Abstract
Background
In women, single-dose nevirapine for prophylaxis against mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1) selects for nevirapine-resistant HIV-1, which subsequently decays rapidly. We hypothesized that the selection, acquisition, and decay of nevirapine-resistant HIV-1 differs in infants, varying by the timing of HIV-1 infection.
Methods
We conducted a prospective, observational study of 740 Mozambican infants receiving single-dose nevirapine prophylaxis and determined the timing of infection and concentrations of nevirapine-resistant HIV-1 over time.
Results
Infants with established in utero infection had a high rate (87.0%) of selection of nevirapine-resistant HIV-1 mutants, which rapidly decayed to undetectable levels. The few without nevirapine resistance received zidovudine with single-dose nevirapine and/or their mothers took alternative antiretroviral drugs. Infants with acute in utero infection had a lower rate of nevirapine-resistant HIV-1 (33.3%; P =.006, compared with established in utero infection), but mutants persisted over time. Infants with peripartum infection also had a lower rate of nevirapine-resistant HIV-1 (38.1%; P =.001, compared with established in utero infection) but often acquired 100% mutant virus that persisted over time (P =.017, compared with established in utero infection).
Conclusions
The detection and persistence of nevirapine-resistant HIV-1 in infants after single-dose nevirapine therapy vary by the timing of infection and the antiretroviral regimen. In infants with persistent high-level nevirapine-resistant HIV-1, nevirapine-based antiretroviral therapy is unlikely to ever be efficacious because of concentrations in long-lived viral reservoirs. However, the absence or decay of nevirapine-resistant HIV-1 in many infants suggests that nevirapine antiretroviral therapy may be effective if testing can identify these individuals.
Almost half a million children are infected annually with human immunodeficiency virus type 1 (HIV-1) [1]. Most acquire HIV-1 through mother-to-child transmission (MTCT). Without interventions, 5%–10% of at-risk infants acquire HIV-1 in utero, 10%–20% in the peripartum period, and 5%–20% from breast milk [2].
Single-dose nevirapine has been among the strategies recommended by the World Health Organization to prevent MTCT of HIV-1 [3]. This intervention provides 1 dose of nevirapine to the mother during labor and 1 dose of nevirapine to the infant within 72 h of birth and can reduce by 40% the overall rate of MTCT in breast-feeding populations [4]. An undesirable outcome of single-dose nevirapine is the selection of HIV-1 mutants resistant to nevirapine, which has been detected in 20%–69% of mothers [5–9] and 46%–87% of infants [5, 10] by consensus genotyping when evaluated 6–8 weeks after single-dose nevirapine treatment. More sensitive assays detect resistant viruses in an even greater proportion of mothers and infants [10–14].
Drug-resistant mutants selected by single-dose nevirapine may diminish the success of later antiretroviral treatment (ART) in women [15–17] and infants [16, 18] (http://www3.niaid.nih.gov/news/newsreleases/2009/P1060.html), particularly if initiated shortly after single-dose nevirapine treatment, suggesting that mutants may decay to clinically insignificant levels over time. In contrast, resistant viruses selected when ART fails [19–21] or acquired at the time of HIV-1 infection [22, 23] can persist for years. If mutant concentrations wane below the threshold of detection in the absence of drug pressure, the mutants can persist as viable proviruses, even after only a single dose of nevirapine [24], and if selective pressure is resumed, these can replicate and repopulate the blood [25]. Together, these data suggest that prolonged selection during viral replication and acute infection with mutant viruses both favor the persistence of drug-resistant mutants.
In this prospective study, we evaluated whether the prevalence and persistence of nevirapine-resistant HIV-1 in single-dose nevirapine–treated infants differed according to whether nevirapine selective pressure acted on an established or acute HIV-1 infection. We hypothesized that the timing of infant HIV-1 acquisition relative to the selective pressure of single-dose nevirapine would determine the size of the drug-resistant HIV-1 reservoir and, thus, the persistence of mutants. Understanding the dynamics of nevirapine-resistant viruses in infants has broad implications because many countries with a high prevalence of HIV-1 infection use single-dose nevirapine for the prevention of MTCT.
MATERIALS AND METHODS
Study design
We conducted an observational cohort study of newborns born to HIV-1–infected mothers in Beira, Mozambique, who took single-dose nevirapine at birth. One of 4 antiretroviral regimens taken to prevent MTCT was administered by clinicians following Ministry of Health guidelines: maternal single-dose nevirapine during labor and infant single-dose nevirapine at <72 h of age, infant single-dose nevirapine plus 1 week of zidovudine when mothers did not receive single-dose nevirapine, maternal and infant single-dose nevirapine plus daily maternal zidovudine from as early as 32 weeks’ gestation for mothers with CD4 cell counts >350 cells/μL (introduced in Beira in early 2006), or maternal combination ART (most commonly with zidovudine, lamivudine, and nevirapine) for women with CD4 cell counts <350 cells/μL. Whether maternal single-dose nevirapine at the time of delivery and an additional antiretroviral drug were administered was determined by mothers’ self-report at postpartum study visits and confirmed by review of clinical registries. Infant receipt of single-dose nevirapine was determined by review of infants’ medical records for documentation by a nurse of the time and date of administration. Infants’ whole blood was collected on filter paper cards at birth and 2, 4, 6, and 8 weeks of age and by venipuncture from HIV-1–infected infants when 3, 4, 5, 6, 8, 10, 12, 18, and 24 months of age.
The study was approved by the institutional review boards of Seattle Children’s Hospital (Seattle, WA) and the Mozambique Ministry of Health (Maputo, Mozambique). Informed consent was obtained from all mothers before enrollment.
