Abstract
Mice with a deletion of the p53 gene have normal antibody titers against sheep red blood cells and normal switching to all Ig isotypes. In older mice (11 and 16 weeks old) the somatic hypermutation (SHM) frequencies are progressively reduced. In young mice (8 weeks old) with p53 deletion, the SHM frequencies are normal. However, the mutation pattern is changed in all p53−/− mice: mutations at A are increased. Surprisingly, deletion of the Ung2 gene in addition to the deletion of p53 corrected the A mutation frequencies to those of control mice. Known interactions of p53 protein with several proteins involved in error-prone BER during SHM may explain these findings. There is no indication that the absence of p53 affects the function of AID. Inactivation of p21 does not alter SHM, supporting the idea that the p53 protein is involved in SHM by its direct association with the SHM process. There is no significant change of mutations at T. Thus, the hypermutability at A is strand-biased (transcription? replication?). The translesion polymerase pol eta has so far been found to be the sole mutator at A and T in mice. However, the pattern in p53−/− mice is compatible with the possible inhibition by p53 of another translesion polymerase, pol iota, which in the absence of p53 may be recruited to error-prone repair of abasic sites in SHM.
Keywords: Somatic hypermutation, p53, AID, Ig genes, knockout mice, pol eta, pol iota
1. Introduction
The activation-induced cytosine deaminase (AID) initiates the process of somatic hypermutation (SHM) of Ig genes by creating uracils in the variable (V) region and flanking DNA sequences (reviewed in (Longerich et al., 2006). Many of the uracils are repaired in an error-free mode, however, an as yet unknown proportion of uracils are treated by invoking error-prone mechanisms (Liu et al., 2008; Longerich et al., 2006; Storb et al., 2009). Lesion-bypass polymerases introduce mutations at the uridine and sequences within a dozen or so nucleotides in the vicinity of the uridine (Longerich et al., 2006). Both mismatch repair (MMR) and base excision repair (BER) proteins are involved in the error-processes (Longerich et al., 2006). Nucleotide excision repair has not been found to play a role in SHM (Kim et al., 1997).
Since p53 is a major monitor of genome integrity, it can be expected that p53 is involved in the process of SHM. p53 is expressed in mouse germinal center B cells (Ranuncolo et al., 2007). p53 has been shown to be involved directly in BER (Seo and Jung, 2004). It has been proposed that p53 would facilitate DNA repair by allowing cells to remain in G1 before resuming cycling (Smith and Seo, 2002; Vousden and Prives, 2009). Mutations during SHM have been shown to occur in the G1 phase (Faili et al., 2002b) and Gasior, S. and U.S., unpublished). Would one expect then that the mutation frequency is increased when p53 is inactivated because cells with AID-induced uracils would have shorter times in G1 and therefore less time to repair the lesions? Certainly, general mutations have been found to increase in the absence of p53 (Zhou et al., 2001). However, since DNA repair by MMR and BER is co-opted in SHM into introducing mutations, the opposite may occur, namely that in the absence of p53 and due to the shorter time in G1 fewer mutations would occur. Furthermore, the pattern of mutations may partly depend on interactions of p53 with MMR and BER, as well as influencing the targeting of various lesion-bypass polymerases.
In order to obtain an insight into a possible role of p53 in SHM we have determined the effects of the inactivation of the p53 gene on the frequency and pattern of SHM.
2. Materials and methods
2.1 Mice
The p53 knockout mice (Donehower et al., 1992), the p21 knockout mice (Brugarolas et al., 1995) and their age-matched wildtype littermates were obtained from The Jackson Laboratory and were further bred in our mouse facility. The p53−/− mice were C57BL/6. Ung−/− mice were a gift of D. Barnes and T. Lindahl (Imperial Cancer Research Fund, Clare Hall Laboratories, South Mimms, U.K.), and were bred in our mouse facility with B6 mice. The ung−/− mice in this study were obtained by crossing p53−/−, p53+/−, or p53+/+ mice on a B6 background with ung−/− or ung+/− mice on a mainly B6 background but some with a mixed (F3) B6 and 129/Sv (129) background. Three mice were a mix of B6 and 129 at the Ig heavy chain locus. Based on the IgH sequences we obtained (see below in section 3.3), 26, 26, and 35% of the mutated IgH genes were 129 in the ung−/−, the p53+/−ung−/−, and the p53−/−ung−/− mouse 2 (mice 8, 9, and 11, Tables 2A, 2B), respectively. The 129 strain has an inactivated pol iota gene. We determined the proportion of the active and inactive 129 iota gene and found that all of the ung−/− mice (mice #8–11 Tables 2A, 2B) had only the active pol iota gene.
Table 2A.
SHM is altered in p53 −/− mice
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | wt1 | p53−/−1 | wt2 | p53−/−2 | wt3 | p53−/− 3 | wt ung+ (a) |
ung−/− |
p53+/− ung−/− |
p53−/− ung−/− 1 |
p53−/− ung−/− 2 |
| Tot cls(b) | 50 | 87 | 37 | 123 | 123 | 106 | 66 | 60 | 80 | 98 | 90 |
| Mut cls(c) | 24 | 7 | 22 | 20 | 61 | 63 | 43 | 37 | 42 | 42 | 42 |
| Mut/tot(d) | 0.48 | 0.08 | 0.59 | 0.16 | 0.49 | 0.59 | 0.65 | 0.62 | 0.53 | 0.43 | 0.47 |
| Tot muts(e) | 153 | 18 | 189 | 91 | 219 | 270 | 285 | 302 | 216 | 370 | 196 |
| Mut/103(f) | 5.13 | 2.07 | 6.92 | 3.66 | 2.89 | 3.45 | 5.34 | 6.57 | 4.14 | 7.09 | 3.76 |
| 2<1 p3×10−7 | 4<3 p7×10−5 | ||||||||||
| Age | 16 weeks | 16 weeks | 11 weeks | 11 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks |
wt littermate of ung −/− mice
Total clones sequenced
Total clones with mutations
Proportion of total clones that had mutations
Total mutations in all sequences
Mutations per 103 nucleotides in mutated sequences. The decreased mutation frequencies in mice p53 −/− 2 and 4, compared with their wt littermates is highly significant.
Table 2B.
