Abstract
We reported previously that hypertonic saline (HS) treatment can prevent or upregulate the function of polymorphonuclear neutrophils (PMN) via A2a adenosine receptors (A2aR) or A3 adenosine receptors (A3R), respectively. A3R translocate to the cell surface upon PMN stimulation and thus HS promotes PMN responses under conditions of delayed HS treatment. Here we investigated if inhibition of A3R improves the protective effects of HS resuscitation in a mouse sepsis model. We found that HS nearly triples extracellular adenosine concentrations in whole blood and that inhibition of A3R with the selective antagonist MRS-1191 dose-dependently improves the inhibitory effect of HS. MRS-1191 at a concentration of 1 nM enhanced the inhibitory effect of HS and reduced stimulatory effects of delayed HS treatment. Using a mouse model of cecal ligation and puncture (CLP)-induced sepsis, we found that MRS-1191 reduces acute lung injury and PMN accumulation in lung tissue. While delayed HS treatment (4 ml/kg of 7.5 % NaCl) of mice 1 h after CLP aggravated PMN accumulation, lung tissue damage, and mortality 24 h after CLP, infusion of MRS-1191 (2 ng/kg body weight) combined with HS reduced these detrimental effects of delayed HS treatment. Our data thus show that A3 receptor antagonists can strengthen the beneficial effects of HS resuscitation by avoiding stimulatory side effects that result from delayed HS administration.
Keywords: Polymorphonuclear neutrophils, hypertonic saline, neutrophil elastase, A3 adenosine receptor antagonist, sepsis, acute lung injury
INTRODUCTION
Acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and multiple organ failure (MOF) are major clinical complications that increase mortality after trauma, burns, and sepsis 1,2,3. Polymorphonuclear neutrophils (PMN) play an important role in the pathogenesis of these complications. PMN are vital for a successful immune defense under normal conditions, but in trauma patients, PMN can cause severe host tissue damage by excessive release of cytotoxic mediators such as neutrophil elastase and reactive oxygen species.
Various therapeutic modalities aimed at ameliorating post-traumatic complications resulting from excessive PMN activation have been explored in experimental settings and clinical studies. One such treatment option involves the use of hypertonic saline (HS) solutions that can be administered as resuscitation fluids. Since the early 1980’s, interest in the concept of small-volume HS resuscitation has grown because of beneficial hemodynamic effects 5,6,7, but also because of potential immunomodulatory effects of HS that were originally identified in our laboratory 8-14. In clinical and experimental settings, HS is typically administered as 7.5% NaCl solutions at a dose of 4 ml/kg body weight. This can increase plasma tonicity by up to 40 mM, which we have found to strongly influence PMN functions. Numerous studies have demonstrated that HS has protective anti-inflammatory effects that involve inhibition of PMN activation. However, these protective effects of HS are diminished when HS treatment occurs in a delayed fashion after PMN activation has occurred 15-17. Moreover, such delayed administration of HS can enhance PMN responses, which may exacerbate PMN-induced host tissue damage 15, 18-20. The unpredictability as to whether and under which clinical conditions HS treatment may prevent or aggravate PMN responses in trauma patients limits the use of HS resuscitation as a therapeutic tool to reduce post-traumatic inflammation.
In our previous studies, we focused on the molecular mechanisms by which HS affects PMN responses. These studies have revealed that ATP release, hydrolysis of ATP to adenosine, and activation of adenosine receptors play important roles in the response of leukocytes to HS 21-23. The generation of adenosine in the extracellular environment of PMN results in the stimulation of A2a-type adenosine receptors (A2aR) of PMN. A2aR suppress PMN through a negative feedback mechanism that involves up-regulation of intracellular cAMP and protein kinase A signaling which block cell activation 22, 23. In addition to A2aR, we found that PMN also express A3-type adenosine receptors (A3R) that have an opposing effect on PMN, namely by triggering mitogen-activate protein kinase signaling that promotes PMN activation 21. Cell stimulation, for example with formyl peptide (fMLP), induces rapid translocation of A3R from the cytosol to the cell surface, making these receptors available for subsequent stimulation by extracellular adenosine generated in response to HS 4, 20.
Thus, A3R may diminish the protective properties of HS resuscitation by reducing the inhibitory effects of HS on PMN responses. The purpose of the current study was to investigate whether A3 receptor antagonists can be used to improve the beneficial efficacy of HS resuscitation in a mouse sepsis model.