Diagnosis of HIV-1 infection and viral concentration in infants
HIV-1 was diagnosed by detection of viral DNA in dried whole blood spotted on filter paper cards (FTA filter paper cards; Whatman) by nested polymerase chain reaction (PCR) of HIV-1 pol [26] and confirmed by testing longitudinally collected specimens (Table 1). Specimens that tested negative for HIV-1 were evaluated for PCR inhibitors by amplification of β-globin.
Table 1.
Reagents and Conditions Used for Human Immunodeficiency Virus (HIV)–1 DNA Polymerase Chain Reaction (PCR) and Oligonucleotide Ligation Assay (OLA)
| Assay | Primers and probes (5′→ 3′) | Conditions |
|---|---|---|
| HIV-1 pol DNA qualitative PCR for infant diagnosis | ||
| First round | Forward, CCTACACCTGTCAACATAATTGG; reverse, AACTTCTGTATATCATTGACAGTCCA | 35 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min |
| Second round | Forward, AATTAAAGCCAGGAATGGATGG; reverse, CATTTATCAGGATGGAGTTCATA | 35 cycles at 95°C for 30 s, 52°C for 30 s, and 72°C for 1 min |
| HIV-1 gag DNA real-time PCR | ||
| First round | Forward, ACAGTGGGGGGACATCAAGC; reverse CCTGAAGGGTACTAGTAGTTCCTGCTAT | 23 cycles at 95°C for 15 s and 57°C for 1 min |
| Second round | Forward, GACATCAAGCAGCCATGCAAATGTT; reverse, TGCTATGTCACTTCCCCTTGGTTCTCT | 35 cycles at 95°C for 15 s and 60°C for 1 min |
| Probe | FAM-ACCATCAATGAGGAGGCTGCAGAATGGGA-TAMRA | … |
| Human β-globin DNA real-time PCR | ||
| First round | Forward, TCTGTCCACTCCTGATGCTGT; reverse ACGTGCAGCTTGTCACAGTG | 16 cycles at 95°C for 15 s and 60°C for 1 min |
| Second round | Forward, TGAAGGCTCATGGCAAGAAA; reverse GCTCACTCAGTGTGGCAAAGG | 35 cycles at 95°C for 15 s and 60°C for 1 min |
| Probe | FAM-TCCAGGTGAGCCAGGCCATCACTA-TAMRA | … |
| OLA | Codon 103, Wt dig-ACATCCCGCAGGGTTAAAAAAGAAR, Mut f-ACATCCCGCAGGGTTAAAAAAGAAC, and Com p-AAATCAGRAACAGTACTGGATGTGGGGGAT-bio; codon 106, Wt dig-CCAGCAGGGTTAAAAAAGAAAAAATCAG, Mut f-CCAGCAGGGTTAAAAAAGAAAAAATCAA, Com p-TRACAGTACTGGATGTGGGGGATGCATAT-bio; codon 181, Wt dig-ACAAAATCCAGAAATAGTCATCTA, Mut f-AMAAAATCCAGAAATAGTCATCTG, and Com p-TCAATACATGGATGATTTGTATGTA-bio; codon 190, Wt dig-CATGGATGACTTGTATGTAGG, Mut f-CATGGATGACTTGTATGTAGC, and Com p-ATCTGAYTTAGAAATAGGGCAG-bio | Ligation reaction: 10 cycles at 95°C for 30 s and 37°C for 10 min |
NOTE. Nucleotides comprising the codons of interest are in boldface type. bio, biotin; Com, common; dig, digoxigenin; f, fluorescein; Mut, mutant; p, phosphate; Wt, wild type.
The HIV-1 DNA concentration was estimated by amplification of HIV-1 gag and human β-globin DNA in each specimen by real-time PCR [27] adapted to filter paper. Specimens with low concentrations of gag (<2.4 log10/106 cells) also had the HIV-1 pol concentration assessed by limiting dilution PCR [28].
Timing of infant HIV-1 infection
In utero infection was defined by detection of HIV-1 DNA in the infant’s birth specimen [29] and peripartum infection by a negative birth specimen with positive specimens between 2 and 8 weeks of age. In utero infection was categorized post hoc as established if the HIV-1 DNA concentration was >2.4 log10/106 white blood cells in birth or acute if the birth HIV-1 DNA concentration was less than this threshold and in subsequent specimens increased by >1 log10.
Estimating the size of nevirapine-resistant HIV-1 populations
Nevirapine-resistant HIV-1 mutants were detected and the size of the populations estimated using an oligonucleotide ligation assay (OLA) [30–33] (http://depts.washington.edu/idimmweb/faculty/frenkel/OLAmanual1305april04.pdf). OLA reagents were adapted to detect 4 mutant codons in HIV-1 subtype C pol (K103N, V106M, Y181C, and G190A), conferring high-level resistance to nevirapine (Table 1). The OLA was performed on 100–300 copies of HIV-1 DNA from each specimen to ensure the detection of mutants at low concentrations, using mutant standards of 0%, 2%, 5%, 10%, 25%, 50%, 75%, and 100%. An optical density exceeding that of the mutant and wild-type mixture of 2% was considered positive for mutant.
Testing for nevirapine-resistant HIV-1 was conducted on all samples available from infants with in utero or peripartum infection through 8 weeks of age (longitudinal analysis). When nevirapine-resistant HIV-1 was detected in an infant during this initial analysis, the proportion of mutant viruses was quantified over time when at least 1 specimen was collected from the infant between 6 weeks and 24 months of age (decay analysis).