The pattern of SHM is altered in p53 −/− mice
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | wt1 | p53−/−1 | wt2 | p53−/−2 | wt3 | p53−/−3 | wt ung+ (a) |
ung−/− |
p53+/− ung−/− |
p53−/− ung−/− 1 |
p53−/− ung−/− 2 |
| A mut %(a) | 32 | 72 | 33 | 50 | 30 | 45 | 36 | 31 | 34 | 30 | 30 |
| A-G %(b) | 13 | 22 | 15 | 25 | 14 | 23 | 17 | 15 | 20 | 13 | 15 |
| 2+4+6 > 1+3+5 p4×10−4(c) | 10+11 = 8+9 p0.13 | ||||||||||
| A-C %(b) | 11.8 | 22.2 | 9.5 | 13.2 | 7.3 | 11.1 | 7 | 7.9 | 7.4 | 6.8 | 8.7 |
| A-T %(b) | 6.5 | 28 | 8.5 | 11 | 8.7 | 11 | 11.6 | 7.6 | 6.9 | 10.3 | 6.1 |
| 2+4+6>1+3+5 p0.06(d) | 10+11 = 8+9 p0.38 | ||||||||||
| C-T %(b) | 5 | 0 | 10 | 7 | 7 | 11 | 10 | 14 | 14 | 19 | 18 |
| 8+9+10+11>1+3+5+7 p8.5×10−8(g) | |||||||||||
| C-A %(b) | 4.6 | 0 | 2.6 | 0 | 5.5 | 1.9 | 1.1 | 2.6 | 3.2 | 0.2 | 0.5 |
| 2+4+6< 1+3+5 p0.01(e) | 10+11 < 8 + 9 p8×10−4(e) | ||||||||||
| C-G %(b) | 1.3 | 0 | 2.6 | 3.3 | 4.6 | 1.1 | 3.9 | 0 | 0 | 0.3 | 0.5 |
| G-A %(b) | 9 | 6 | 15 | 10 | 15 | 7 | 14 | 30 | 26 | 29 | 20 |
| 2+4+6<1+3+5 p0.01(f) | 10+11 = 8+9 p0.41 8+9+10+11>1+3+5+7 p3.4×10−13(g) |
||||||||||
| G-C %(b) | 13.7 | 11.1 | 7.9 | 7.7 | 6.4 | 7.4 | 6 | 0.7 | 0.5 | 0.5 | 1 |
| G-T %(b) | 9.8 | 0 | 3.7 | 5.5 | 4.1 | 1.9 | 4.9 | 0.3 | 0 | 1.1 | 0 |
| 2+4+6<1+3+5 p0.03(h) | 10+11 = 8+9 p0.37 | ||||||||||
| T mut % (a) | 25.1 | 11.5 | 25.3 | 17.3 | 25 | 23.9 | 23.7 | 20.6 | 21.5 | 19.7 | 29.7 |
| T-C %(b) | 12 | 6 | 11 | 3 | 14 | 12 | 10 | 8 | 16 | 8 | 17 |
| T-A %(b) | 8.5 | 5.5 | 9 | 6.6 | 7.3 | 7.8 | 7.7 | 7.3 | 3.2 | 6.8 | 7.1 |
| T-G %(b) | 4.6 | 0 | 5.3 | 7.7 | 3.7 | 4.1 | 6 | 5.3 | 2.3 | 4.9 | 5.6 |
| A/T(i) | 1.26 | 6.5 | 1.29 | 2.81 | 1.25 | 1.85 | 1.52 | 1.48 | 1.82 | 1.49 | 1.02 |
| C/G(j) | 0.34 | 0 | 0.58 | 0.43 | 0.75 | 0.84 | 0.58 | 0.59 | 0.68 | 0.63 | 0.9 |
| Age | 16 weeks | 16 weeks | 11 weeks | 11 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks |
Proportion of total mutations that were at A or at T
Proportion of total mutations that were the respective changes
The increase in A-G in the three p53 −/− mice (#2, 4, 6) compared with their wt littermates is highly significant (Fisher's exact test).
The increase in A-T in the three p53 −/− mice (#2, 4, 6) compared with their wt littermates is borderline signficant. The increase in A-C mutations is not significant (p0.16)
The decrease in C-A in the three p53 −/− mice (#2, 4, 6) and two p53 −/−ung−/− mice (#10, 11) compared with their p53 -wt littermates is significant
The decrease in G-A in the three p53 −/− mice (#2, 4, 6) compared with their wt littermates is significant
C-T and G-A transitions are significantly different between all ung −/− mice and all wt mice
The decrease in G-T in the three p53 −/− mice (#2, 4, 6) compared with their littermates is significant
% mutations at A/% mutations at T
% mutations at C/% mutations at G
Bold: mutations in p53 −/− mice are significantly different (> or <) from p53 -wt
A male Msh6+/− mouse was obtained from the Mouse Repository at the National Cancer Institute (Rockville, MD) and bred with B6 in our facility. p53 +/− mice were mated either to Msh6 +/− mice or Msh6 −/− in an attempt to obtain p53−/−Msh6−/− mice. The primers used for genotyping the mice are shown in Supplementary Table 1a.
2.2 Immunization
The mice were immunized by an intra-peritoneal injection of sheep red blood cells (2 ×108 cells) followed by a secondary immunization after 21 days. The spleens and blood were collected after 21+ 4 days.
2.3 Analysis of SHM
PNA-high GL7+ B220+ (germinal center, mutating B cells) cells and an equivalent number of PNA-low GL7-low B220+ (non-germinal center B cells) cells were obtained from spleens of immunized mice as described (Longerich et al., 2005). DNA was extracted using DNeasy columns (QIAGEN). VHJ558-rearranged IgH genes were amplified using primers VHJ558BLF (GGAATTCGCCTGACATCTGAGGACTCTGC) and JCIntronR (GACTAGTCCTCTCCAGTTTCGGCTGAATCC) as described (Longerich et al., 2005). Around 10,000 cell equivalents of template DNA were used for the PCR reaction at the following PCR cycling conditions: 1 cycle at 95 °C for 4 min, 95 °C for 40 s, and 64–58 °C for 40 s (touchdown annealing), 13 cycles at 72 °C for 4 min, followed by 27 cycles at 95 °C for 40 s, 57 °C for 40 s, 72 °C for 4 min, and a final extension at 72 °C for 7 min.The PCR products were resolved by agarose gel electrophoresis. The band encompassing the JH4 rearrangement of the VHJ558 IgH gene was excised, and DNA was purified using a gel extraction kit (Qiaquick; QIAGEN). PCR products were cloned using the Zero Blunt Topo PCR Cloning Kit (Invitrogen). Individual colonies were picked for automated DNA preparation and sequencing at the University of Chicago Cancer Research Center DNA Sequencing Facility.
2.4 Real-time RT-PCR
Total RNA was made from PNA-high and PNA-low B-cells with RNA STAT-60 (Tel-Test Inc). cDNA was made by the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) using oligo-dT. Real-time PCRs were run using gene specific primers and analyzed on a MYiQ system with SYBR Green SuperMix (Bio-Rad Laboratories). PCR conditions were 95°C for 30 s, 58°C for 45 s, and 72°C for 60 s for 40 cycles. Mouse beta actin was used as a reference for the relative quantification of pol eta, pol iota and Msh2 using the Pfaffl method (Pfaffl, 2001). The analysis was done in duplicates. The primers used in this analysis are shown in Supplementary Table 1b.
