Abstract
Primary dystonia is a movement disorder characterized by sustained muscle contractions and in which dystonia is the only or predominant clinical feature. TOR1A (DYT1) and the transcription factor THAP1 (DYT6) are the only genes identified thus far for primary dystonia. Using electromobility shift assays and chromatin immunoprecipitation (ChIP)-qPCR, we demonstrate a physical interaction between THAP1 and the TOR1A promoter that is abolished by pathophysiologic mutations. Our findings provide the first evidence that causative genes for primary dystonia intersect in a common pathway and raise the possibility of developing novel therapies targeting this pathway.
Introduction
Mutations in the transcription factor THAP1 were recently found to cause dystonia type 6 1-7, Many of the mutations that have been identified are located within the THAP domain, a sequence-specific DNA-binding domain belonging to the zinc-finger superfamily 8-11. Our previous study showed that a single point mutation (F81L) in the DNA binding domain of THAP1 impaired its DNA binding affinity 1, suggesting that THAP1 mutations may cause a transcriptional dysregulation of THAP1 downstream targets.
THAP1 has been shown to be a modulator of Ribonucleoside-diphosphate reductase large subunit 1 (RRM1) and several other pRB/E2F cell-cycle target genes in endothelial cells, including mitotic arrest deficient 2 (MAD2), survivin (BIRC5), hyaluronan-mediated motility receptor (HMMR), Ribonucleoside-diphosphate reductase large subunit 2 (RRM2), cell division cycle 2 (CDC2), cyclin B1, and Disks Large Homolog 7 (DLG7) 12, but these genes have not been linked to dystonia. As clinically defined forms of primary dystonia have been mapped to distinct loci with no obvious physiological link, no common pathways have emerged. However, the phenotypic overlap among forms of primary dystonia suggests that THAP1 could represent a link via transcriptional regulation of other dystonia genes. We therefore investigated whether the TOR1A gene mutated in dystonia type 1, might be a transcriptional target gene for THAP1. In the present study we demonstrated that TOR1A is a direct target of endogenous THAP1 in human primary cells and in mouse brain.
Results
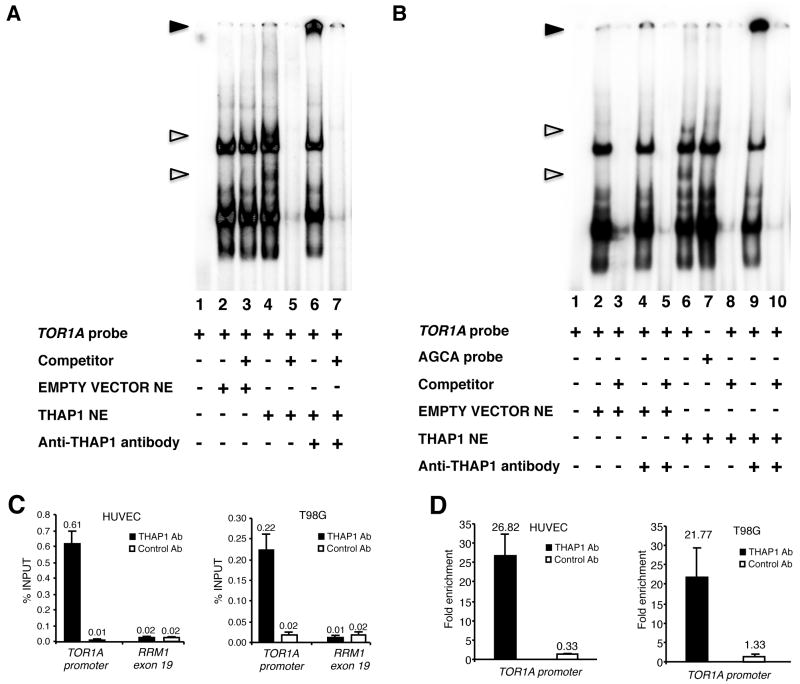
A previous study 9 has determined that THAP1 binds to an 11-bp consensus DNA sequence, the THAP-domain-binding sequence (THABS). Sequence evaluation of the human TOR1A minimal gene promoter 13 for putative THABS predicted an inverted THABS at nucleotides -111/-101 relative to the translation initiation site of the TOR1A gene: cTGCCctgAag. To determine whether THAP1 binds to the TOR1A promoter, nuclear extracts from 293T cells transfected with wild type human THAP1 were subjected to electrophoretic mobility shift assays using the human TOR1A fragment -159/-80 as a probe. We detected two protein/DNA complexes that were specific to THAP1 over-expression and were competed off by an excess of unlabelled probe (Fig 1A), indicating that these interactions were specific. The presence of THAP1 in the complexes was confirmed by their supershift with an antibody to THAP1 (Fig 1A). The GGCA core motif of the THABS was previously shown to be required for THAP-domain–THABS interaction 9. Consistently, EMSA with the TOR1A probe -159/-80 mutated in its core consensus site (AGCA probe) did not reveal a shift with the same cellular extract (Fig 1B).