Statistical analyses
The rate of in utero HIV-1 transmission was calculated and a 95% confidence interval (CI) estimated by the exact binomial method, and peripartum HIV-1 transmission was calculated using the Kaplan-Meier method to account for censored follow-up times. The proportion of infants with nevirapine-resistant HIV-1 was compared using the χ2 test or the Fisher exact test. The longitudinal measures of the proportion of nevirapine-resistant mutants in each infant’s total HIV-1 population were compared between infant groups using a generalized estimating equation model.
RESULTS
Infant enrollment and rates of MTCT of HIV-1
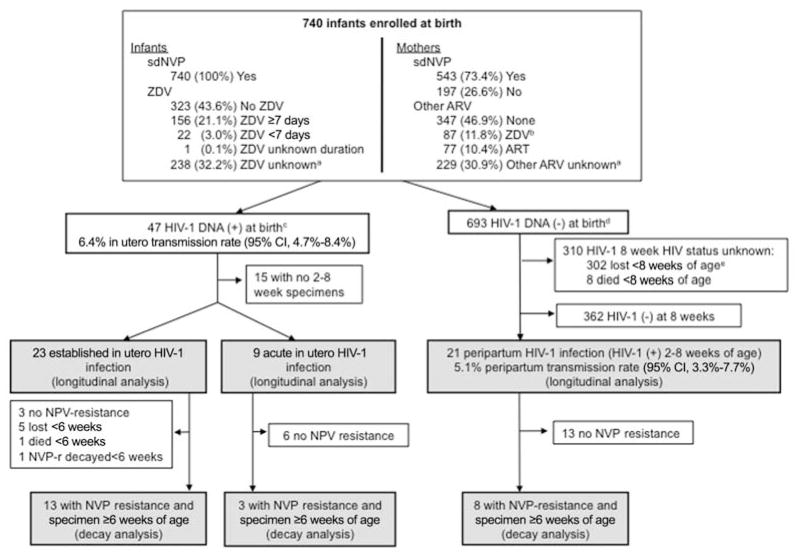
A total of 740 infants were enrolled in the study from June 2005 to February 2008. All infants (100%) received single-dose nevirapine, and 179 (24.2%) also received zidovudine after delivery. Most infants’ mothers (73.4%) received single-dose nevirapine, at times in combination with other antiretroviral drugs (Figure 1). HIV-1 was transmitted to 47 infants in utero (6.4%; 95% CI, 4.7%–8.4%) and 21 infants peripartum (5.1%; 95% CI, 3.3%–7.7%). Among 502 infants in whom feeding practices were assessed, most (397, 79.1%) were breast-fed at the initial postbirth study visit.
Figure 1.
Infant enrollment, study follow-up, and human immunodeficiency virus type 1 (HIV-1) status through 8 weeks of age. A total of 740 infants born to HIV-1–infected mothers were enrolled in the study during the period June 2005–February 2008. Of the 47 (6.4.%) infected in utero, 32 had follow-up beyond birth and were analyzed for the selection of nevirapine (NVP)–resistant HIV-1 prior to 8 weeks of age (longitudinal analysis), including 23 with established in utero infection (HIV-1 DNA level, >2.4 log10/106 white blood cells in birth specimen) and 9 with acute in utero infection (HIV-1 DNA was less than this threshold at birth and increased by >1 log10 in subsequent specimens). Of these, only 13 and 3 infants with established and acute in utero infection, respectively, had NVP resistance prior to 8 weeks of age and sufficient samples beyond 6 weeks of age for analysis of decay of NVP-resistant HIV-1 (decay analysis). Of the 693 infants with negative HIV-1 DNA at birth, 21 had peripartum HIV-1 infection (HIV-1 DNA positive at 2–8 weeks of age) and were analyzed for detection of NVP-resistant HIV-1 (longitudinal analysis). Of these, 8 had detectable NVP-resistant mutations, and all had sufficient samples for analysis of NVP resistance decay (decay analysis). ART, antiretroviral therapy; ARV, antiretroviral; sdNVP, single-dose NVP; ZDV, zidovudine. aUnknown because mother/infant had no postbirth follow-up visit, when prepartum and postpartum ARVs were ascertained and/or confirmed. bOne mother also received ZDV and lamivudine for 1 week after delivery. cBirth specimens were available for 82%, 93%, 94%, and 96% within day of life 1–5, respectively; includes 2 infants with high concentrations of HIV-1 DNA detected in their first specimen collected at 8 and 11 days of age, suggesting established in utero infection. dIncludes 7 infants without birth specimens who were HIV-1 DNA negative in subsequent specimens. eHIV-1 polymerase chain reaction result was negative for the latest specimen tested.
Selection and transmission of nevirapine-resistant HIV-1
Sufficient (100–300 copies) HIV-1 DNA was available in birth specimens collected at a median of 1 day (range, 0–5) from 39 infants with in utero HIV-1 infection to evaluate nevirapine resistance; all had exclusively wild-type HIV-1 at the 4 codons evaluated: K103, V106, Y181, and G190.
Twenty-three of 30 infants with established in utero infection had sufficient specimens for longitudinal analysis, and 20 (87.0%; 95% CI, 66.4%–97.2%) selected nevirapine-resistant HIV-1 (Figure 2). The most common mutation selected was Y181C (16 [80.0%] of 20), followed by K103N (13 [65.0%] of 20), V106M (7 [35.0%] of 20), and G190A (4 [20.0%] of 20), with multiple mutant codons selected in 14 (70.0%) of 20. In most infants with nevirapine-resistant HIV-1, resistant viruses were first detected at 2 weeks of age (14 [93.3%] of 15, with available 2-week specimens). The 3 infants without nevirapine-resistant HIV-1 were born to the only 3 mothers who received either zidovudine or ART and who either did not take single-dose nevirapine or took single-dose nevirapine in the context of nevirapine-containing ART initiated before delivery. All 3 of these infants also took zidovudine after delivery. Although significant relationships were found between the selection of nevirapine resistance in infants exposed to maternal single-dose nevirapine and infant zidovudine (Table 2), the small sample sizes do not allow distinction between these factors.
