical and laboratory characteristics in disseminated penicilliosis marneffei in patients with and without human immunodeficiency virus infection. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31(10):740–746. [PubMed] [Google Scholar]
- 60.Taniguchi T, Ogawa Y, Kasai D, et al. Three cases of fungemia in HIV-infected patients diagnosed through the use of mycobacterial blood culture bottles. Internal Medicine. 2010;49(19):2179–2183. doi: 10.2169/internalmedicine.49.3811. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman L, Standard PG, Jalbert M, Kantipong P, Limpakarnjanarat K, Mastro TD. Diagnostic antigenemia tests for Penicilliosis marneffei. Journal of Clinical Microbiology. 1996;34(10):2503–2505. doi: 10.1128/jcm.34.10.2503-2505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chongtrakool P, Chaiyaroj SC, Vithayasai V, et al. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. Journal of Clinical Microbiology. 1997;35(9):2220–2223. doi: 10.1128/jcm.35.9.2220-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao L, Chen DL, Lee C, et al. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. Journal of Clinical Microbiology. 1998;36(10):3028–3031. doi: 10.1128/jcm.36.10.3028-3031.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao L, Chan KM, Chen D, et al. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. Journal of Clinical Microbiology. 1999;37(4):981–986. doi: 10.1128/jcm.37.4.981-986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desakorn V, Smith MD, Walsh AL, et al. Diagnosis of Penicillium marneffei infection by quantitation of urinary antigen by using an enzyme immunoassay. Journal of Clinical Microbiology. 1999;37(1):117–121. doi: 10.1128/jcm.37.1.117-121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desakorn V, Simpson AJH, Wuthiekanun V, et al. Development and evaluation of rapid urinary antigen detection tests for diagnosis of Penicilliosis marneffei. Journal of Clinical Microbiology. 2002;40(9):3179–3183. doi: 10.1128/JCM.40.9.3179-3183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuen K, Wong SS, Tsang DN, Chau P. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344(8920):444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]
- 68.Huang YT, Hung CC, Liao CH, Sun HY, Chang SC, Chen YC. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. Journal of Clinical Microbiology. 2007;45(9):2858–2862. doi: 10.1128/JCM.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pornprasert S, Praparattanapan J, Khamwan C, et al. Development of TaqMan real-time polymerase chain reaction for the detection and identification of Penicillium marneffei. Mycoses. 2009;52(6):487–492. doi: 10.1111/j.1439-0507.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- 70.Vanittanakom N, Vanittanakom P, Hay RJ. Rapid identification of Penicillium marneffei by PCR-based detection of specific sequences on the rRNA gene. Journal of Clinical Microbiology. 2002;40(5):1739–1742. doi: 10.1128/JCM.40.5.1739-1742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanittanakom N, Merz WG, Sittisombut N, Khamwan C, Nelson KE, Sirisanthana T. Specific identification of Penicillium marneffei by a polymerase chain reaction/hybridization technique. Medical Mycology. 1998;36(3):169–175. [PubMed] [Google Scholar]
- 72.Pongpom M, Sirisanthana T, Vanittanakom N. Application of nested PCR to detect Penicillium marneffei in serum samples. Medical Mycology. 2009;47(5):549–553. doi: 10.1080/13693780802484875. [DOI] [PubMed] [Google Scholar]
- 73.LoBuglio KF, Taylor JW. Phylogeny and PCR identification of the human pathogenic fungus Penicillium marneffei. Journal of Clinical Microbiology. 1995;33(1):85–89. doi: 10.1128/jcm.33.1.85-89.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boon-Long J, Mekha N, Poonwan N, et al. In vitro antifungal activity of the new triazole DO 870 against Penicillium marneffei compared with that of amphotericin B, fluconazole, itraconazole, miconazole and flucytosine. Mycoses. 1996;39(11-12):453–456. doi: 10.1111/j.1439-0507.1996.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 75.Sekhon AS, Garg AK, Padhye AA, Hamir Z. In vitro susceptibility of mycelial and yeast forms of Penicillium marneffei to amphotericin B, fluconazole, 5-fluorocytosine and itraconazole. European Journal of Epidemiology. 1993;9(5):553–558. doi: 10.1007/BF00209535. [DOI] [PubMed] [Google Scholar]
- 76.Nakai T, Uno J, Ikeda F, Tawara S, Nishimura K, Miyaji M. In vitro antifungal activity of micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrobial Agents and Chemotherapy. 2003;47(4):1376–1381. doi: 10.1128/AAC.47.4.1376-1381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Supparatpinyo K, Nelson KE, Merz WG, et al. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrobial Agents and Chemotherapy. 1993;37(11):2407–2411. doi: 10.1128/aac.37.11.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radford SA, Johnson EM, Warnock DW. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrobial Agents and Chemotherapy. 1997;41(4):841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Supparatpinyo K, Schlamm HT. Voriconazole as therapy for systemic Penicillium marneffei infections in AIDS patients. American Journal of Tropical Medicine and Hygiene. 2007;77(2):350–353. [PubMed] [Google Scholar]