Figure 1.
THAP1 binds the TOR1A promoter in vitro and in vivo. (A,B) Nuclear extracts (NE) from 293T cells transfected with wildtype human THAP1 were subjected to electrophoretic mobility shift assay (EMSA) using the TOR1A promoter region −159/−80 as a probe in A, or the same probe mutated in the THAP1 core consensus site (AGCA probe) in B. Unfilled triangles indicate specific nuclear complexes. Filled triangles indicate the supershifted bands. (C,D) ChIP-qPCR assays were used to analyze association of THAP1 with the TOR1A promoter in vivo. Cross-linked chromatin from HUVEC primary cells or T98G cells was subjected to immunoprecipitation with antibodies against THAP1 (THAP1 Ab1) or negative control antibodies (Control Ab). The human RRM1-exon 19 genomic region was used as a control genomic region. (C) Immunoprecipitated DNA was quantified by qPCR using the percent of input method. (D) Fold enrichment of THAP1 on the TOR1A promoter was calculated by dividing the amount of TOR1A promoter DNA precipitated by anti-THAP1 antibodies to the amount of DNA precipitated from the RRM1-exon 19 genomic region. ChIP results are shown as means with SD from three separate datapoints.
Next, to assess the association of THAP1 with the TOR1A promoter in vivo, we performed chromatin immunoprecipitation (ChIP)-qPCR assays in human primary cells (human umbilical vein endothelial cell, HUVECs) and the T98G glioblastoma cell line. In both cell types, THAP1 bound to the endogenous TOR1A promoter, but not to a control genomic site (RRM1 Exon 19) that does not contain a THABS motif (Fig 1C). Consistent with the specificity of our ChIP assays, the control antibodies did not immunoprecipitate significant levels of either DNA sequence. Quantification indicated an approximate 26- and 21-fold enrichment of THAP1 on the TOR1A promoter (compared to the control genomic site) in HUVECs and T98G cells, respectively (Fig 1D). ChIP-qPCR assays in cells treated with THAP1 siRNAs revealed a significant reduction of THAP1 association with the TOR1A promoter, consistent with reduced levels of THAP1 protein (see Supplementary Fig 1). Taken together with the EMSA DNA-binding assays, these in vivo ChIP assays indicate that TOR1A is a direct target of endogenous THAP1.