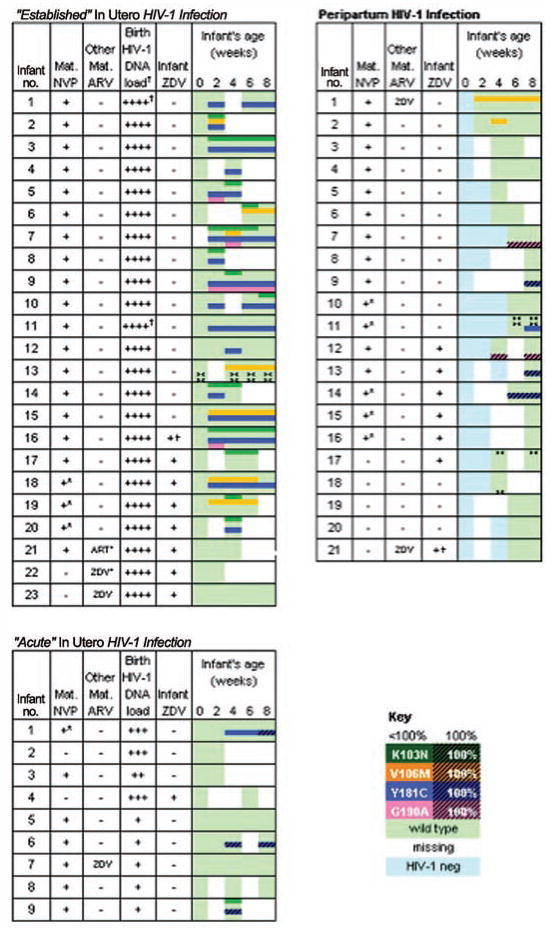
Figure 2.
The rate of nevirapine (NVP)–resistant human immunodeficiency virus type 1 (HIV-1) infection in infants exposed to single-dose NVP, varying by timing of infants’ HIV-1 infection. All infants were infected in utero had wild-type viruses at birth (left panel). Selection of mutants among infants with in utero infections differed by the timing of HIV-1 infection. A higher rate of resistance was detected in infants with “established” versus “acute” infection (P =.006), characterized, respectively, by a high/stable versus low/increasing HIV-1 DNA load at the time of birth (left, upper and left, lower panels, respectively). Administration of zidovudine (ZDV) diminished selection of NVP-resistant HIV-1 (P =.02) among infants with established in utero infections. Mutants were not selected in many infants with acutely acquired HIV-1, suggesting minimal viral diversity at the time of NVP selective pressure. Infants infected peripartum appeared to have acquired either wild-type or mutant viruses from their mother (right panel), with mutants detected less frequently, compared with established in utero infections (P =.001). +*, Mother’s NVP at <1 h prior to delivery. ART*, Mother started NVP-containing antiretroviral therapy (ART) 5 days prior to delivery. ZDV*, Mother took only 1 dose of ZDV on day of delivery. +†, Infant took only 1 dose of ZDV after delivery. ><, oligonucleotide ligation assay results indeterminate; results by consensus sequencing. Infant HIV-1 DNA concentration at birth: ++++, gag >300 c/106 cells or pol replicates >90%; +++, gag <300 c/106 cells and pol >70% to 90%; ++, gag <300 c/106 cells and pol >50% to 70%; and +, gag <300 c/106 cells and pol >0% to 50%. †Birth specimens were from ≤5 days of life, except for “Established.”
Table 2.
Nevirapine Resistance Compared between Infants Acquiring In Utero Human Immunodeficiency Virus (HIV)–1 Infection Either Well or Immediately before Birth or Peripartum
| Parameter | Nevirapine-resistant infants by timing of HIV-1 infection | P | ||
|---|---|---|---|---|
| In utero | Peripartum | |||
| Established | Acute | |||
| Resistance detected in ≥1 specimen | 20/23 ( 87)a,b | 3/9 (33)a | 8/21 (38)b | .006,a .001b |
| Y181C | 16/23 (70) | 3/9 (33) | 4/21 (19) | |
| K103N | 13/23 (57) | 1/9 (11) | 0/21 (0) | |
| V106M | 7/23 (30) | 0/9 (0) | 2/21 (10) | |
| G190A | 4/23 (17) | 0/9 (0) | 2/21 (10) | |
| Multiple mutations | 14/23 (61) | 1/9 (11) | 0/21 (0) | |
| Only wild-type HIV-1 in birth (in utero) or first specimen with virus (peripartum) | 30/30 (100)b | 9/9 (100)c | 2/8 (25)b,c | <.001,b .002c |
| 100% nevirapine-resistant HIV-1 in first positive specimen | 0/20 (0)b | 0/3 (0) | 5/8 (62)b | <.001b |
| Infant administered zidovudine (all had single-dose nevirapine) | ||||
| Yes | 4/7 (57)d | 0/1 (0) | 3/6 (50) | .02d |
| No | 16/16e (100)d | 3/8d (38) | 5/15 (33) | |
| Maternal ingestion of single-dose nevirapine | ||||
| Before delivery | 20/20 (100)b,d | 3/7 (43) | 8/16 (50)b, f | <.001b |
| No ingestion of single-dose nevirapineg | 0/3 (0)d | 0/2 (0) | 0/5 (0)f | <.001d |
| Maternal ingestion of other antiretroviral drug | ||||
| Zidovudine or ART | 0/3 (0)d | 0/1 (0) | 1/2 (50) | <.001d |
| None | 20/20 (100)d | 3/8 (38) | 7/19 (37) | |
| Mean ± SD concentration of nevirapine-resistant mutant across infants by age | ||||
| 6–8 weeks | 56.3 ± 37.8 | … | 56.9 ± 48.7 | .98 |
| 3–4 months | 55.1 ± 44.5 | … | 60.2 ± 54.5 | .89 |
| 5–6 months | 16.2 ± 23.0 | … | 72.5 ± 48.6 | .017 |
NOTE. Data are number (percentage) of patients unless otherwise indicated. ART, antiretroviral therapy; SD, standard deviation.
