Abstract
We used Drosophila olfactory memory in order to understand in vivo the molecular basis of cognitive defect in Fragile X syndrome. We observed that Fragile X protein (FMRP) was required acutely and interacted with argonaute1 and staufen in long-term memory (LTM). Occlusion of long-term memory formation in Fragile X mutants could be rescued by protein synthesis inhibitors, suggesting that excess baseline protein synthesis could impact negatively on cognition.
Keywords: Long-term memory, Fragile X, Argonaute 1, RNAi, Drosophila
INTRODUCTION
Fragile X mental retardation syndrome, caused by the absence of FMRP, is the most frequent cause of heritable mental retardation in males. Lack of FMRP has been associated with increases in general protein synthesis 1 downstream of metabotropic glutamate receptor (mGluR) activation 2. FMRP is associated with elements of LTM such as protein synthesis, staufen 3, 4 and the RNAi pathway 5, 6 but the link between excess protein synthesis and cognition remains unclear.
RESULTS
Fmr1 is involved in olfactory learning and long-term memory formation
We took advantage of two null alleles of Fmr1, Fmr13 and Fmr1B55 to investigate memory formation after Pavlovian olfactory conditioning 7 in Drosophila . We confirmed that FMRP is not detected in brains of these Fmr1 mutants (Fig. S1a,b). Nonetheless, these mutants respond normally to the odors and footshock (Fig. S1c,d).
After repetitive training sessions, two distinct forms of long-lasting memory can be distinguished 7. Massed training (MT) (ten training sessions without rest interval) produces a decremental, cycloheximide-insensitive memory (ARM), while spaced training (ST) (ten training sessions with a 15-min rest interval) produces both ARM and a