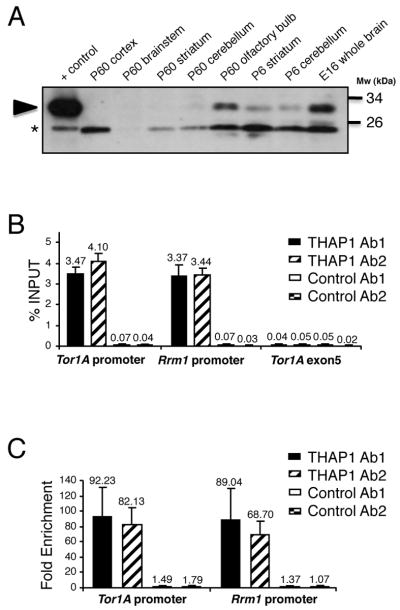
Physiological interaction between THAP1 and the TOR1A gene was further investigated in vivo in mouse brain tissue. Immunoblot analysis revealed that THAP1 concentration was highest in embryonic whole brain tissue and declined after birth in compared brain regions (Fig 2A). This pattern is similar to what has been reported for torsinA 14, 15, the protein encoded by TOR1A. In addition, ChIP-qPCR assays with adult mouse brain chromatin revealed robust binding of endogenous THAP1 to the Tor1A and Rrm1 (positive control) promoters (> 3% of total input DNA precipitated with THAP1 antibodies), whereas binding of THAP1 to a control genomic site (Tor1A exon 5) was similar to the background levels obtained with control antibodies (Fig 2B). Quantification indicated a > 65-fold enrichment of THAP1 on the Tor1A and Rrm1 promoters in mouse brain (Fig 2C). Importantly, very similar results were obtained with two independent THAP1 antisera and two distinct control antibodies (Fig 2B and 2C). We concluded that THAP1 is expressed in the brain and binds to the Tor1A promoter in vivo.
Figure 2.
Endogenous THAP1 is bound to the Tor1A promoter in mouse brain. (A) Immunoblot analysis of THAP1 expression using anti-THAP Ab2 in nuclear fractions (80 μg/lane) of wildtype mouse brains structures obtained at indicated ages. Nuclear extracts (10 μg) of cells transfected with THAP1 were used as positive control. Arrowhead indicates band of the predicted size for THAP1. *Nonspecific immunoreactive band. (B,C) Cross-linked chromatin from adult mouse brain was subjected to immunoprecipitation with two distinct THAP1 (THAP1 Ab1 and THAP1 Ab2) and negative control antibodies (Control Ab1 and Control Ab2). The mouse Rrm1 promoter and Tor1A-exon 5 genomic region were used as positive and negative control regions, respectively. Immunoprecipitated DNA was quantified by qPCR (B) and fold enrichment (C) was calculated by dividing the amount of Tor1A or Rrm1 promoter DNA precipitated by anti-THAP1 antibodies to the amount of DNA precipitated from the Tor1A-exon 5 genomic region. Results are shown as means with SD from three separate datapoints.
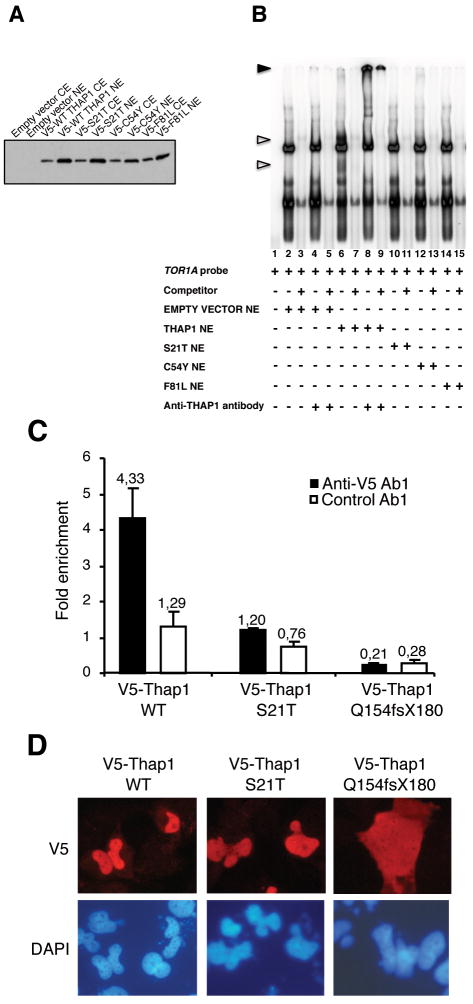
We further hypothesized that disruption of the THAP1/TOR1A interaction may be involved in the molecular pathogenesis of dystonia type 6. To examine this hypothesis, we carried out EMSA experiments using nuclear extracts of 293T cells transfected with the previously characterized F81L THAP1 mutant, as well as two other pathophysiologic THAP1 missense mutants, C54Y (Fuchs T. et al. in preparation) and S21T 2 all of which are located within the THAP domain. Immunoblots from these nuclear extracts verified that the THAP1 mutants were efficiently expressed (Fig 3A). EMSA studies with these extracts showed that each of the three distinct missense mutations abolish binding of THAP1 to TOR1A (Fig 3B). Furthermore, ChIP-qPCR assays using T98G cells expressing V5-tagged wild type or S21T mutant THAP1 proteins confirmed that genetically identified point mutations within the THAP domain abolish THAP1/TOR1A interactions in vivo (Fig 