Comparison between in utero established and in utero acute groups.
Comparison between in utero established and peripartum groups.
Comparison between in utero acute and peripartum groups.
Comparison within in utero established group.
Includes 1 infant who had only 1 day of zidovudine therapy.
Comparison within peripartum group.
One mother took single-dose nevirapine in addition to ART before delivery.
Among the 9 infants with acute in utero infection, all of whom underwent longitudinal analysis, 3 (33.3%; 95% CI, 7.5%–70.1%) had nevirapine-resistant HIV-1 detected at <8 weeks. The rate of selection was lower compared with infants with established in utero infections (P =.006). The Y181C mutant was selected in all 3 infants and K103N in 1 infant. The Y181C mutation expanded to nearly 100% of the virus population in all 3 infants before 8 weeks of age. Among this group of 9 infants, only 2 mothers did not take single-dose nevirapine and 1 mother took zidovudine, making comparisons on the basis of these variables unreliable.
All 21 infants with peripartum infection had sufficient specimens for longitudinal analysis, and 8 (38.1%; 95% CI, 18.1%–61.6%) had nevirapine-resistant HIV-1 detected at <8 weeks. The prevalence of resistance was lower compared with infants with established in utero infections (P =.001). A comparison of only infants born to women who took single-dose nevirapine (excluding 1 woman also receiving ART) confirmed a higher proportion of resistance in infants with established in utero infections compared with peripartum infections (20 [100%] of 20 [95% CI, 83.2%–100.0%] vs 8 [50.0%] of 16 [95% CI, 24.7%–75.3%]; P <.001), as did the same comparison of only infants whose mother’s took single-dose nevirapine >1 h before delivery (17 [100%] of 17 [95% CI, 80.5%–100.0%] for established in utero infected infants and 6 [54.6%] of 11 [95% CI, 23.4%–83.3%] for peripartum-infected infants; P =.005).
Similar to infants infected in utero, Y181C (4 [50.0%] of 8) was the most common mutation among peripartum-infected infants, with V106M and G190A each detected in 2 infants. Only a single mutant variant was detected in each peripartum-infected infant with nevirapine-resistant HIV-1, suggesting transmission (as opposed to selection) of mutant virus. Other observations suggesting transmission of nevirapine-resistant viruses include (1) a higher rate of mutant virus in the first specimen with HIV-1 detected in infants with peripartum compared with established in utero infections (P <.001), whose mutants were selected later and generally at several codons; (2) the detection of 100% mutant viral populations throughout the period of observation in most (5 [62.5%] of 8; 95% CI, 24.5%–91.5%) peripartum-infected infants compared with none (0 [0%] of 23; 95% CI, 0%–14.8%) with established in utero infection (P <.001); (3) a tendency for a greater frequency of mutant viruses in infants whose mothers took single-dose nevirapine (8 [50.0%] of 16; 95% CI, 24.7%–75.3%), compared with those whose mothers did not (0 [0%] of 5; 95% CI, 0%–52.2%; P =.11); and (4) that the addition of infant zidovudine to single-dose nevirapine did not appear to affect the rate of nevirapine-resistance compared with single-dose nevirapine alone (3 [50.0%] of 6 [95% CI, 11.8%–88.2%] vs 5 [33.3%] of 15 [95% CI, 11.8%–61.6%]; P =.63).
Decay of nevirapine-resistant HIV-1
Of the infants with nevirapine-resistant HIV-1 detected in the first 8 weeks of life, 24 (13 with established in utero infection, 3 with acute in utero infection, and 8 with peripartum infection) had at least 1 sample collected between 6 weeks and 24 months of age and were evaluated for decay of nevirapine resistance (Figure 3). Mutants decayed rapidly among infants with established in utero infection. In contrast, mutants persisted in most infants with acute in utero infection or peripartum infection. Among the 3 infants with peripartum infections with nevirapine-resistant HIV-1 populations detected at <100% between 2 and 8 weeks, only 1 had sufficient specimens collected for evaluation, and his mutant virus decayed during the first few months of life similar to infants with established in utero infections.
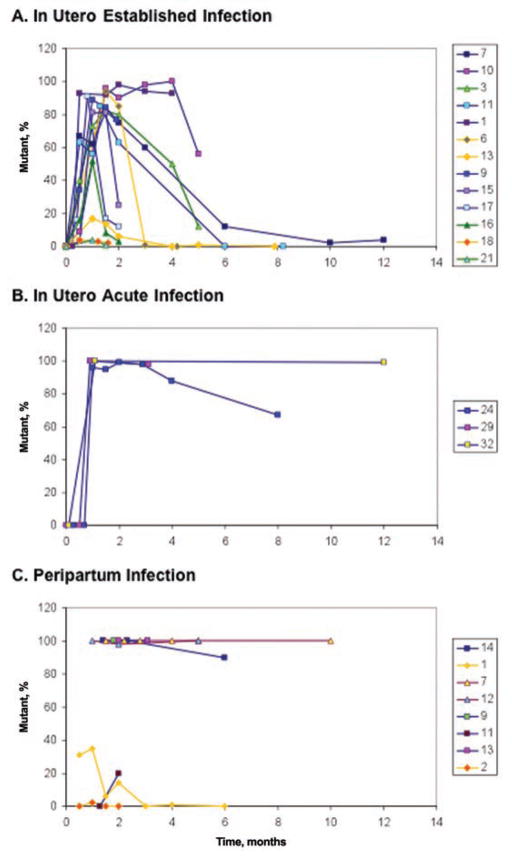
Figure 3.