- 80.Odabasi Z, Paetznick VL, Rodriguez JR, Chen E, Ostrosky-Zeichner L. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrobial Agents and Chemotherapy. 2004;48(5):1912–1915. doi: 10.1128/AAC.48.5.1912-1915.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao C, Liu W, Li R, Wan Z, Qiao J. In vitro interactions of micafungin with amphotericin B, itraconazole or fluconazole against the pathogenic phase of Penicillium marneffei. Journal of Antimicrobial Chemotherapy. 2009;63(2):340–342. doi: 10.1093/jac/dkn494. [DOI] [PubMed] [Google Scholar]
- 82.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Morbidity and Mortality Weekly Report. 2009;58, RR.4 [PubMed] [Google Scholar]
- 83.Supparatpinyo K, Hirunsri P, Uthammachai C, et al. An efficacy study of itraconazole in the treatment of Penicillium marneffei infection. Journal of the Medical Association of Thailand. 1992;75(12):688–691. [PubMed] [Google Scholar]
- 84.Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectios Diseases. 7th edition. Philadelphia, Pa, USA: Churchill Livingstone; 2010. [Google Scholar]
- 85.Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. European Journal of Clinical Pharmacology. 1997;52(3):235–237. doi: 10.1007/s002280050280. [DOI] [PubMed] [Google Scholar]
- 86.Smith D, van de Velde V, Woestenborghs R, Gazzard BG. The pharmacokinetics of oral itraconazole in AIDS patients. Journal of Pharmacy and Pharmacology. 1992;44(7):618–619. doi: 10.1111/j.2042-7158.1992.tb05478.x. [DOI] [PubMed] [Google Scholar]
- 87.Barone JA, Moskovitz BL, Guarnieri J, et al. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy. 1998;18(2):295–301. [PubMed] [Google Scholar]
- 88.Barone JA, Moskovitz BL, Guarnieri J, et al. Enhanced bioavailability of itraconazole in hydroxypropyl-β- cyclodextrin solution versus capsules in healthy volunteers. Antimicrobial Agents and Chemotherapy. 1998;42(7):1862–1865. doi: 10.1128/aac.42.7.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.AIDSinfo. Itraconazole. 2010, http://www.aidsinfo.nih.gov/DrugsNew/DrugDetailT.aspx?int_id=44&ClassID=0&TypeID=0.
- 90.Andrade RA, Evans RT, Hamill RJ, Zerai T, Giordano TP. Clinical evidence of interaction between itraconazole and nonnucleoside reverse transcriptase inhibitors in HIV-infected patients with disseminated histoplasmosis. Annals of Pharmacotherapy. 2009;43(5):908–913. doi: 10.1345/aph.1L624. [DOI] [PubMed] [Google Scholar]
- 91.Abel S, Russell D, Taylor-Worth RJ, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. British Journal of Clinical Pharmacology. 2008;65(1):27–37. doi: 10.1111/j.1365-2125.2008.03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, December 2009, http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 93.Gupta S, Mathur P, Maskey D, Wig N, Singh S. Immune restoration syndrome with disseminated Penicillium marneffei and cytomegalovirus co-infections in an AIDS patient. AIDS Research and Therapy. 2007;4, article 21 doi: 10.1186/1742-6405-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saikia L, Nath R, Biswanath P, Hazarika D, Mahanta J. Penicillium marneffei infection in HIV infected patients in Nagaland and immune reconstitution after treatment. Indian Journal of Medical Research. 2009;129(3):333–334. [PubMed] [Google Scholar]
- 95.Manosuthi W, Chaovavanich A, Tansuphaswadikul S, et al. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. Journal of Infection. 2007;55(5):464–469. doi: 10.1016/j.jinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Supparatpinyo K, Perriens J, Nelson KE, Sirisanthana T. A controlled trial of itraconazole to prevent relapse of Penicillium marneffei infection in patients infected with the human immunodeficiency virus. New England Journal of Medicine. 1998;339(24):1739–1743. doi: 10.1056/NEJM199812103392403. [DOI] [PubMed] [Google Scholar]
- 97.Sun HY, Chen MY, Hsiao CF, Hsieh SM, Hung CC, Chang SC. Endemic fungal infections caused by Cryptococcus neoformans and Penicillium marneffei in patients infected with human immunodeficiency virus and treated with highly active anti-retroviral therapy. Clinical Microbiology and Infection. 2006;12(4):381–388. doi: 10.1111/j.1469-0691.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 98.Chaiwarith R, Charoenyos N, Sirisanthana T, Supparatpinyo K. Discontinuation of secondary prophylaxis against penicilliosis marneffei in AIDS patients after HAART. AIDS. 2007;21(3):365–367. doi: 10.1097/01.aids.0000253374.19966.f9. [DOI] [PubMed] [Google Scholar]