3C). Interestingly, the THAP1 Q154fsX180 mutant also failed to associate with the TOR1A promoter in the ChIP-qPCR assay, despite the fact that this THAP1 mutant contains an intact DNA-binding domain. The THAP1 Q154fsX180 mutant protein is predicted to lack the nuclear localization sequence (NLS) required to enter the nucleus. Accordingly, immunofluorescence studies in both T98G (Fig 3D) and 293T (data not shown) cells indicated that this mutant THAP1 protein localizes to the cytoplasm, contrary to the wild type or S21T point mutant THAP1 proteins that exhibit nuclear localization. We concluded that the different THAP1 mutations described in dystonia type 6 patients, which affect either the DNA-binding domain or remove the NLS, have the same functional consequence i.e. the abrogation of THAP1 binding to TOR1A in vivo. This may explain the lack of genotype-phenotype correlations in dystonia type 6 patients to date.
Figure 3.
Causative mutations of DYT6 dystonia disrupt the THAP1/TOR1A interaction. (A) Immunoblot with monoclonal anti-V5 antibody showing similar levels of expression of the wildtype and mutant THAP1 proteins in cytoplasmic and nuclear extracts of 293T cells transfected with the indicated expression vectors. (B) Electrophoretic mobility shift assay (EMSA) showing that DYT6 patient point mutations disrupt physical interaction between THAP1 and TOR1A promoter region. Nuclear extracts from 293T cells transfected with the indicated expression vectors were used. Unfilled triangles indicate specific nuclear complexes. Filled triangles indicate the supershifted bands. (C,D) Cross-linked chromatin from T98G cells transfected with the indicated expression vectors was subjected to immunoprecipitation with anti-V5 or control antibodies. Immunoprecipitated DNA was quantified as described above (see Fig 1 legend) and fold enrichment of wildtype THAP1 or mutant proteins over the TOR1A promoter (C) was calculated by dividing the amount of TOR1A promoter DNA precipitated by anti-V5 antibodies to the amount of DNA precipitated from the RRM1-exon 19 genomic region. The results are shown as means ± SD from three separate datapoints. (D) Transfected T98G cells were analyzed by indirect immunofluorescence microscopy with anti-V5 antibodies (red). DNA was counterstained with DAPI (blue)
To determine whether THAP1 modulates TOR1A expression, we analyzed TOR1A mRNA levels in lymphoblast-derived cell lines from dystonia type 6 patients. However, we did not detect a specific modulation of torsinA expression in the lymphoblast cell lines from patients compared to unaffected controls (Supplementary Fig 2). In addition, we did not detect any significant effect on torsinA expression after over-expression of THAP1 by transfection and by retroviral delivery in 293T cells and HUVECs, respectively, despite the fact high levels of THAP1 were measured by western blot analysis and qPCR (Supplementary Fig 3). Similarly, no significant effects on torsinA mRNA levels were found after knockdown of THAP1 in HUVECs (Supplementary Fig 3), suggesting regulation may only occur in specific regions of the brain or during development.