2.5 ELISA assays
ELISA assays were performed using the SBA clonotyping System/AP kit according to the manufacturer’s instructions (cat # 5300-04, Southern Biotechnology Associates) as described (Shen et al., 2006). The concentration of the immunoglobulins in the samples was calculated based on the standard readings. The analysis was done in duplicates.
2.6 Hemagglutinin assays
Serum was diluted 1:1 with 1× PBS and incubated for 30 minutes at 50° C. Serial dilutions of 1:2 were mixed with equal volumes of sheep red blood cells (2 × 108 cells/ml). The highest dilution at which agglutination was seen is noted in Table 1.
Table 1.
General status of p53−/−, p21−/−, and wildtype mice
| Mousea | Age | Anti-SRBCb | No. of PNA-hi B cells |
Total no. of nucleated spleen cells |
|---|---|---|---|---|
| (1)wildtype mouse1 |
11 weeks | 1/1024 | 195,428 | 72 × 106 |
| (2) p53−/− mouse 1 |
11 weeks | 1/1024 | 205,616 | 74 × 106 |
| (3) wildtype mouse2 |
16 weeks | 1/1024 | 251,645 | 84 × 106 |
| (4) p53−/− mouse 2 |
16 weeks | 1/1024 | 270,228 | 226 × 106 |
| (5) wildtype mouse 3 |
8 weeks | 1/2048 | 229,312 | 98 × 106 |
| (6) p53−/− mouse 3 |
8 weeks | 1/2048 | 240,041 | 104 × 106 |
| (7) ung control mouse |
8 weeks | 1/2048 | 265,482 | 84 × 106 |
| (8) ung−/− mouse |
8 weeks | 1/2048 | 227,856 | 80 × 106 |
| (9) p53+/− ung−/− mouse |
8 weeks | 1/1024 | 215,221 | 96 × 106 |
| (10) p53−/− ung−/− mouse 1 |
8 weeks | 1/1024 | 220,662 | 89 × 106 |
| (11) p53−/− ung−/− mouse 2 |
8 weeks | 1/1024 | 218,016 | 92 × 106 |
|
p21 wildtype mouse |
8 weeks | 1/1024 | ~185,000 | 90 × 106 |
|
p21−/− mouse |
8 weeks | 1/1024 | ~180,000 | 83 × 106 |
The numbers in parenthesis refer to the mouse numbers in Table 2A.
Hemaglutinin assay
2.7 Statistical Analyses
The p-values were calculated using Fisher's exact test for 2×2 contingency tables. For example, for the WRC, GYW hotspot comparison, a 2×2 table of mutation counts was constructed, where the rows index mice (p53−/− versus controls) and the columns index type of mutations (mutations in WRC, GYW hotspots versus other mutations at C and G).
3. Results
3.1 General immune status of p53−/− mice
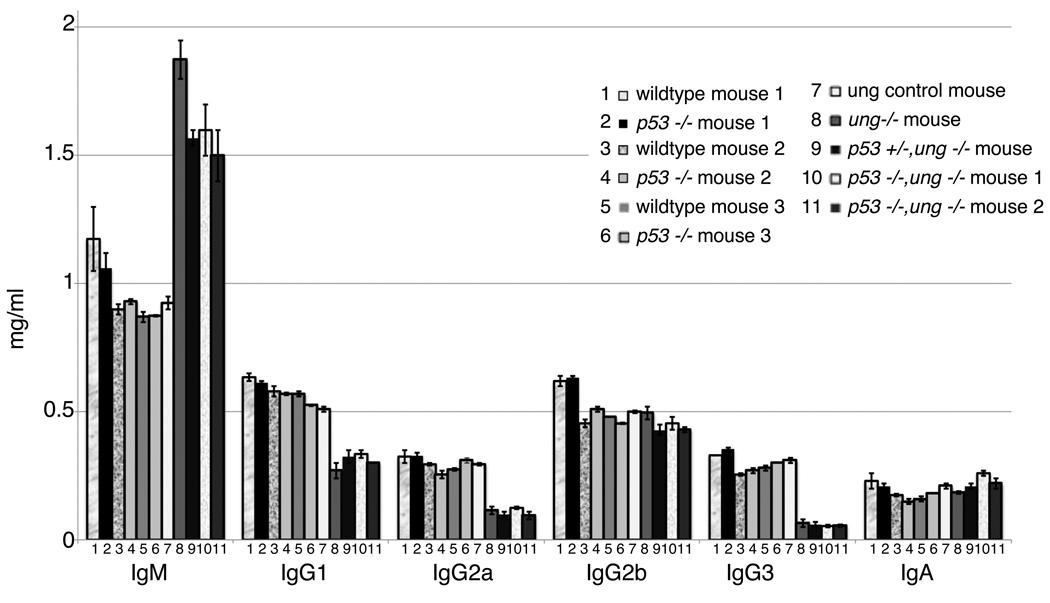
All mice in this study responded with essentially equal anti-sheep red blood cell (SRBC) titers four days after a secondary immunization with SRBC (Table 1). The serum levels of isotype switched Igs were the same in p53−/− mice as in their wildtype littermates (Fig. 1). Others also found no effect of p53 inactivation on class switch recombination (Ramiro et al., 2006). As expected, in all ung−/− mice IgM in the serum was raised considerably, and the levels of IgG1, IgG2a and IgG3 were at least two-fold lower than in ung wildtype littermates. These isotype changes are as in previous studies with ung−/− mice (Rada et al., 2002; Shen et al., 2006). As before, we found no decrease in IgG2b in ung−/− mice (Shen et al., 2006). Normal levels of IgA had been noticed before in young ung−/− mice, but not in very old mice (Rada et al., 2002). The mice in our study were young. These findings suggest that the overall immune responsiveness was not dependent on p53. Furthermore, lack of p53 did not ameliorate the inhibition of isotype switching in ung−/− mice (Fig. 1). The total numbers of nucleated spleen cells were similar in all mice, except that they were drastically increased in the oldest, 16 week old p53−/− mouse (Table 1). Surprisingly though, the numbers of total PNA-hi (= germinal center) B cells were similar in the oldest p53−/− mouse and its wildtype littermate (Table 1).
Fig.1. CSR activity in wildtype and p53−/− mice.
The data shown are an average of two independent ELISA experiments. The numbers on the x-axis refer to the mouse numbers in Table 2A. The y-axis shows the concentration of specific immunoglobulins.