The dynamics of nevirapine (NVP)–resistant human immunodeficiency virus type 1 (HIV-1) varying by time of infection. Wild-type virus was detected at birth in all infants infected in utero. Mutants selected by NVP replicated to high concentrations in infants with established in utero infections (panel A; infant identification numbers listed in boxes to the right of the graphs) then waned after NVP pressure subsided. NVP-resistant HIV-1 replicated to high concentrations in infants with acute in utero infections (panel B) and persisted as the majority genotype during the first months of life. A single mutant variant was apparently transmitted to most infants with peripartum infection (panel C), and this genotype persisted at high concentrations over time, compared with established in utero infections (P =.017). The prolonged persistence of mutants in both infants with acute in utero and peripartum infections suggests that mutants infected a large population of susceptible cells during primary infection.
A comparison of the concentration of nevirapine-resistant HIV-1 in individual infants with established in utero and peripartum infections revealed no significant difference at 6–8 weeks (P =.98) or 3–4 months (P =.89) of age but was greater in infants with peripartum infection at 5–6 months of age (P =.017). Insufficient data were available from infants with peripartum infection at >6 months of age for formal statistical comparisons; however, mutants remained at high concentrations in these infants and were very low or undetectable in those with established in utero infections. Similarly, data from infants with acute in utero infection were too sparse for statistical comparisons. Although only the dominant mutation in each individual was used in the longitudinal analyses, secondary mutations were rare at the later time points and when detected were at lower concentrations than the dominant mutant.
DISCUSSION
The timing of infants’ HIV-1 infection relative to nevirapine-selective pressure determined 1 of 3 distinct dynamic patterns of nevirapine-resistant HIV-1. Notably, all infants with in utero infections and available birth specimens initially had wild-type HIV-1. The first pattern, observed in those with established in utero infection defined by elevated viral loads at birth, was characterized by a high rate of selection followed by rapid decay of nevirapine-resistant HIV-1. The second pattern, in infants with low but escalating viral loads at birth, consistent with acute in utero infection, included lower rates of resistance, but resistant viruses persisted over time. The third pattern occurred in infants with peripartum infection and consisted of a substantial fraction acquiring nevirapine-resistant HIV-1, which persisted at high concentrations. As expected, use of infant zidovudine, maternal single-dose nevirapine, and other maternal antiretroviral agents influenced the rates of nevirapine-resistant virus detected among infants.
According to accepted models, a single [34, 35] or several [35] HIV-1 variants may infect an infant, and within a few weeks of primary infection virus populates a large number of susceptible cells [36]. Once infection is established, a high rate of viral replication and a lack of proofreading activity by HIV-1 polymerase generate mutations at all positions in the viral genome [21]. Archiving of mutants results in individuals harboring all single-base changes that confer high-level resistance to nevirapine shortly after infection [21] that can be rapidly selected by administration of nevirapine monotherapy [37].
In keeping with these models, infants who already harbor large viral populations at the time single-dose nevirapine is administered, as in the case of established in utero infection, should rapidly select archived mutants that confer nevirapine resistance. Indeed, consistent with the expected selective forces [38], multiple mutant variants emerged in nearly every infant with a high viral load at birth, peaked by 2–4 weeks of age with concentrations approaching 100%, and subsequently decayed. An exception to this pattern occurred in infants also administered zidovudine or whose mothers took combinations of antiretroviral drugs. Viral replication under multidrug therapy requires multiple mutations, and as suggested by models [39], it appears unlikely that such multidrug-resistant viruses evolve through random mutations.
The infrequent selection of nevirapine-resistant HIV-1 in infants experiencing acute in utero infection, the second pattern, is consistent with infants harboring a small HIV-1 population at birth, typically with limited viral diversity [34, 35]. An absence of nevirapine-resistant HIV-1 in their viral quasispecies would preclude the rapid selection of mutants. Among the few infants with selection of nevirapine-resistant HIV-1, the rapid expansion of mutants to high concentrations that persisted over time corresponds to viruses populating a large number of susceptible cells during acute infection [36].
The third pattern, acquisition of 100% nevirapine-resistant HIV-1 that persisted at high concentrations, was prominent in infants with peripartum infection. However, because more than half of peripartum-infected infants acquired wild-type viruses, transmissions in these infants may have occurred before the selection of maternal mutants or from mothers who did not take nevirapine. Infant zidovudine did not appear to affect whether an infected infant had nevirapine-resistant HIV-1, which would be consistent with acquisition of nevirapine-resistant virus. Too few mothers of peripartum-infected infants took zidovudine in combination with single-dose nevirapine to evaluate its effect on the selection of nevirapine-resistant HIV-1, which we anticipate would decrease the selection of mutants as observed in infants with established in utero infections.
The rapid decay of nevirapine-resistant HIV-1 among infants with established in utero infections parallels the decay in post-partum women [16], whereas the persistence of mutants in acutely infected infants mirrors adults acquiring drug-resistant HIV-1 [22, 40]. During acute infection, HIV-1 rapidly populates a large population of vulnerable cells [36], which comprise much of the long-lived HIV-1 reservoirs [19]. Therefore, the persistence of nevirapine resistance is expected when these mutants predominate during acute infection.