nondecremental cycloheximide-sensitive (protein synthesis-dependent) long-term memory (LTM). Mutants homozygous for either the Fmr13 or Fmr1B55 allele showed defects in one-day memory after ST but not after MT, and this LTM defect is rescued in Fmr13 mutants expressing a genomic transgene containing Fmr1+ driven by its endogenous promoter (Fig. 1 a,b). Mutants heteroallelic for Fmr13 and Fmr1B55 were also defective for one-day memory after ST but not after MT. (Fig. 1 c,d).
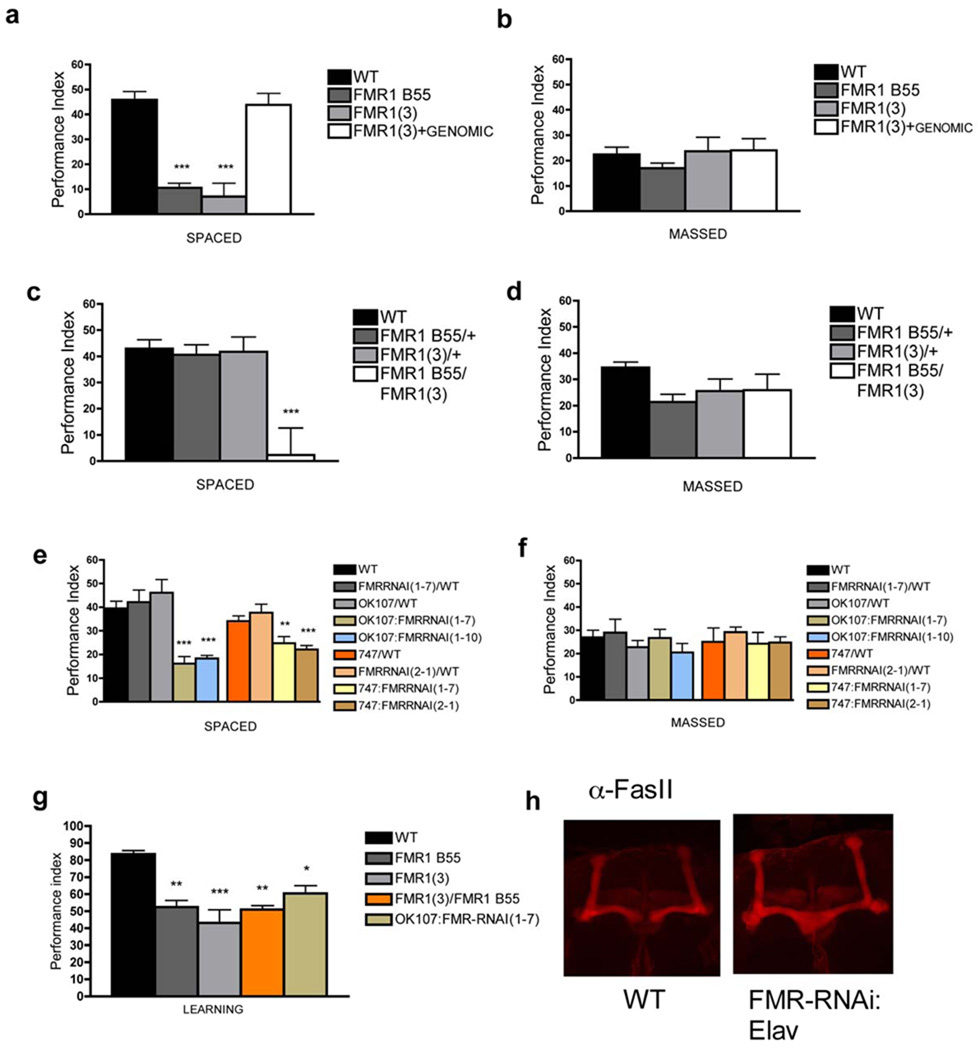
Figure 1. Drosophila FMRP is required for olfactory learning and 1-day memory after ST.
A) One-day memory after ST is defective in FMR1(3) (vs. WT, P < 0.0001) and FMR B55 (vs. WT, P < 0.0001) and rescued with FMR1(3)+GENOMIC (vs. Fmr13, P < 0.0001; vs. WT, P = 0.715). B) In contrast, one-day memory after MT was normal. N = 8 PIs per group. C) Defective one-day memory after ST in FMR B55/FMR 1(3) (vs. WT, P = 0.0001), D) but normal after MT. N = 8 PIs per group. E) One-day memory after ST was significantly lower than WT in OK107;FMRRNAi(1-7) (vs. WT, P < 0.0001), OK107;FMRRNAi(1-10) (vs. WT, P = 0.001), 747;FMRRNAi(1-7) (vs. WT, P = 0.0046) and in 747;FMRRNAi(2-1) (vs. WT, P = 0.001) flies, but normal in the control genotypes: OK107/WT , FMRRNAi(1-7)/WT, 747/WT and FMRRNAi(2-1)/WT. N = 8, 8, 4, 8, 8, 8, 8 and 8 PIs. F) One-day memory after MT for any of the groups in E) was normal. N = 4–8 PIs for each genotype. G) Learning was significantly lower than WT in FMR B55 (vs. WT, P = 0.0062), FMR 1(3) (vs. WT, P = 0.0002), FMR B55/FMR 1(3) (vs. WT, P = 0.0045) or OK107;FMR-RNAi(1-7) (vs. WT, P = 0.034). N = 4 PIs per group. All graphs depict mean +/− S.E.M. H) elavGAL4/+;UAS-FmrRNAi (1-7)/+ present MB midline crossing defect (N=20).