3.2 Production of p53−/− MMR or BER double-deficient mice
Since the major DNA repair systems involved in SHM are mismatch repair (MMR) and base excision repair (BER) (reviewed in (Longerich et al., 2006) we wanted to produce double-knockout mice of essential components of these systems and p53 to help explain the mutation patterns described below. We were unable to obtain any double-knockout mice for Msh6, the partner of Msh2 in the repair of single base-mismatches. Among 115 offspring of multiple crosses, we would have expected to obtain ~17 Msh6−/−p53−/− pups. None were obtained. Thus, deletion of both genes may be embryonic lethal (Young et al., 2007). We obtained the expected ratios of p53−/− offspring. Double-knockout offspring for p53 and ung were at about 50% of the expected ratios. We did not find tumors in any of the p53−/− mice, regardless of the ung genotype. Presumably they were too young. Tumors have been seen in p53−/− mice before 10 weeks of age, but the average time to tumor appearance was 20 weeks (Donehower et al., 1992). We analyzed most of the mice at only 8 weeks of age since their SHM frequency was normal at that time (Table 2A). The oldest p53−/− mouse in this study was sacrificed at 16 weeks. It had no obvious tumors, but an enlarged spleen.
3.3 Somatic hypermutation of Ig genes in p53−/− mice
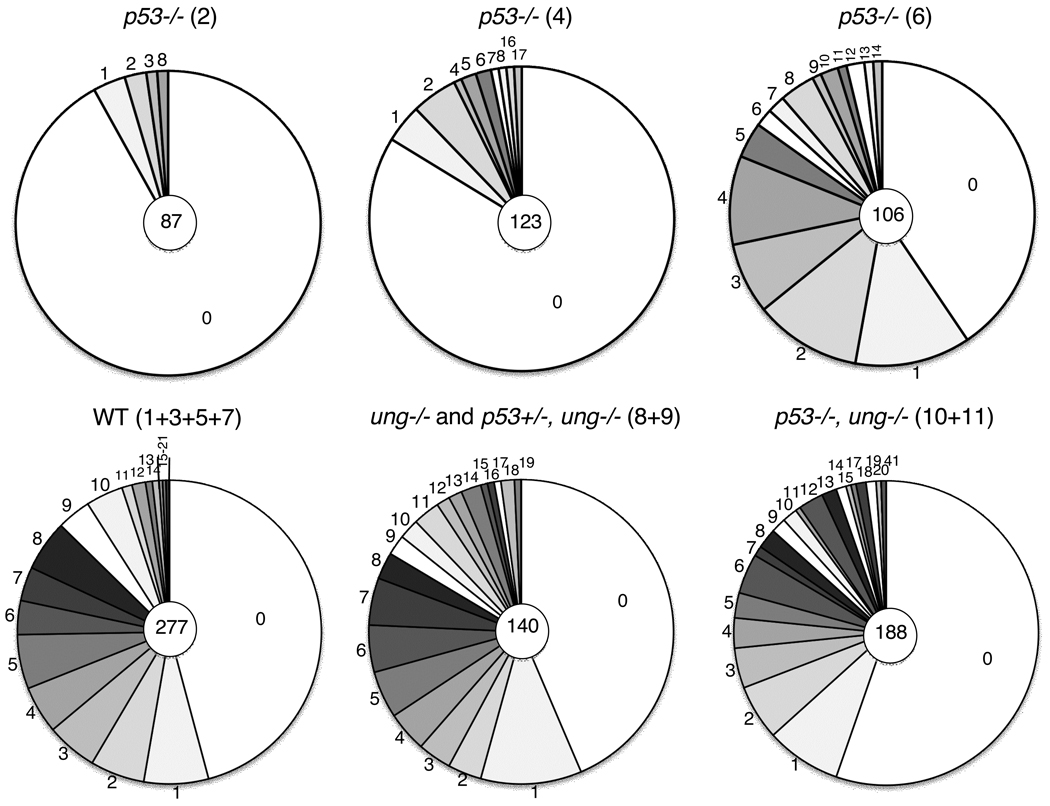
PNA-hi B cells were isolated from mice four days after secondary immunization with SRBCs and DNA sequences of cells with JH4 rearrangements of the VHJ558 gene were determined (Jolly et al., 1997). Strain 129 IgH sequences from the three mice with 129 and B6 IgH genes were compared to the 129 sequence, and B6 sequences were compared to B6, respectively. All mice had mutations in the 5’ region of the JH4-Cmu intron. In wildtype controls 48 to 65% of the sequences had mutations (Table 2A (d), Mut/total clones). This measure of mutability was greatly reduced to 16 and 8% of the sequences in the older p53−/−mice (Table 2A (d) #2 and 4 compared with #1 and 3), but not in the youngest mice, regardless of their p53 and/or ung genotype (Table 2A (d) #6, 10 and 11 compared with #5, 8, and 9, respectively). Also, the mutation frequencies per 103 nucleotides in sequences with mutations were reduced 2-fold or more in the older p53−/− mice compared with their wt littermates, but not in the youngest mice (Table 2A (e) #2 and 4, compared with their wt littermates, #1 and 3). We assume that SHM in the young (8 week old) mice represents the most direct effect of the deletion of p53 on SHM and have therefore analyzed the effect of additional ung-deletion only in 8 week old mice. The reduction of mutations in the older mice (Fig. 2) may be a signature of cumulative events in the B cells already before immunization. In addition, the drastically reduced mutability in the oldest p53−/− mouse may be due to stress of SHM in the overall more severely compromised environment that then eliminated cells with a high mutation load. This may relate mainly to non-Ig genes. E.g. the tumor suppressor gene Bcl6 is a relatively efficient target of AID (Pasqualucci et al., 1998; Peng et al., 1999; Shen et al., 1998) and many other non-Ig genes are subject to SHM (Liu et al., 2008; Storb et al., 2009).
Fig.2. Mutation frequencies in wildtype and p53−/− mice.
The pie charts depict the number of point mutations per DNA clone (1.24 kb). The numbers in the central circle are the total number of clones sequenced. The numbers in parentheses ( ) refer to the mouse numbers in Table 2A.
We have no evidence that p53 is involved in the targeting of AID. The mutation frequencies for C + G in the p53−/−ung−/− mice is 4.51×10−3 nts, compared with 4.64×10−3 for the p53 wt, ung−/− controls. Most of these are transition mutations, an even more directly correlated measure of AID targeting. The mutation frequencies for C+G transitions were 4.3×10−3 and 4.27 × 10−3 in the p53−/−ung−/− versus their p53 wt, ung−/− controls, respectively. The lack of an effect of p53 inactivation on AID targeting is also supported by the finding that isotype switching is not altered (Fig. 1). Finally, there is no change in the targeting of AID to the known AID hotspots (Supplementary Table 2). The Ig class switch recombination (CSR) in p53−/− mice is not altered (Fig.1) presumably because AID and ung are sufficient, and lesion bypass polymerases are not required for CSR.