Finally, we analyzed THAP1 mRNA levels in DYT1 patient fibroblasts, but did not detect a change in THAP1 expression in these cells when compared to healthy controls (Supplementary Fig 4).
Discussion
Despite the identification of several gene loci associated with dystonia, its pathophysiology remains poorly understood. TOR1A and THAP1 are the only genes identified thus far for primary dystonia. In this study, we demonstrate that THAP1 protein physically interacts with the TOR1A gene promoter both in vitro and in vivo. Our findings provide the first evidence for what may be a functional link between two forms of primary dystonia and suggest the mechanism of transcriptional dysregulation as a cause of primary dystonia.
While we show in the present study that dystonia type 1 and 6 intersect in a common pathway, the difference in age- and distribution-related spectrum of primary dystonia remains obscure. The fact that THAP1 is an upstream component of the pathway may explain at least in part some differences observed between both phenotypes, including the more frequent oral presentation of patients with dystonia type 6. THAP1 may regulate other potential gene targets, which could also influence phenotype. Nevertheless, even within one particular dystonia type, the age of onset and the distribution of the disease vary greatly, indicating that other factors must be involved in the development of symptoms.
A single transcription factor can act as both a repressor and enhancer of transcription, depending on the genomic context, tissue and/or cell type specificity, and availability of co-factors16, 17. Although both THAP1 and torsinA are ubiquitously expressed, they exhibit regional specificity. It is possible, therefore, that disruption of the THAP1 – TOR1A interaction may be relevant to disease only in the central nervous system, and perhaps only in specific regions and neuronal subtypes18-20. This possibility is supported by our observations that torsinA mRNA levels were not modulated in non-neuronal cells after THAP1 knockdown or overexpression, and in lymphoblastoid cell lines from dystonia type 6 patients. THAP1 regulation of TOR1A remains to be tested in brains from dystonia type 6 patients and in mouse models of dystonia type 6, neither of which is currently available. Despite the fact this question remains to be addressed, the present study offers important new insights into molecular mechanisms of primary dystonia pathogenesis and raises the possibility that novel therapeutics targeting this common pathway may be effective for the treatment of different forms of dystonia. Pharmacologic therapy for dystonia remains unsatisfactory21, and transcription factors are selectively enriched as drug targets among the current FDA approved drugs22, making THAP1 an obvious and attractive target. Moreover, THAP1 is known to interact with other proteins23, 24 and characterization of further transcriptional targets, particularly other genes related to dystonia, and interactors may lead to discovery of additional drug targets for dystonia.
Supplementary Material
Acknowledgments
We thank Melanie Plaza, Samira Chandwani, Sheo Singh, Sierra White, and Hannah Lederman for technical help and Meenakshisundaram Ananthanarayanan for helpful discussions. We are grateful to Pascale Mercier for providing mouse brain tissues for ChIP assays. This work was supported by research grants from the Dystonia Medical Research Foundation (LJO, TF), the Bachmann-Strauss Dystonia and Parkinson Foundation (LJO and MEE), the Ligue Nationale Contre le Cancer (‘Equipe labellisée Ligue 2009’ to JPG), the National Institute of Neurological Disorders and Stroke (NS037409 to LJO).