We determined a spatial requirement for FMRP by evaluating LTM formation using RNA interference. Western blot analysis from adult brain after pan-neuronally restricted expression of the UAS-FmrRNAi construct with Elav-GAL4 revealed a significant decrease (40% of baseline) in FMRP level (Fig. S1g,h). Furthermore, we replicated the ß-lobe midline crossing defect (classified as in 8 : 4 severe, 3 moderate and 2 mild in 20 brains for transgenic flies, compared to 0 for each category in 20 brains from wild-type flies) (Fig. 1h). We then evaluated the effects on LTM formation of FMRP knockdowns restricted to the MB, because of its previously demonstrated role in olfactory LTM formation 9. Using two different MB PGAL4 drivers and two different UAS-FmrRNAi constructs, knockdown of FMRP yielded defects in one-day memory after ST but not after MT (Fig. 1 e,f). Interestingly, we observed decreased FMRP level in Kenyon cells (Fig. S2e) but no ß-lobe midline crossing when expression of UAS-Fmr-RNAi was restricted to the MB (using either OK107-Gal4 or 747-Gal4; N=20 flies per genotype; Fig. S2f). In contrast to these results, knockdown of FMRP in the central complex (Feb170) or projection neurons from antennal lobe (GH146) did not yield defects in one-day memory after ST (Fig. S1f).
Considering the high prevalence of learning disability in Fragile X patients , defects in MBs of Fmr1 mutants 8 and their role in learning 10, we asked whether olfactory learning was impaired in Fmr1 mutants and observed significant defects in Fmr13, Fmr1B55 homozygotes, the Fmr13/Fmr1B55 heteroallelic mutant and in flies expressing UAS-FmrRNAi in MB (Fig. 1g).
FMRP is required physiologically in adult for LTM
To dissect the involvement of FMRP in brain development (see Fig. 1h) from its physiological role during adult LTM formation, we modulated FMRP expression using conditional transgenic tools. Acute adult heat shock-induced overexpression of wild-type FMRP produced a significant decrement in one-day memory after ST but not after MT (Fig. 2 a,c, d). In contrast, overexpression of FMRP after ST beyond memory consolidation (Fig. 2b) did not affect one-day memory (Fig. 2c). Interestingly, learning appeared normal at 3 or 24 hours after overexpression of Fmr1+ in adult (Fig. S2a).
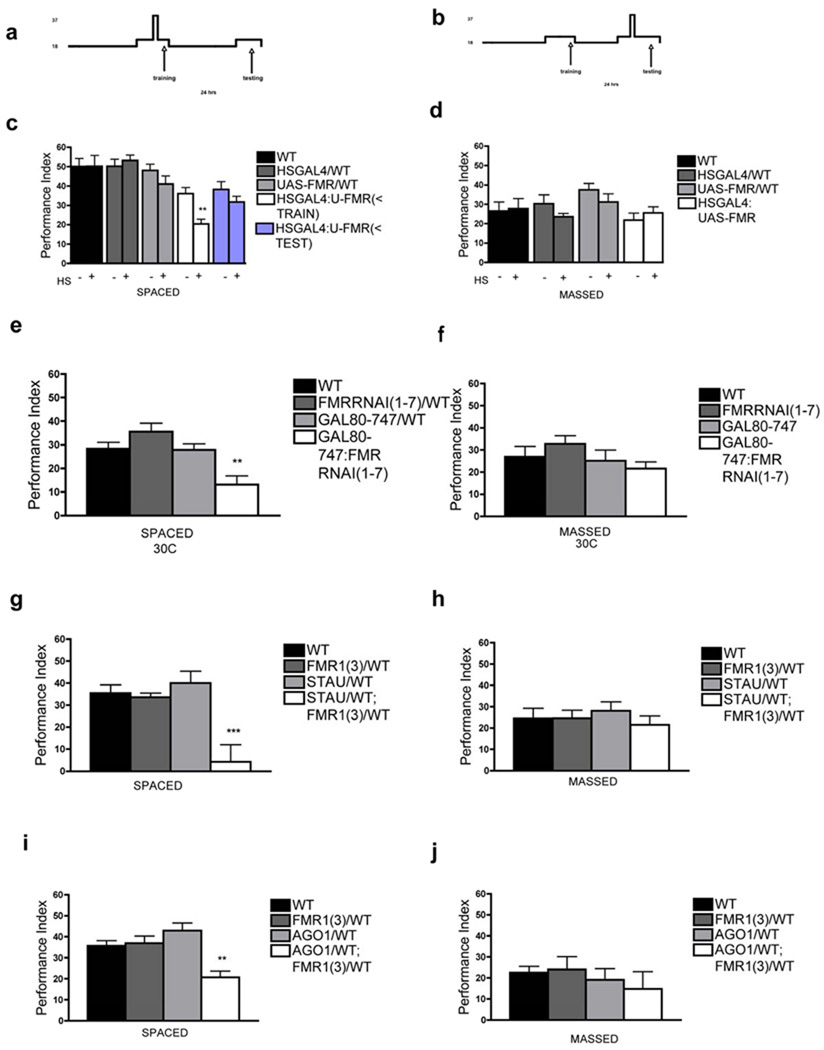
Figure 2. Drosophila FMRP is required acutely for LTM formation and interacts with Staufen and Argonaute 1.