However, there are significant changes in mutation patterns in the p53−/− mice. A major change in the pattern of SHM was seen with mutations at A. The percentage of A mutations over total mutations in mutated sequences ranged narrowly between 30 and 36% in the p53 wt mice, similar to what is generally found for SHM in normal mice (Table 2B (a), # 1, 3, 5, and 7; summarized in Table 2C). In the p53−/− mice, however, mutations at A were at 45 to 72% of total mutations (Table 2B (a), # 2, 4, 6); summarized in Table 2C). The most significant change in mutations at A were A to G transitions (Table 2B (b); summarized in Table 2C). Surprisingly, when ung was deleted in addition to the deletion of p53, the A mutations over total mutations and the % mutations at A to G were normal: only 30 % of all mutations were at A and 13 or 15% of all mutations were A to G (Table 2B (b), #10–11; summarized in Table 2C). This suggests that when p53 is deleted, the increased mutability at A depends on ung.
Table 2C.
Summary of a subset of mutations
| Mice | A mutations (%) | A to G (%) | T to C (%) | C to T (%) | G to A (%) |
|---|---|---|---|---|---|
| Wt | 33 | 15 | 12 | 8 | 13 |
| p53−/− | 56 | 23 | 7 | 6 | 8 |
| P-value* | 4×10−4 | 4×10−4 | 0.25 | 0.34 | 0.01 |
| p53−/− ung −/− | 30 | 14 | 13 | 19 | 25 |
| P-value** | 0.6 | 0.13 | 1.0 | 0.10 | 0.41 |
| Ung−/− | 31 | 16 | 12 | 16 | 26 |
| P-value* | 0.38 | 0.42 | 0.31 | 9×10−8 | 3×10−13 |
P-value
versus wt;
versus ung−/−
Bold, increased mutations at A and A-to-G in p53−/− versus wt
Individual mouse data are in Table 2B: wt = #1, 3, 5, 7; p53−/− = #2, 4, 6; p53−/−ung−/− = #11, 12; ung−/− = #8–11
As observed previously (Rada et al., 2002; Shen et al., 2006), all ung−/− mice have increased C to T and G to A transition mutations (Table 2B (b), #8–11; summarized in Table 2C). This is not affected by the p53 status.
There is also a significant reduction of C to A transversion mutations in p53−/− mice. Unlike the A-G transitions, this C-A transversion is independent of ung since the reduction of C -A is still prominent in ung−/− mice (Table 2B (b)). The C-A changes may relate to a p53 effect on mismatch repair during transcription or while the leading strand DNA is replicated. The C-A change may be also strand-biased since the complementary reductions of G to T transversions are significant in p53−/− mice only in the presence of ung. However, this interpretation is complicated by the very low G-T transversion mutation frequencies in ung−/− mice. While G-A and C-T transitions are high in ung−/− mice, as expected, it is not clear why there is an imbalance in C-A versus G-T tranversions in ung−/ mice with wt p53 (Table 2B (b)).
Mice deficient in p21 did not show the SHM changes observed in the p53−/− mice (Table 2D). This suggests that the pathway from p53 through p21 is not involved in SHM. Most likely, p53 affects SHM because p53 protein interacts with proteins involved in SHM (Table 3; see Discussion).
Table 2D.
SHM in p21 −/− mice is normal
| Mouse | wt | p21 −/− |
|---|---|---|
| Tot clones(a) | 24 | 22 |
| Mut clones(b) | 10 | 8 |
| Mut/tot clones(c) | 42 | 36 |
| Tot muts(d) | 45 | 51 |
| Muts/103(e) | 3.62 | 5.1 |
| A mutations(f) | 14 | 17 |
| A muts %(g) | 31 | 33 |
| C mutations(f) | 4 | 6 |
| C muts %(g) | 9 | 12 |
| G mutations(f) | 17 | 15 |
| G muts %(g) | 38 | 29 |
| T mutations(f) | 10 | 13 |
| T muts %(g) | 22 | 25 |
| Age | 8 weeks | 8 weeks |
Total clones sequenced
Total clones with mutations
Proportion of total clones that had mutations
Total mutations
Mutations per 103 nucleotides in mutated sequences.
Number of mutations at the indicated nucleotide
Proportion of total mutations that were at the indicated nucleotide
There are no significant differences between the wt and p21 −/− littermates.
Table 3.
List of proteins, genes and mechanisms that are likely involved in SHM and that are regulated by p53
| p53 | Reference |
|---|---|
| a.Expressed in germinal center B cells | {Ranuncolo, 2007 #143} |
| b.Enhances BER | {Offer, 2001 #163; Offer, 1999 #164} {Zhou, 2001 #141} {Smith, 2002 #142} |
| c.Decreases mutagenesis | {Zhou, 2001 #141} |
| d.Pol beta almost absent in p53−/− mice | {Seo, 2002 #162} |
| e.Interaction with pol beta required for enhanced BER | {Zhou, 2001 #141} |
| f.Decreases Ung2 activity due to activation of phosphatase | {Lu, 2004 #77} } |
| g.Decreases efficiency of translesion DNA synthesis | {Avkin, 2006 #161} |
| h.Deletion of p53 increases mutagenesis by translesion pols. | {Avkin, 2006 #161} |
| i.Reduces transcription of APE1 gene | {Zaky, 2008 #56} |
| j.Increases transcription of Msh2 gene | {Scherer, 2000 #55} |
| k.Increases transcription of PCNA gene | {Shan, 2005 #54} |
4) Discussion
4.1 Increased mutations at A
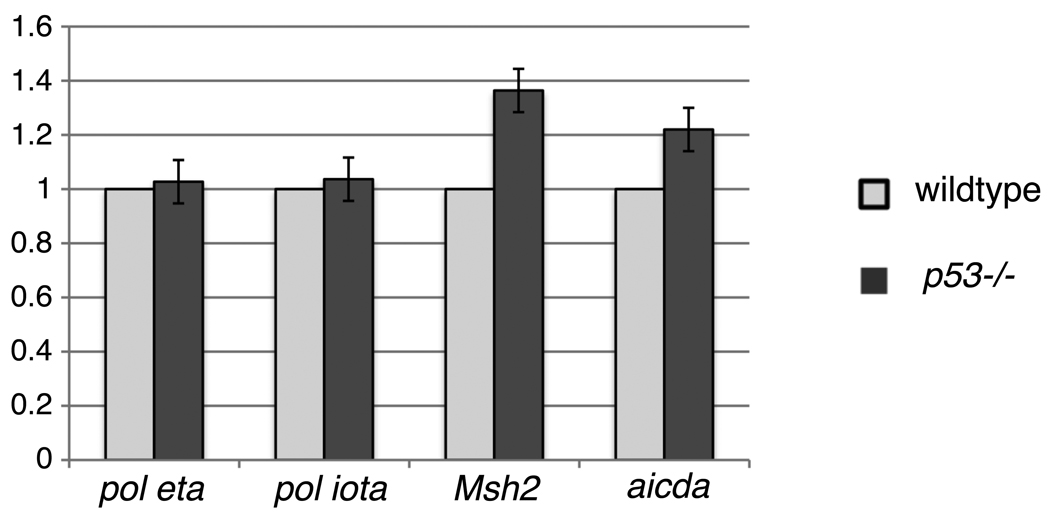