References
- 1.Fuchs T, Gavarini S, Saunders-Pullman R, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 2.Bressman SB, Raymond D, Fuchs T, et al. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–446. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djarmati A, Schneider SA, Lohmann K, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–452. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- 4.Houlden H, Schneider SA, Paudel R, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 74:846–850. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti M, Barzaghi C, Brancati F, et al. Mutation screening of the DYT6/THAP1 gene in Italy. Mov Disord. 2009;24:2424–2427. doi: 10.1002/mds.22861. [DOI] [PubMed] [Google Scholar]
- 7.Paisan-Ruiz C, Ruiz-Martinez J, Ruibal M, et al. Identification of a novel THAP1 mutation at R29 amino-acid residue in sporadic patients with early-onset dystonia. Mov Disord. 2009;24:2428–2429. doi: 10.1002/mds.22849. [DOI] [PubMed] [Google Scholar]
- 8.Roussigne M, Kossida S, Lavigne AC, et al. The THAP domain: a novel protein motif with similarity to the DNA-binding domain of P element transposase. Trends Biochem Sci. 2003;28:66–69. doi: 10.1016/S0968-0004(02)00013-0. [DOI] [PubMed] [Google Scholar]
- 9.Clouaire T, Roussigne M, Ecochard V, et al. The THAP domain of THAP1 is a large C2CH module with zinc-dependent sequence-specific DNA-binding activity. Proc Natl Acad Sci U S A. 2005;102:6907–6912. doi: 10.1073/pnas.0406882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessiere D, Lacroix C, Campagne S, et al. Structure-function analysis of the THAP zinc finger of THAP1, a large C2CH DNA-binding module linked to Rb/E2F pathways. J Biol Chem. 2008;283:4352–4363. doi: 10.1074/jbc.M707537200. [DOI] [PubMed] [Google Scholar]
- 11.Sabogal A, Lyubimov AY, Corn JE, et al. THAP proteins target specific DNA sites through bipartite recognition of adjacent major and minor grooves. Nat Struct Mol Biol. 17:117–123. doi: 10.1038/nsmb.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol C, Lacroix C, Mathe C, et al. The THAP-zinc finger protein THAP1 regulates endothelial cell proliferation through modulation of pRB/E2F cell-cycle target genes. Blood. 2007;109:584–594. doi: 10.1182/blood-2006-03-012013. [DOI] [PubMed] [Google Scholar]
- 13.Armata IA, Ananthanarayanan M, Balasubramaniyan N, Shashidharan P. Regulation of DYT1 gene expression by the Ets family of transcription factors. J Neurochem. 2008;106:1052–1065. doi: 10.1111/j.1471-4159.2008.05465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevan A, Breakefield XO, Bhide PG. Developmental patterns of torsinA and torsinB expression. Brain Res. 2006;1073-1074:139–145. doi: 10.1016/j.brainres.2005.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jungwirth M, Dear ML, Brown P, et al. Relative tissue expression of homologous torsinB correlates with the neuronal specific importance of DYT1 dystonia-associated torsinA. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp557. [DOI] [PubMed] [Google Scholar]
- 16.Chong JL, Wenzel PL, Saenz-Robles MT, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montemayor C, Montemayor OA, Ridgeway A, et al. Genome-wide analysis of binding sites and direct target genes of the orphan nuclear receptor NR2F1/COUP-TFI. PLoS One. 5:e8910. doi: 10.1371/journal.pone.0008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wichmann T. Commentary: Dopaminergic dysfunction in DYT1 dystonia. Exp Neurol. 2008;212:242–246. doi: 10.1016/j.expneurol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–229. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasques X, Cif L, Gonzalez V, et al. Factors predicting improvement in primary generalized dystonia treated by pallidal deep brain stimulation. Mov Disord. 2009;24:846–853. doi: 10.1002/mds.22433. [DOI] [PubMed] [Google Scholar]
- 22.Ma'ayan A, Jenkins SL, Goldfarb J, Iyengar R. Network analysis of FDA approved drugs and their targets. Mt Sinai J Med. 2007;74:27–32. doi: 10.1002/msj.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussigne M, Cayrol C, Clouaire T, et al. THAP1 is a nuclear proapoptotic factor that links prostate-apoptosis-response-4 (Par-4) to PML nuclear bodies. Oncogene. 2003;22:2432–2442. doi: 10.1038/sj.onc.1206271. [DOI] [PubMed] [Google Scholar]
- 24.Mazars R, Gonzalez-de-Peredo A, Cayrol C, et al. The thap-zinc finger protein thap1 associates with coactivator HCF-1 and O-GLcNAc transferase: A link between DYT6 and DYT3 dystonias. J Biol Chem. 2010;285:13364–13371. doi: 10.1074/jbc.M109.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.