MATERIALS AND METHODS
Purification of human PMN
The Human Research Program of the Beth Israel Deaconess Medical Center approved all experiments described in this study. Peripheral venous blood was obtained from healthy human volunteers. PMN were isolated from the peripheral blood as described previously 4. Briefly, heparinized blood was mixed with 5% (wt/vol) Dextran 500 dissolved in normal saline solution (Pharmacia, Piscataway, NJ) and allowed to sediment for 30 min at room temperature. The cells in the supernatant were separated by Percoll gradient centrifugation according to the manufacturer’s recommendations (Amersham Biosciences, Piscataway, NJ). The PMN layer was collected and washed twice with Hank’s balanced salt solution (HBSS) containing Ca2+ and Mg2+ (HyClone, Logan, UT). PMN were suspended in HBSS to a concentration of 107 cells/ml and used for experiments immediately. Cell isolation and all subsequent experiments were performed under sterile and strictly pyrogen-free conditions.
In vitro assay of PMN activation
PMN were stimulated with HS by adding appropriate dilutions of pyrogen-free and sterile HBSS containing an additional 1 M NaCl so as to increase the tonicity of the final cell culture medium by 10, 20, or 40 mM beyond isotonic levels. This range of hypertonicity was chosen because it corresponds to plasma hypertonicity levels found in trauma patients after infusion of HS resuscitation fluids 24. Different concentrations of the A3R antagonist, 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS-1191; Sigma) were prepared in HBSS and used at final concentrations of 0, 0.01, 0.1, or 1 nM. These levels of MRS-1191 were chosen based on initial pilot studies. PMN (106) were pre-incubated for 30 min at 37°C with 5 μM cytochalasin B (Sigma, St. Louis, MO) as described previously 21,25. The cells were stimulated with 100 nM fMLP either 5 min before or after exposure to HS in the absence or presence of MRS-1191. After incubation for another 1 h, samples were placed on ice for 10 min, centrifuged 10 s at 16,000×g, and elastase activity released into the supernatant was measured as described previously 21. Briefly, 40 μl of supernatants was mixed with 160 μl of a buffer consisting of 50 mM Tris/HCl and 100 mM NaCl, pH 7.4, containing 0.05% (vol/vol) Triton X-100. Enzymatic reactions were started by the addition of the elastase-specific chromogenic substrate N-methoxysuccinyl-(L-alanyl)2-L-prolyl-L-vaniline 4-nitroanilide (Sigma) at a final assay concentration of 1 mM. After 30 min at room temperature, changes in optical density were measured at a wavelength of 405 nm using a UV-1201 spectrophotometer (Shimazu Corporation, Colombia, MD).
HS-induced extracellular adenosine formation
Heparinized human whole blood samples were diluted with an equal volume of HBSS, pre-warmed for 30 min at 37°C, and then treated with HS to elevate extracellular tonicity by 40 mM. Adenosine accumulation in the extracellular space was determined with high performance liquid chromatography (HPLC) as described previously 4.
Animal model
The use of laboratory animals was in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. All studies were performed with adult male C57BL/6J wild-type mice (Charles River Laboratories, Wilmington, MA), 8-10 weeks old, weighing 20-25 g. Sepsis was induced by cecal ligation and puncture (CLP) as previously described 17. Briefly, general anesthesia was induced and maintained with 1-2% Isoflurane using a precision vaporizer (V-1 Table Top System; Vet Equip, Pleasanton, CA) and a catheter was implanted into the right femoral artery either before or after induction of sepsis as described below. Prior to implantation, the right inguinal area was shaved and disinfected with Povidone iodine. Then the femoral artery was exposed through a 3-mm incision. The artery was ligated on the distal side using a 5-0 thread. The artery was opened lengthwise using a No. 10 blade and a PE10 catheter was inserted (Becton Dickinson, Sparks, MD). CLP was performed by placing a 1-cm midline abdominal incision. The exposed cecum was ligated at its distal side to the ileocecal valve with 4-0 silk, avoiding intestinal obstruction. The cecum was punctured twice with a 22-G needle, returned into the abdomen, and the abdominal wall was closed in layers. After full recovery, the mice were returned to their cages and allowed access to standard diet and water ad libitum.
Treatment with hypertonic saline and A3 receptor antagonist
Before or after induction of sepsis, 4 ml of a 7.5% NaCl solution with or without the A3R antagonist MRS-1191 was administered via the femoral catheter. The animals were randomly divided into the following groups:
Untreated animals were subjected to catheter implantation and CLP but no fluid infusion.
Animals pre-treated with HS received 4 ml/kg of 7.5% HS 10 min prior to CLP (HS→ CLP).
Animals pre-treated with HS + MRS-1191 received HS and 2 ng/kg MRS-1191 10 min before CLP (HS + MRS-1191 →CLP).
Animals subjected to delayed treatment with HS received CLP followed by HS after 60 min (CLP→HS)
Animals subjected to delayed treatment with HS and MRS-1191 received CLP followed by administration of HS + 2 ng/kg MRS-1191 60 min after CLP (CLP→ HS + MRS-1191)
Animals treated only with MRS-1191 received 2 ng/kg MRS-1191 but not HS 10 min prior to CLP (MRS-1191).