There are several known functions of p53 that may relate to factors known to be involved in SHM. p53 protein interacts directly or indirectly with a considerable number of factors, and p53 can act as an activator or suppressor of transcription by interacting with regulatory DNA sequences, as summarized in Table 3. Deletion of p53 decreases BER (Table 3 b.; (Offer et al., 2001; Offer et al., 1999; Smith and Seo, 2002; Zhou et al., 2001). Reduced general BER would be expected especially due to reduced activity of pol beta (Table 3 d., e.; (Seo et al., 2002; Zhou et al., 2001). However, SHM invoking error-prone polymerases may be more active in p53−/− mice because the reduced activity of the relatively high fidelity pol beta may allow error-prone lesion bypass polymerases, such as pol eta or pol iota (see below), to be more efficiently recruited. In addition, ung activity would be increased, and the transcription of the APE1 gene would be increased, thus helping the resolution of the AID-induced uracil by error-prone BER (Table 3 f., i.; (Lu et al., 2004; Zaky et al., 2008). Furthermore, it has been shown in cell lines that p53 decreases the efficiency of translesion DNA synthesis, but increases its fidelity (Table 3 g.; (Avkin et al., 2006). Complementing this finding, deletion of p53 increased the mutagenesis by translesion polymerases (Table 3 h.; (Avkin et al., 2006). Thus, error-prone repair during SHM would increase in the absence of p53. Apparently, the expected decreased transcription of the Msh2 and PCNA genes in the absence of p53 (Table 3 j., k.; (Scherer et al., 2000; Shan and Morris, 2005) is not sufficiently severe to reduce SHM below the levels of p53 wild type mice, except perhaps in the older mice, but see above (first paragraph in section 3.3). In fact, we found a slight increase of stable Msh2 mRNA in p53−/− mice (Fig. 3).
Fig. 3. Relative mRNA expression in wildtype and p53−/− mice.
The data shown are an average of two independent real time RT-PCR assays. The pol eta, pol iota, Msh2 and AID (aicda) mRNA levels were normalized to beta actin mRNA (shown as 1). The y-axis shows the mRNA levels of pol eta, pol iota, Msh2 and aicda in p53−/− mouse relative to levels in wildtype mouse.
These considerations lead to the conclusion that the increased mutation frequencies at A are mainly due to less restricted recruitment of translesion DNA polymerases in p53-deficient cells during SHM. Any changes of SHM in p53−/− mice that are normalized in the absence of ung are likely due to BER that depends on the creation of an abasic site by ung.
The dependence of A mutations on ung is contrary to the current dogma that most A (and T) mutations are due to coupling of pol eta with mismatch repair (MMR), not with ung-initiated base excision repair (BER) (Delbos et al., 2005; Longerich et al., 2006). However, in the p53−/− mice only the mutations at A, not those at T are increased. Thus, there is an A>T strand bias (Table 2B (i)) which is even higher than normal for SHM (Longerich et al., 2006; Storb et al., 1998). We assume that mutations at A indicate that a T in the transcribed (template) strand has been copied erroneously. Meaning that the non-transcribed (coding) strand had acquired an AID-induced uracil and that a “long” patch of nucleotides had been replaced by error-prone DNA synthesis, rather than only the uracil (Klungland and Lindahl, 1997). If the excess of mutations at A over T was created by the same mechanism that mutates C and G, one would expect a corresponding strand-bias for mutations at C and G, namely increased mutagenesis at C over G, representing error-prone copying of G on the transcribed strand. However, while in the p53−/− mice the A/T ratio increases compared with wildtype, the C/G ratio decreases in the two oldest mice and remains about the same in the youngest mouse (Table 2B (j) #2, 4, 6 versus #1, 3, 5). This suggests that the effect of p53 is strand-biased for A mutations, but a correlated strand bias for C/G mutations does not occur. The increase of mutations at A in p53−/− mice depends on ung and appears to involve the recruitment of translesion polymerases preferably during the error-prone synthesis in the non-transcribed strand.
AID targeting of the non-transcribed strand would require that this strand be single-stranded at the time of AID action. During transcription of linear, double-stranded DNA in vitro, AID has been found to mainly target the non-transcribed strand, suggesting that it attacks DNA in the transcription bubble (Chaudhuri et al., 2003; Pham et al., 2003). However, in the case of AID action in double-stranded supercoiled DNA that was transcribed, a strand bias was not observed (Shen at el, 2005; Shen and Storb, 2004; Shen et al., 2009). This suggested that AID may interact on both DNA strands with flipped-out cytosines created in negative supercoils behind the transcribing RNA polymerase. Since there is no evidence that AID targeting is altered in p53−/− mice, the strand bias is unlikely related to altered strand-specific AID targeting. It may therefore relate to the recruitment of BER or MMR or lesion bypass DNA polymerases. Strand specificity in the treatment of AID created uracils could relate to transcription. Alternatively, it could relate to DNA replication. There is a strong origin of replication at the 3’ end of the IgH locus in mature B cells (Zhou et al., 2002). Thus in the mutating B cells, the leading strand would be the non-transcribed strand. In summary, the reason for the observed strand bias of A mutations in p53−/− mice currently has not been resolved.