The threshold concentrations above which drug-resistant HIV-1 populations have clinical consequences have not been defined. The rapid decay of nevirapine-resistant HIV-1 among the infants with established in utero infections in our study suggests that after an interval nevirapine ART may be effective in these infants, as suggested in studies of African women [16, 41] and infants [16, 18].
The absence of nevirapine-resistant HIV-1 in nearly half (42%) of the infants we studied using a highly sensitive assay is notable because nevirapine ART would most likely effectively treat these infants. However, only 73% of mothers received single-dose nevirapine, and because maternal single-dose nevirapine increased the rate of resistance over infant-only single-dose nevirapine, a greater maternal uptake should increase the rate of nevirapine-resistant HIV-1 among their infants. Alternatively, the rate of nevirapine resistance should decrease with greater use of zidovudine and ART, as recently recommended by the World Health Organization [42]. Given the opposing effects of maternal single-dose nevirapine and infant zidovudine on the rate of nevirapine-resistant HIV-1 in infants, along with our data showing wide variability in the decay of mutants, a cautious approach is warranted in estimating the likelihood of nevirapine-resistant HIV-1 in particular infants. Until studies can define these parameters and evaluate the utility of susceptibility testing for guiding the selection of infant ART, the current recommendation to use protease inhibitor–based ART for treatment of single-dose nevirapine–exposed infants [43] is the safest approach to treating infants’ HIV-1 infection.
This study has several limitations. First, although the overall study was large, the small number of infants in some subgroups and variable follow-up rates limited our power to compare infants exposed to different maternal and infant antiretroviral regimens. Variable follow-up rates may have additionally reduced our capture of transient nevirapine-resistant HIV-1 and may therefore have resulted in underestimations of resistance. Lastly, our ability to determine which antiretrovirals (other than single-dose nevirapine) were taken by mothers and infants was limited by the accuracy of mothers’ self-report. Although maternity clinic records were checked, data were sparse when participants did not follow up after delivery.
Despite these limitations, we found that after use of single-dose nevirapine for prophylaxis against HIV-1 infection, the detection and decay of nevirapine-resistant HIV-1 in infants follow predictable patterns linked to the timing of HIV-1 infection. The reduced rates of nevirapine-resistant mutants we observed among in utero infected infants exposed to additional antiretroviral agents identify an additional benefit from combination therapies and argue against nevirapine-only programs for the prevention of MTCT. The absence of nevirapine-resistant HIV-1 and the rapid decay of mutants in a substantial proportion of infants suggest that nevirapine ART has the potential to effectively treat many infants. However, the persistence of nevirapine-resistant HIV-1 at high concentrations in certain infants differs from the rapid decay observed in women [5–9] and suggests that nevirapine-based ART is unlikely to ever be efficacious in this subgroup. Testing for drug resistance may identify infants whose viral replication could be suppressed by nevirapine ART; however, first the thresholds for clinically relevant nevirapine mutations must be defined.
Acknowledgments
We thank the participating women and infants, the Mozambican Ministry of Health staff, the study team (Emilia Almeida, Victoria Neves, Mfila Nhamutavira, Maria Serra, and Sandra Pereira), the laboratory staff (Joaquim Chidacaje and Jossefa Sairosse; Mozambique Ministry of Health), and Dr Joseph E. Fitzgibbon at the US National Institutes of Health for important contributions to this study.
Financial support. This study was supported by a National Institutes of Health grant award to Dr. Frenkel (RO1 AI058723). M.A.M. was also supported in part by a National Institute of Health STD/AIDS Research Training Grant (T32 AI07140).
Footnotes
Presented in part: the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, California, 27 February 2007 (abstract 92); the 45th Annual Meeting of the Infectious Disease Society of America, San Diego, California, 6 October 2007 (abstract 875); and the 17th International HIV Drug Resistance Workshop Sitges, Spain, 13 June 2008 (abstract 71).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Joint UN Programme on HIV/AIDS and World Health Organization. AIDS epidemic update. Geneva, Switzerland: Joint UN Programme on HIV/AIDS and World Health Organization; Dec, 2007. [Google Scholar]
- 2.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings: towards universal access: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 4.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 5.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 6.Eshleman SH, Guay LA, Mwatha A, et al. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV-1 6–8 weeks after single-dose nevirapine (HIVNET 012) J Acquir Immune Defic Syndr. 2004;35:126–130. doi: 10.1097/00126334-200402010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Lee EJ, Kantor R, Zijenah L, et al. Breast-milk shedding of drug-resistant HIV-1 subtype C in women exposed to single-dose nevirapine. J Infect Dis. 2005;192:1260–1264. doi: 10.1086/444424. [DOI] [PubMed] [Google Scholar]
- 8.Eshleman SH, Hoover DR, Chen S, et al. Nevirapine (NVP) resistance in women with HIV-1 subtype C, compared with subtypes A and D, after the administration of single-dose NVP. J Infect Dis. 2005;192:30–36. doi: 10.1086/430764. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro RL, Thior I, Gilbert PB, et al. Maternal single-dose nevirapine versus placebo as part of an antiretroviral strategy to prevent mother-to-child HIV transmission in Botswana. AIDS. 2006;20:1281–1288. doi: 10.1097/01.aids.0000232236.26630.35. [DOI] [PubMed] [Google Scholar]