A surgery control group received all surgical interventions but not CLP and no fluid infusion (sham group).
The effects of HS and MRS-1191 treatment on PMN accumulation in the lungs was determined using 6 mice per treatment group and survival experiments were carried out using 11 mice per treatment group.
Neutrophil accumulation in lung tissue
As a marker of PMN recruitment to the lungs, neutrophil elastase (NE) activity in lung tissue sample homogenates was determined 2 h after CLP. The choices of using ME as a neutrophil marker rather than myeloperoxidase (MPO) and of the 2-h time point were based on our previous experience with this animal model that has shown that ME is a more reliable marker than MPO and that PMN accumulation reaches a peak at 2 h 17. To harvest lung tissue samples, mice were anesthetized as described above and euthanized by cervical dislocation. The pulmonary circulatory system was flushed with 10 ml HBSS through the right ventricle after exsanguination by cardiac puncture. Then the whole lung was carefully removed, snap-frozen in liquid nitrogen, and stored at −80°C until analysis. Lungs were weighed, placed in 5 ml ice cold 50 mM potassium phosphate buffer, pH 6.0 containing 0.5% hexadecyl-trimethyl-ammonium bromide (Sigma), and homogenized on ice using a manual Dounce tissue homogenizer. The homogenates were centrifuged at 20,000×g and 4°C for 30 min and 20 μl of the supernatants was added in duplicate into a 96-well plate. NE activity was measured as described previously 17.
Survival and lung tissue damage
To compare the survival rates in animals receiving HS pre-treatment vs. delayed HS treatment with or without MRS-1191, a separate set of mice with 11 animals per group was subjected to CLP and treatment with HS and MRS-1191 as described above. Survival rates were monitored for 48 h after CLP. In addition, a small set of 2-3 animals per group was used to evaluate lung tissue damage using histological examination. Controls included un-manipulated healthy mice and mice subjected to CLP without fluid treatment. Lungs of these animals were collected 24 h after CLP and histological examination was performed as previously described 20.
Statistical analysis
All values are expressed as mean ± standard deviation (SD). Statistical analyses were performed with ANOVA for comparisons of differences between categories, Student’s t-test with Bonferroni correction for multiple comparisons within categories, or Kaplan-Meier assay as indicated. Differences between groups were considered statistically significant at p values < 0.05.
RESULTS
HS treatment induces rapid accumulation of extracellular adenosine
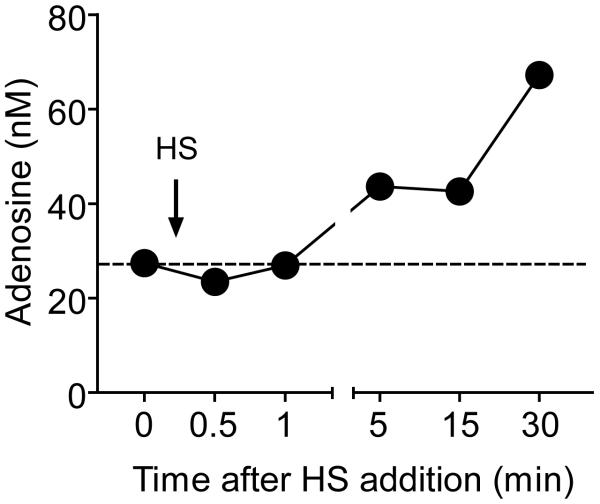
We have previously shown with purified human PMN that HS regulates PMN functions by the release of cellular ATP and formation of extracellular adenosine that regulates PMN responses though autocrine feedback mechanisms involving A2a and A3 adenosine receptors 21, 23. Under physiological conditions, adenosine can be generated from ATP released by PMN but also by neighboring cells that respond to HS and that can therefore indirectly regulate PMN responses in a paracrine fashion. We investigated the kinetics of extracellular adenosine formation in human whole blood in response to addition of clinically relevant level of HS. HS at a hypertonicity level of 40 mM beyond isotonicity induced rapid accumulation of extracellular adenosine (Fig. 1). Adenosine concentrations rose within 5 min and gradually increased to ~70 nM, which is nearly 3-fold higher than the baseline concentrations measured under isotonic conditions. Adenosine at these concentrations has profound effects on PMN responses 4, 21, 23. These findings suggest that HS infusion leads to elevated adenosine concentrations that have strong modulatory effects on PMN function in circulating blood.
Fig. 1. HS induces adenosine accumulation in human whole blood.
Heparinized human whole blood was treated with 40 mM HS for the indicated times and adenosine accumulation in the extracellular space was determined by HPLC analysis.