4.2 Does pol iota play a role in SHM of p53−/− mice?
Mutations at A and T during SHM in mice were found to be entirely due to pol eta (Delbos et al., 2007). Since the increase of mutations at A in p53−/− mice could have been due to increased pol eta, we determined the levels of stable pol eta transcripts in PNAhi B cells of p53−/− and wt control mice (Fig. 3). There was no difference. Pol iota, on the other hand, seemed unlikely to play a role in SHM of mice, since the pattern and frequencies of mutations during SHM were reported to be the same in the 129 strain mice that are deficient in pol iota as in pol iota wildtype mice (C57BL/6) (McDonald et al., 2003). However, in a human B cell line, pol iota-deficiency caused a major reduction in SHM that was remedied by the expression of a transfected pol iota gene (Faili et al., 2002a). Interestingly, the human cell line assayed in that study, BL2, is deficient in p53 (Gao et al., 1999). It is therefore possible, that pol iota was allowed to be active in this cell during SHM, whereas in the presence of p53 (in germinal center B cells) pol iota may be prohibited from access to post-C-deamination processes. Levels of pol iota mRNAs were not changed in p53−/− mice. Comparing the error rates of pol iota with those of pol eta at different nucleotides (Table 4) shows that A to G mutations are a hallmark of the error rates of pol iota (Tissier et al., 2000). When pol iota copies undamaged DNA, it copies T with G three times more often than copying it with the correct A. Table 4 summarizes some of the mutation patterns in p53−/− mice in parallel with the error rates of pol iota versus those of pol eta. Both pol iota and pol eta are candidates for causing the increase in A to G mutations in p53−/− mice. However, pol iota is 52x more error prone for this process. On the other hand, pol iota is highly error-free when copying A, resulting in very few T to N mutations (Table 4 (c)), while pol eta has error rates for copying A that are only slightly lower for copying A with G or even higher for copying A with A or C than its error rates for copying T (Kunkel et al., 2003).
Table 4.
Altered mutation pattern in p53−/− mice may be due to activation of pol iota
| Observed mutations | Reported error rates of translesion polymerases | ||||||
|---|---|---|---|---|---|---|---|
| Mutation | % of total mutations | Change(a) | P-value(b) | pol iota error rate(c) | pol eta error rate(d) | error rate iota/eta(e) | |
| wt | p53−/− | ||||||
| A to G | 15 | 23 | UP | 0.0004 | 3×100 | 5.8×10−2 | 52× UP |
| A to T | 8 | 17 | UP | 0.06 | 6.7×10−1 | 6×10−3 | 112× UP |
| T to N | 27.3 | 17.6 | DOWN | 0.25 | 1–2×10−4 | 8–14×10−3 | 40–141× DOWN |
Increased or decreased mutations in p53−/− versus wt mice
P-value of mutations in p53−/− versus wt mice
Increased or decreased error rate of pol iota compared with pol eta
Clearly, there is no increase in mutations at T in p53−/− mice (Table 2B (a), (b); Table 4) as would have been expected if the mutations were due to increased activity of pol eta. Thus it is possible that mutations are created at A and T by the action of pol eta in the presence of p53, but in the absence of p53, mutations are increased at A and decreased at T because pol iota partially replaces pol eta.
The effects of p53 inactivation on SHM reveal another novel role added to the vast repertoire of p53 functions.
Supplementary Material
Acknowledgments
We are grateful to B. Kee for technical advice and use of her instrument for Real Time PCR, W. Buikema and C. Hall for DNA sequencing, and R. Duggan for flow cytometric cell sorting. We also thank T.E Martin for many suggestions for the manuscript. The work was supported by National Institutes of Health grants AI047380 and AI053130. S.R. was supported by a postdoctoral fellowship from the Cancer Research Institute.
Appendix
There are three Supplementary Tables 1A, 1B, and 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Avkin S, Sevilya Z, Toube L, Geacintov N, Chaney SG, Oren M, Livneh Z. p53 and p21 regulate error-prone DNA repair to yield a lower mutation load. Mol Cell. 2006;22:407–413. doi: 10.1016/j.molcel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt F. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- Delbos F, Aoufouchi S, Faili A, Weill JC, Reynaud CA. DNA polymerase eta is the sole contributor of A/T modifications during immunoglobulin gene hypermutation in the mouse. J Exp Med. 2007;204:17–23. doi: 10.1084/jem.20062131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbos F, De Smet A, Faili A, Aoufouchi S, Weill J-C, Reynaud C-A. Contribution of DNA polymerase eta to immunoglobulin gene hypermutation in the mouse. J. Exp. Med. 2005;201:1191–1196. doi: 10.1084/jem.20050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Faili A, Aoufouchi S, Flatter E, Guéranger Q, Reynaud CA, Weill JC. Induction of somatic hypermutation in immunoglobulin genes is dependent on DNA polymerase iota. Nature. 2002a;419:944–947. doi: 10.1038/nature01117. [DOI] [PubMed] [Google Scholar]
- Faili A, Aoufouchi S, Guéranger Q, Zober C, Leon A, Bertocci B, Weill JC, Reynaud CA. AID-dependent somatic hypermutation occurs as a DNA single-strand event in the BL2 cell line. Nat Immunol. 2002b;3:815–821. doi: 10.1038/ni826. [DOI] [PubMed] [Google Scholar]
- Gao C, Nakajima T, Taya Y, Tsuchida N. Activation of p53 in MDM2-overexpressing cells through phosphorylation. Biochem Biophys Res Commun. 1999;264:860–864. doi: 10.1006/bbrc.1999.1611. [DOI] [PubMed] [Google Scholar]
- Jolly CJ, Klix N, Neuberger MS. Rapid methods for the analysis of immunoglobulin gene hypermutation: application to transgenic and gene targeted mice. Nucleic Acids Res. 1997;25:1913–1919. doi: 10.1093/nar/25.10.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kage K, Matsuda F, Lefranc MP, Storb U. B lymphocytes of xeroderma pigmentosum or Cockayne syndrome patients with inherited defects in nucleotide excision repair are fully capable of somatic hypermutation of immunoglobulin genes. J Exp Med. 1997;186:413–419. doi: 10.1084/jem.186.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungland A, Lindahl T. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) Embo J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Pavlov YI, Bebenek K. Functions of human DNA polymerases eta, kappa and iota suggested by their properties, including fidelity with undamaged DNA templates. DNA Repair (Amst) 2003;2:135–149. doi: 10.1016/s1568-7864(02)00224-0. [DOI] [PubMed] [Google Scholar]
- Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- Longerich S, Basu U, Alt F, Storb U. AID in somatic hypermutation and class switch recombination. Curr Opin Immunol. 2006;18:164–174. doi: 10.1016/j.coi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Longerich S, Meira L, Shah D, Samson LD, Storb U. Alkyyladenine DNA glycosylase (Aag) in somatic hypermutation and class switch recombination. DNA Repair. 2007;6:1764–1773. doi: 10.1016/j.dnarep.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longerich S, Tanaka A, Bozek G, Nicolae D, Storb U. The very 5' end and the constant region of Ig genes are spared from somatic mutation because AID does not access these regions. J Exp Med. 2005;202:1443–1454. doi: 10.1084/jem.20051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Nguyen TA, Appella E, Donehower LA. Homeostatic regulation of base excision repair by a p53-induced phosphatase: linking stress response pathways with DNA repair proteins. Cell Cycle. 2004;3:1363–1366. doi: 10.4161/cc.3.11.1241. [DOI] [PubMed] [Google Scholar]
- McDonald JP, Frank EG, Plosky BS, Rogozin IB, Masutani C, Hanaoka F, Woodgate R, Gearhart PJ. 129-derived strains of mice are deficient in DNA polymerase iota and have normal immunoglobulin hypermutation. J Exp Med. 2003;198:635–643. doi: 10.1084/jem.20030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer H, Milyavsky M, Erez N, Matas D, Zurer I, Harris CC, Rotter V. Structural and functional involvement of p53 in BER in vitro and in vivo. Oncogene. 2001;20:581–589. doi: 10.1038/sj.onc.1204120. [DOI] [PubMed] [Google Scholar]
- Offer H, Wolkowicz R, Matas D, Blumenstein S, Livneh Z, Rotter V. Direct involvement of p53 in the base excision repair pathway of the DNA repair machinery. FEBS Lett. 1999;450:197–204. doi: 10.1016/s0014-5793(99)00505-0. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Migliazza A, Fracchiolla N, William C, Neri A, Baldini L, Chaganti RS, Klein U, Küppers R, Rajewsky K, Dalla-Favera R. BCL-6 mutations in normal germinal center B cells: evidence of somatic hypermutation acting outside Ig loci. Proc Natl Acad Sci U S A. 1998;95:11816–11821. doi: 10.1073/pnas.95.20.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HZ, Du MQ, Koulis A, Aiello A, Dogan A, Pan LX, Isaacson PG. Nonimmunoglobulin gene hypermutation in germinal center B cells. Blood. 1999;93:2167–2172. [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham P, Bransteitter R, Petruska J, Goodman M. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- Rada C, Williams G, Nilsen H, Barnes D, Lindahl T, Neuberger M. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr. Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- Ramiro AR, Jankovic M, Callen E, Difilippantonio S, Chen HT, McBride KM, Eisenreich TR, Chen J, Dickins RA, Lowe SW, Nussenzweig A, Nussenzweig MC. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranuncolo SM, Polo JM, Dierov J, Singer M, Kuo T, Greally J, Green R, Carroll M, Melnick A. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol. 2007;8:705–714. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- Scherer SJ, Maier SM, Seifert M, Hanselmann RG, Zang KD, Muller-Hermelink HK, Angel P, Welter C, Schartl M. p53 and c-Jun functionally synergize in the regulation of the DNA repair gene hMSH2 in response to UV. J Biol Chem. 2000;275:37469–37473. doi: 10.1074/jbc.M006990200. [DOI] [PubMed] [Google Scholar]
- Seo YR, Fishel ML, Amundson S, Kelley MR, Smith ML. Implication of p53 in base excision DNA repair: in vivo evidence. Oncogene. 2002;21:731–737. doi: 10.1038/sj.onc.1205129. [DOI] [PubMed] [Google Scholar]
- Seo YR, Jung HJ. The potential roles of p53 tumor suppressor in nucleotide excision repair (NER) and base excision repair (BER) Exp Mol Med. 2004;36:505–509. doi: 10.1038/emm.2004.64. [DOI] [PubMed] [Google Scholar]
- Shan B, Morris G. Binding sequence-dependent regulation of the human proliferating cell nuclear antigen promoter by p53. Exp Cell Res. 2005;305:10–22. doi: 10.1016/j.yexcr.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Shen H, Peters A, Baron B, Zhu X, Storb U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science. 1998;280:1750–1752. doi: 10.1126/science.280.5370.1750. [DOI] [PubMed] [Google Scholar]
- Shen HM, Ratnam S, Storb U. Targeting of the activation-induced cytosine deaminase is strongly influenced by the sequence and structure of the targeted DNA. Mol. Cell. Biol. 2005;25:10815–10821. doi: 10.1128/MCB.25.24.10815-10821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Storb U. Activation-induced cytidine deaminase (AID) can target both DNA strands when the DNA is supercoiled. Proc. Natl. Acad. Sci. 2004;101:12997–13002. doi: 10.1073/pnas.0404974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HM, Tanaka A, Bozek G, Nicolae D, Storb U. Somatic hypermutation and class switch recombination in Msh6(−/−)Ung(−/−) double-knockout mice. J Immunol. 2006;177:5386–5392. doi: 10.4049/jimmunol.177.8.5386. [DOI] [PubMed] [Google Scholar]
- Shen HM, Poirier MG, Allen MJ, North J, Lal R, Widom J, Storb U. The activation-induced cytidine deaminase (AID) efficiently targets DNA in nucleosomes but only during transcription. J Exp Med. 2009;206:1057–1071. doi: 10.1084/jem.20082678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Seo YR. p53 regulation of DNA excision repair pathways. Mutagenesis. 2002;17:149–156. doi: 10.1093/mutage/17.2.149. [DOI] [PubMed] [Google Scholar]
- Storb U, Peters A, Klotz E, Kim N, Shen HM, Kage K, Rogerson B, Martin TE. Somatic hypermutation of immunoglobulin genes is linked to transcription. Curr Top Microbiol Immunol. 1998;229:11–19. doi: 10.1007/978-3-642-71984-4_2. [DOI] [PubMed] [Google Scholar]
- Storb U, Shen HM, Nicolae D. Somatic hypermutation: processivity of the cytosine deaminase AID and error-free repair of the resulting uracils. Cell Cycle. 2009;8:3097–3101. doi: 10.4161/cc.8.19.9658. [DOI] [PubMed] [Google Scholar]
- Tissier A, McDonald JP, Frank EG, Woodgate R. poliota, a remarkably error-prone human DNA polymerase. Genes Dev. 2000;14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Young LC, Keuling AM, Lai R, Nation PN, Tron VA, Andrew SE. The associated contributions of p53 and the DNA mismatch repair protein Msh6 to spontaneous tumorigenesis. Carcinogenesis. 2007;28:2131–2138. doi: 10.1093/carcin/bgm153. [DOI] [PubMed] [Google Scholar]
- Zaky A, Busso C, Izumi T, Chattopadhyay R, Bassiouny A, Mitra S, Bhakat KK. Regulation of the human AP-endonuclease (APE1/Ref-1) expression by the tumor suppressor p53 in response to DNA damage. Nucleic Acids Res. 2008;36:1555–1566. doi: 10.1093/nar/gkm1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ahn J, Wilson SH, Prives C. A role for p53 in base excision repair. Embo J. 2001;20:914–923. doi: 10.1093/emboj/20.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ermakova OV, Riblet R, Birshtein BK, Schildkraut CL. Replication and subnuclear location dynamics of the immunoglobulin heavy-chain locus in B-lineage cells. Mol Cell Biol. 2002;22:4876–4889. doi: 10.1128/MCB.22.13.4876-4889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.