- 10.Troyer RM, Lalonde MS, Fraundorf E, et al. A radiolabeled oligonucleotide ligation assay demonstrates the high frequency of nevirapine resistance mutations in HIV type 1 quasispecies of NVP-treated and untreated mother-infant pairs from Uganda. AIDS Res Hum Retroviruses. 2008;24:235–250. doi: 10.1089/aid.2007.0138. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, Li JF, Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 12.Palmer S, Boltz V, Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci U S A. 2006;103:7094–7099. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flys T, Nissley DV, Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192:24–29. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 14.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–1082. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 16.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 17.Lockman S A5208/OCTANE Study Team. Lopinavir/ritonavir+tenofovir/emtricitabine is superior to nevirapine + tenofovir/emtricitabine for women with prior exposure to single-dose nevirapine: A5208 (“OCTANE”). Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. Alexandria, VA: Foundation for Retrovirology and Human Health; 2009. Paper 955. [Google Scholar]
- 18.Barlow-Mosha L, Ajunua P, Mubiru M, et al. Early effectiveness of a NVP-based HAART regimen among HIV-infected children with and without prior single-dose NVP exposure. Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. Alexandria, VA: Foundation for Retrovirology and Human Health; 2008. Abstract 583. [Google Scholar]
- 19.Frenkel LM, Wang Y, Learn GH, et al. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol. 2003;77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer S, Boltz V, Maldarelli F, et al. Selection and persistence of non-nucleoside reverse transcriptase inhibitor-resistant HIV-1 in patients starting and stopping non-nucleoside therapy. AIDS. 2006;20:701–710. doi: 10.1097/01.aids.0000216370.69066.7f. [DOI] [PubMed] [Google Scholar]
- 21.Coffin J. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 22.Pao D, Andrady U, Clarke J, et al. Long-term persistence of primary genotypic resistance after HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2004;37:1570–1573. doi: 10.1097/00126334-200412150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Delaugerre C, Chaix ML, Blanche S, et al. Perinatal acquisition of drug-resistant HIV-1 infection: mechanisms and long-term outcome. Retrovirology. 2009;6:85. doi: 10.1186/1742-4690-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wind-Rotolo M, Durand C, Cranmer L, et al. Identification of nevirapine-resistant HIV-1 in the latent reservoir after single-dose nevirapine to prevent mother-to-child transmission of HIV-1. J Infect Dis. 2009;199:1301–1309. doi: 10.1086/597759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–285. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 26.Beck IA, Drennan KD, Melvin AJ, et al. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol. 2001;39:29–33. doi: 10.1128/JCM.39.1.29-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gantt S, Shetty AK, Seidel KD, et al. Laboratory indicators of mastitis are not associated with elevated HIV-1 DNA loads or predictive of HIV-1 RNA loads in breast milk. J Infect Dis. 2007;196:570–576. doi: 10.1086/519843. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo AG, Goracke PC, Rowhanian K, Mullins JI. Quantitation of target molecules from polymerase chain reaction-based limiting dilution assays. AIDS Res Hum Retrovir. 1997;13:737–742. doi: 10.1089/aid.1997.13.737. [DOI] [PubMed] [Google Scholar]
- 29.Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1 [letter] N Engl J Med. 1992;327:1246–1247. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 30.Frenkel LM, Wagner LE, II, Atwood SM, Cummins TJ, Dewhurst S. Specific, sensitive, and rapid assay for human immunodeficiency virus type 1 pol mutations associated with resistance to zidovudine and didanosine. J Clin Microbiol. 1995;33:342–347. doi: 10.1128/jcm.33.2.342-347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelstein RE, Nickerson DA, Tobe VO, Manns-Arcuino LA, Frenkel LM. Oligonucleotide ligation assay for detecting mutations in the human immunodeficiency virus type 1 pol gene that are associated with resistance to zidovudine, didanosine, and lamivudine. J Clin Microbiol. 1998;36:569–572. doi: 10.1128/jcm.36.2.569-572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck IA, Mahalanabis M, Pepper G, et al. Rapid and sensitive oligonucleotide ligation assay for detection of mutations in human immunodeficiency virus type 1 associated with high-level resistance to protease inhibitors. J Clin Microbiol. 2002;40:1413–1419. doi: 10.1128/JCM.40.4.1413-1419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck IA, Crowell C, Kittoe R, et al. Optimization of the oligonucleotide ligation assay, a rapid and inexpensive test for detection of HIV-1 drug resistance mutations, for non-North American variants. J Acquir Immune Defic Syndr. 2008;48:418–427. doi: 10.1097/QAI.0b013e31817ed7d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolinsky SM, Wike CM, Korber BTM, et al. Selective transmission of HIV-1 variants from mother to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 35.Scarlatti G, Leitner T, Halapi E, et al. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc Natl Acad Sci U S A. 1993;90:1721–1725. doi: 10.1073/pnas.90.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schacker T, Little S, Connick E, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000;181:354–357. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 37.Richman DD, Havlir D, Corbeil J, et al. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68:1660–1666. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirochnick M, Fenton T, Gagnier P, et al. Pediatric AIDS Clinical Trials Group Protocol 250 Team. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1- infected pregnant women and their neonates. J Infect Dis. 1998;178:368–374. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 39.Perelson AS, Essunger P, Ho DD. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS. 1997;11(suppl A):S17–S24. [PubMed] [Google Scholar]
- 40.Chan KC, Galli RA, Montaner JS, Harrigan PR. Prolonged retention of drug resistance mutations and rapid disease progression in the absence of therapy after primary HIV infection. AIDS. 2003;17:1256–1258. doi: 10.1097/00002030-200305230-00020. [DOI] [PubMed] [Google Scholar]
- 41.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Rapid advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva, Switzerland: World Health Organization; Nov, 2009. [Google Scholar]
- 43.World Health Organization. Paediatric HIV/ART Care Guideline Group Meeting: WHO Antiretroviral Therapy for Infants and Children; Geneva, Switzerland. [April 2008]. [Google Scholar]