Inhibition of A3 adenosine receptors reduces the enhancing effect of HS
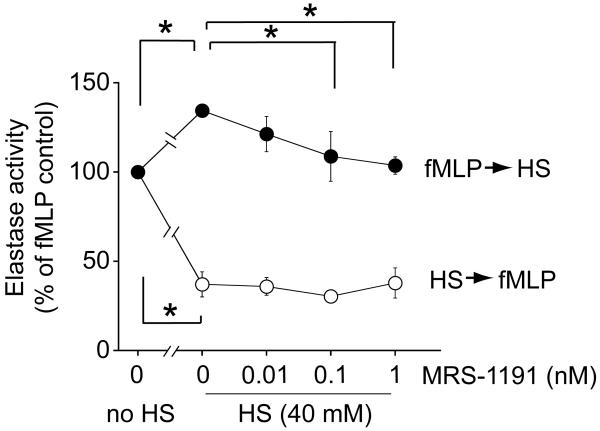
We have previously reported that HS treatment prior to or at the time of cell stimulation blocks PMN activation, while addition of HS after fMLP stimulation enhances PMN responses 8. Our subsequent work has shown that these enhancing effects of HS may be due to A3R receptor activation. A3R receptors rapidly translocate to the cell surface upon PMN stimulation and thus HS can enhance PMN responses via A3R 4, 20. In the current study, we found that treatment of purified human PMN with a combination of HS (40 mM) and the A3R antagonist MRS-1191 (0.01, 0.1, and 1 nM) dose-dependently abolishes these enhancing effects of HS (Fig. 2). However, MRS-1191 treatment did not change the suppressive effect of HS that was observed when PMN were pre-treated with 40 mM HS prior to stimulation with fMLP (Fig. 2). These findings suggest that A3R can be modulated with MRS-1191 only after PMN are stimulated with fMLP. Moreover, our findings suggest that pre-treatment with 40 mM HS can block A3 receptor translocation to the cell surface, which corresponds to our findings published in a previous report 20.
Fig. 2. Inhibition of A3 receptors diminishes the enhancing effect of HS.
Purified human PMN were treated with the indicated concentrations of MRS-1191 and 40 mM HS either 5 min before (open circles) or 5 min after (solid circles) cell stimulation with 100 nM fMLP. PMN activation was determined by assessing degranulation based on elastase release. Data expressed as means ± SD are representative of 3 individual experiments with PMN from different donors. Statistical analysis: ANOVA, Bonferroni; *p<0.01.
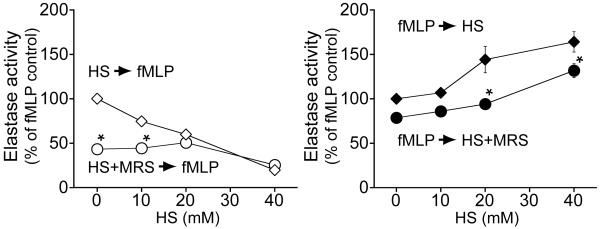
While MRS-1191 did not further suppress cells pre-treated with 40 mM HS, MRS-1191 significantly reduced the suppressive effect of lower concentrations of HS (Fig. 3, left panel). In addition, we found that MRS-1191 treatment also diminished the response of PMN to fMLP in the absence of HS. These findings suggest that A3 receptors may play a role in fMLP-induced PMN responses under isotonic or weakly hypertonic conditions.
Fig. 3. Inhibition of A3 receptors increases the suppressive effect of HS on PMN.
Purified human PMN were treated with the indicated concentrations of HS either with (circles) or without (diamonds) MRS-1191 at a final concentration of 1 nM. HS +/− MRS-1191 was added either 5 min before (open symbols; left panel) or 5 min after cell stimulation (solid symbols; right panel) with fMLP (100 nM). PMN activation was determined by assessing degranulation by measuring elastase release. Data expressed as mean ± SD are representative of at least 3 individual experiments with PMN from different donors. Statistical analysis: ANOVA, Bonferroni; *p<0.01.
Treatment of PMN with MRS-1191 (1 nM) after prior cell stimulation with fMLP significantly reduced the enhancing effect of HS on PMN function (Fig. 3, right panel). These findings indicate that inhibition of A3 receptors is able to diminish the enhancing effects of delayed HS treatment.
Inhibition of A3 adenosine receptors reduces PMN recruitment to the lungs
Based on the in vitro findings described above, we tested if combined treatment with A3R antagonists and HS reduces PMN recruitment to the lungs in a mouse model of CLP-induced sepsis.
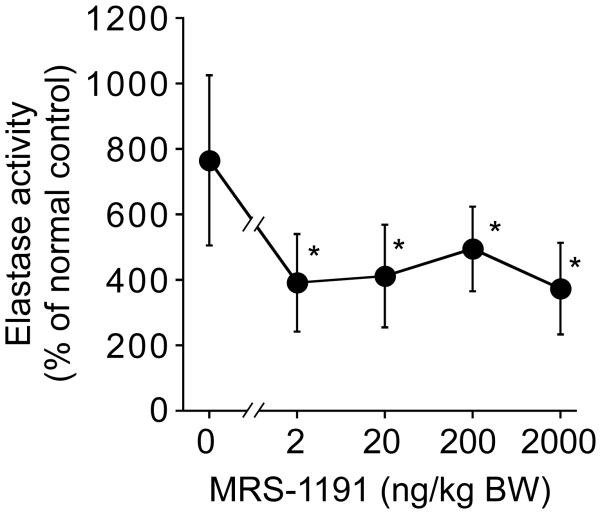
Initially, we determined the most suitable dose of MRS-1191 as shown in Fig. 4. Mice were subjected to CLP allowed to develop sepsis for 60 min and then the animals were treated with 4 ml/kg HS containing either no or 1, 10, 100, or 1,000 nM MRS-1191. These MRS-1191 amounts correspond to doses of 0, 2, 20, 200, or 2,000 ng/kg body weight. MRS-1191 even at the lowest dose tested significantly reduced PMN accumulation in the lungs. Based on these results, we decided to use MRS-1191 at a dose of 2 ng/kg body weight for all subsequent experiments.
Fig. 4. Dose response curve of MRS-1191 on PMN recruitment.
Mice were subjected to CLP and after 60 min treated with HS (4 ml/kg) in combination with the indicated doses of MRS-1191. Neutrophil recruitment to the lungs was evaluated as described in Fig. 4. Data are expressed as mean ± SD and represent results with 4 animals for each data point. Statistical analysis was done with ANOVA, *p<0.05.
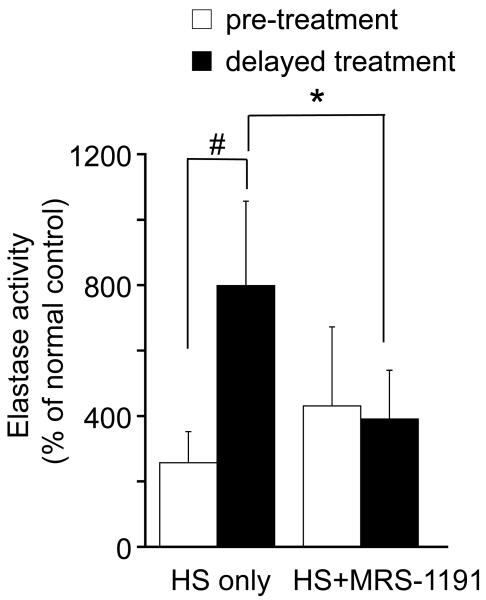
Next, we tested whether MRS-1191 treatment affects PMN accumulation in the lungs of animals that were either pre-treated with HS or the received HS treatment after CLP. Animals that received CLP 60 min before HS treatment showed significantly more pronounced accumulation of PMN in their lungs compared to animals that were pre-treated with HS for 10 min and then subjected to CLP (p<0.05; Fig. 5). However, we found that combined treatment of animals with HS (4 ml/kg body weight) and MRS-1191 (2 ng/kg body weight) completely abrogates the increase in PMN accumulation that was observed in response to delayed HS treatment (p<0.05).
Fig. 5. MRS-1191 reduces PMN recruitment to the lungs of septic mice.
Mice were subjected to sepsis by cecal ligation and puncture (CLP) and treated either with HS alone (4 ml 7.5% NaCl per kg body weight) or with HS in combination with MRS-1191 (2 ng/kg), which was administered either 10 min before (empty bars) or 60 min after CLP (filled bars). PMN accumulation in the lungs was assessed by measuring neutrophil elastase activity in lung tissue homogenates. Data are shown as percent of values measured in untreated normal control animals. Date are expressed as mean ± SD; (n = 6-8 animals per group; ANOVA, #p<0.01, *p<0.05).
MRS-1191 reduces acute lung injury and mortality after CLP
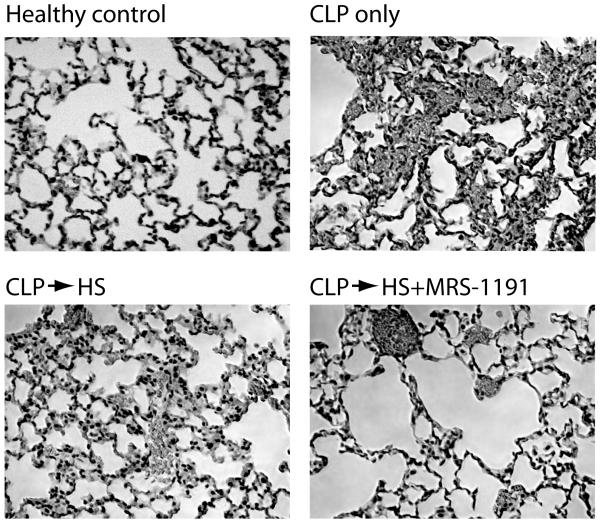
We evaluated signs of lung tissue damage by histological examination of the lungs 24 h after CLP. Histological sections from untreated CLP mice and from animals subjected to CLP and treated with 4 ml/kg HS after 60 min showed characteristic abnormalities such as focal atelectases, hyperemia with congestion, infiltration of PMN into widened alveolar septae, and alveolar spaces containing fluid, debris, and inflammatory cells (Fig. 6). These characteristics of tissue damage were less prominent when animals were treated with a combination of HS and MRS-1191(2 ng/kg), suggesting that inhibition of A3 receptors may improve clinical outcome parameters.
Fig. 6. MRS-1191 reduces acute lung injury.
Animals were subjected to CLP and treated with HS +/− MRS-1191 (2 ng/kg) 60 min after CLP. Control animals were subjected to CLP and remained untreated. Lung tissue samples were obtained 24 h after CLP and evaluated by histological examination of paraffin sections stained with hematoxylin and eosin (HE); magnification ×200.
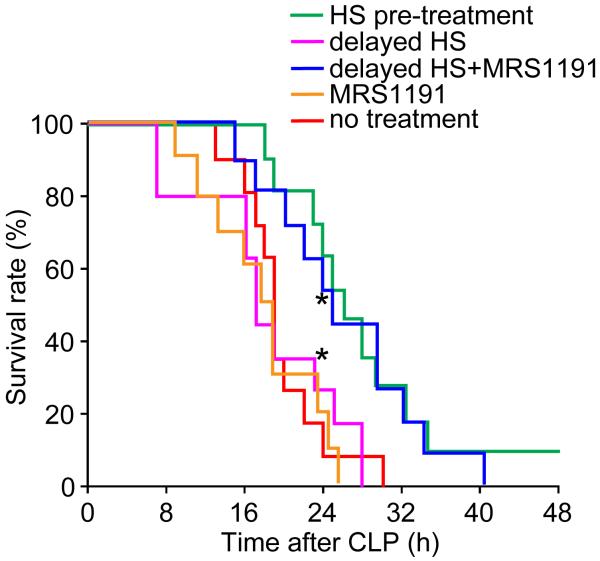
Finally, we studied whether MRS-1191 treatment can improve survival in our CLP mouse model. Mice were treated with 4 ml/kg HS either 10 min before or 60 min after CLP. Delayed HS treatment was provided either with or without MRS-1191 (2 ng/kg). As controls, one group of mice received CLP but no treatment and a second group received only MRS-1191(2 ng/kg) 10 min before CLP, but not HS. Each group consisted of 11 animals and survival was monitored for 48 h after CLP. The 24-h survival rates of animals that received MRS-1191 alone or delayed HS treatment were similar to that of animals without treatment (Fig. 7). By contrast, survival rates 24 h after CLP were significantly higher in the groups that received pre-treatment with HS or that were subjected to delayed HS treatment in combination with MRS-1191 (30% vs. 70%; p<0.05). These results suggest that inhibition of A3 receptors improves the protective efficacy of HS and ameliorates outcome even under adverse clinical circumstances that would otherwise result in exaggerated PMN activation and aggravated host organ damage. The finding that MRS-1191 alone did not improve outcome after sepsis suggests that A3 receptor are not activated in the absence of HS.
Fig. 7. Combined treatment with HS and MRS-1191 improves survival after sepsis.
Mice subjected to CLP were treated with HS (4 ml/kg) with or without MRS-1191 (2 ng/kg), either 10 min before or 60 min after CLP and survival was monitored. At the 24-h time point, survival rates in the HS pretreatment group and the delayed HS treatment group with MRS-1191 differed significantly from all other group (Kaplan-Meier, n=11, *p<0.05).
DISCUSSION
Because of their hemodynamic and many other beneficial properties, hypertonic resuscitation fluids have gained increased attention as potential new therapeutic agents to treat hemorrhagic shock. Our previous work has shown that HS can modulate the immune response after trauma 8-11, 14-16. Specifically, we found that HS can control PMN activation through autocrine mechanisms that involve ATP release and activation of A2a and A3 adenosine receptors 21,23. These two adenosine receptor subtypes elicit opposing effects on PMN responses: A2aR suppress cell activation, while A3R enhance PMN responses. We found that HS exposure triggers accumulation of adenosine in the extracellular space surrounding PMN, thus providing the ligand for both adenosine receptor subtypes. Therefore, the availability and relative distribution of these two receptor subtypes on the cell surface is a key factor that determines whether HS treatment suppresses or enhances PMN responses.
The expression of A3R on the cell surface is a highly dynamic process that seems to depend on the activation state of PMN. Stimulation of PMN with formyl peptide induces the rapid translocation of A3R from the cytosol to the cell surface, which makes this stimulatory receptor available to for activation by adenosine that is generated in response to HS 4, 20. Our current results with MRS-1191 indicate that pre-treatment of PMN with high doses of HS (40 mM) blocks A3R translocation to the cell surface, which is reflected by our finding that MAR-1191 did not alter the response of cells that were pre-treated with HS (Fig. 2). However, the data shown in Fig. 3 (left panel) suggest that at least a limited number of A3R are present on the surface of resting PMN under isotonic or slightly hypertonic conditions. Future studies will be necessary to determine more directly (for example with flow cytometry) how cell stimulation and HS influence the dynamics of A3R translocation and the presence of other important purinergic receptors such as A2aR on the cell surface of PMN.
Our current findings and previous studies have shown that the timing of HS treatment relative to PMN activation or initiation of inflammation determines whether HS resuscitation prevents or enhances PMN responses in vitro 10, 11 and tissue damage in vivo 15, 16. Based on such experimental data, it was assumed that HS resuscitation would be most efficacious in clinical settings where HS treatment is provided during the initial resuscitation of trauma patients 11,19, as this treatment modality would reduce the risk of exacerbating host tissue damage by inadvertently exacerbating PMN-induced post-traumatic inflammatory complications. Unfortunately, unlike with in vitro studies and animal models where the timing of HS treatment can be freely chosen, this is impossible in clinical settings. It is virtually impossible to predict the inflammatory state of trauma patients prior to resuscitation and therefore, although initial HS resuscitation may be beneficial for most trauma patients, it may still exacerbate inflammatory processes in subsets of patients with previously activated PMN. Our current study suggests that A3R activation could be involved in such undesirable side effects of HS resuscitation.
We found that inhibition of A3R increases the anti-inflammatory efficacy of HS, particularly under conditions that would otherwise result in aggravated PMN activation and host tissue injury. This effect was observed with low concentrations of MRS-1191. Thus, adequate antagonist delivery could be achieved by administering MRS-1191 along with HS resuscitation fluids. MRS-1191 at similar concentrations has been shown to reduce various inflammatory processes in several animal models 28-33. Further studies will be needed to test different types of A3R antagonist and to develop appropriate treatment strategies to optimize the beneficial effects of these A3R antagonists for use in conjunction with HS resuscitation. Interestingly, MRS-1191 treatment alone did not improve survival in our mouse model, which suggests that HS must be present to activate A3R and that sepsis itself does not generate sufficient adenosine concentrations induce A3R activation. This conclusion is supported by preliminary results of our ongoing studies, which indicate that plasma adenosine concentrations do not accumulate in our mouse sepsis model despite the fact that we find high plasma levels of ATP.
Acute lung injury and multiple organ failure are among the most crucial complications that contribute to the high mortality after sepsis and severe traumatic injuries 1-3. PMN play an important role in the development of these post-traumatic complications 34-35. Based on our present findings, we conclude that HS in combination with A3R antagonists may represent a novel therapeutic approach to reduce PMN activation and thereby protect critically ill patients from secondary organ damage.
ACKNOWLEDGEMENT
This study was supported in part by National Institute of Health Grant R01 GM-60475 (W.G.J.).
Footnotes
The authors have no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Botha AJ, Moore FA, Moore EE, Sauaia A, Banerjee A, Peterson VM. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J Trauma. 1995;39:411–417. doi: 10.1097/00005373-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Eng J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 4.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 5.Velasco IT, Pontieri V, Rocha e Silva M., Jr Lopes OU: Hyperosmotic NaCl and severe hemorrhagic shock. Am J Physiol. 1980;239:H664–H673. doi: 10.1152/ajpheart.1980.239.5.H664. [DOI] [PubMed] [Google Scholar]
- 6.Velasco IT, Rocha e Silva M, Oliveira MA, Oliveira MA, Silva RI. Hypertonic and hyperoncotic resuscitation from severe hemorrhagic shock in dogs: a comparative study. Crit Care Med. 1989;17:262–264. doi: 10.1097/00003246-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Younes RN, Aun F, Tomida RM, Birolini D. The role of lung innervation in the hemodynamic response to hypertonic sodium chloride solutions in hemorrhagic shock. Surgery. 1985;98:900–9061. [PubMed] [Google Scholar]
- 8.Junger WG, Hoyt DB, Davis RE, Herdon-Remelius C, Namiki S, Junger H, Loomis W, Altman A. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101:2768–2779. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angle N, Hoyt DB, Coimbra R, et al. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9:164–170. doi: 10.1097/00024382-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Angle N, Hoyt DB, Coimbra R, Liu F, Herdon-Remelius C, Loomis W, Junger WG. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42:190–196. [PubMed] [Google Scholar]
- 11.Junger WG, Coimbra R, Liu FC, Herdon-Remelius C, Junger W, Junger H, Loomis W, Hoyt DB, Altman A. Hypertonic saline resuscitation: A tool to modulate immune function in trauma patients? Shock. 1997;8:235–241. [PubMed] [Google Scholar]
- 12.Rizoli SB, Kapus A, Fan J, Li YH, Marshall JC, Rotstein OD. Immunomodulatory effects of hypertonic resuscitation on the development of lung inflammation following hemorrhagic shock. J Immunol. 1998;161:6288–6296. [PubMed] [Google Scholar]
- 13.Rotstein OD. Novel strategies for immunomodulation after trauma: revisiting hypertonic saline as a resuscitation strategy for hemorrhagic shock. J Trauma. 2000;49:580–583. doi: 10.1097/00005373-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Angle N, Hoyt DB, Cabello-Passini R, Herdon-Remelius C, Loomis W, Junger WG. Hypertonic resuscitation reduces neutrophil margination by suppressing neutrophil L selectin expression. J Trauma. 1998;45:7–13. doi: 10.1097/00005373-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Murao Y, Hoyt DB, Loomis W, Namiki S, Patel N, Wolf P, Junger WG. Does the timing of hypertonic saline resuscitation affect its potential to prevent lung damage? Shock. 2000;14:18–23. doi: 10.1097/00024382-200014010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Murao Y, Loomis W, Wolf P, Hoyt DB, Junger WG. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock. 2003;20:29–34. doi: 10.1097/01.shk.0000071060.78689.f1. [DOI] [PubMed] [Google Scholar]
- 17.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciesla DJ, Moore EE, Zallen G, Biffl WL, Silliman CC. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: timing is everything. J Trauma. 2000;48:388–395. doi: 10.1097/00005373-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363:1988–1996. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- 20.Inoue Y, Chen Y, Pauzenberger R, Hirsh MI, Junger WG. Hypertonic saline up-regulates A3 adenosine receptors expression of activated neutrophils and increases acute lung injury after sepsis. Crit Care Med. 2008;36:2569–2575. doi: 10.1097/CCM.0b013e3181841a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Hashiguchi N, Yip L, Junger WG. Hypertonic saline enhances neutrophil elastase release through activation of P2 and A3 receptors. Am J Physiol Cell Physiol. 2006;290:C1051–C1059. doi: 10.1152/ajpcell.00216.2005. [DOI] [PubMed] [Google Scholar]
- 22.Orlic T, Loomis WH, Shreve A, Namiki S, Junger WG. Hypertonicity increases cAMP in PMN and blocks oxidative burst by PKA-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2002;282:C1261–C1269. doi: 10.1152/ajpcell.00479.2001. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76:245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 24.Mattox K, Maningas PA, Moore EE, Mateer JR, Marx JA, Aprahamian C, Burch JM, Pepe PE. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The U.S.A. multicenter trial. 1. Ann Surg. 1991;213:482–491. doi: 10.1097/00000658-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nick JA, Avdi NJ, Young SK, Knall C, Gerwins P, Johnson GL, Worthen GS. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato N, Kashima K, Shimizu H, Uehara Y, Shimomura Y, Mori M. Hypertonic glucose inhibits the production of oxygen-derived free radicals by rat neutrophils. Life Sci. 1993;52:1481–148. doi: 10.1016/0024-3205(93)90109-g. [DOI] [PubMed] [Google Scholar]
- 27.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 28.Hinschen AK, Rose’Meyer RB, Headrick JP. Adenosine receptor subtypes mediating coronary vasodilation in rat hearts. J Cardiovasc Pharmacol. 2003;41:73–80. doi: 10.1097/00005344-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Liu GS, Richards SC, Olsson RA, Mullane K, Walsh RS, Downey JM. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc Res. 1994;28:1057–1061. doi: 10.1093/cvr/28.7.1057. [DOI] [PubMed] [Google Scholar]
- 30.Headrick JP, Peart J. A3 adenosine receptor-mediated protection of the ischemic heart. Vascul Pharmacol. 2005;42:271–279. doi: 10.1016/j.vph.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- 32.Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol. 2006;35:549–558. doi: 10.1165/rcmb.2006-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Kapoian T, Shepard M, Lianos EA. Adenosine-induced apoptosis in glomerular mesangial cells. Kidney Int. 2002;61:1276–1285. doi: 10.1046/j.1523-1755.2002.00256.x. [DOI] [PubMed] [Google Scholar]
- 34.Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003;55:608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- 35.Inoue Y, Seiyama A, Tanaka H, Ukai I, Akimau P, Nishino M, Shimazu T, Sugimoto H. Protective effects of a selective neutrophil elastase inhibitor (Sivelestat) on LPS-induced acute dysfunction of the pulmonary microcirculation. Crit Care Med. 2005;33:1814–1822. doi: 10.1097/01.ccm.0000172547.54086.ad. [DOI] [PubMed] [Google Scholar]