A) Protocol used to overexpress UAS-Fmr+ before training or B) after training. C) One-day memory in HSGAL4;U-FMR flies heat-shocked (+) before ST (<TRAIN) was significantly reduced (P = 0.0015) as opposed to those heat-shocked after ST (<TEST) (P = 0.242). No effect was observed in the genetic controls, HSGAL4/WT or UAS-FMR/WT. N = 8 PIs per group. D) One-day memory after MT did not differ. N = 8 PIs per group. E) Adult UAS-FmrRNAi(1-7) expression in MB of GAL80;747;FMRRNAI(1-7) flies for 3 days caused defect in one-day memory after ST compared to WT (P = 0.0021), FMRRNAI(1-7)/WT) (P < 0.0001) or GAL80;747/WT (P = 0.0028) genetic controls. N = 8 PIs per group. F) One-day memory after MT is not affected. N = 8 PIs per group. G) One-day memory after ST is reduced in STAU/WT;FMR1(3)/WT compared to wild-type flies WT (P = 0.0002), FMR1(3)/WT (P = 0.0004) or STAU/WT (P < 0.0001). N = 8 PIs per group. H) One-day memory after MT was normal in these genotypes. N = 8 PIs per group. I) One-day memory after ST is reduced in AGO1/WT;FMR1(3)/WT compared to controls WT (P = 0.0016), FMR1(3)/WT (P = 0.0007) or AGO1/WT (P < 0.0001). N = 16 PIs per group. J) One-day memory after MT was normal in these same genotypes. N = 8 PIs per group. All graphs depict mean +/− S.E.M.
We confirmed these results with complementary experiments using spatio-temporal control of RNAi-mediate knockdown of FMRP. We used the temperature-sensitive, tubulin-driven GAL80ts transgene, along with MB (747) GAL4-driven expression of UAS-FmrRNAi to disrupt FMRP specifically in adult MB. At restrictive temperature (30°C), when GAL80ts no longer represses GAL4-driven expression of UAS-FmrRNAi, we observed a significant defect in one-day memory after ST but not after MT (Fig. 2e,f). Learning was normal (Fig. S2B). At permissive temperature (18°C), when GAL80ts is capable to inhibit GAL4-driven expression of UAS-FmrRNAi, one-day memory after spaced or massed training was normal (Fig. S2c,d). Despite a significantly decreased FMRP level in Kenyon cells after incubation at restrictive temperature (30C; Fig. S2g), no MB midline defect was observed (0/20 brains) (Fig. S2h).
We observed a significant increase in FMRP expression acutely after ST (P<0.0001) but not after MT (P=0,123) (Fig. S3a,b). Interestingly, we observed that Drosophila adenylyl cyclase mutants, rutabaga1, had lower FMRP level (Fig. S1e).
Staufen and Argonaute 1 interact with FMRP during memory formation
Staufen has been co-localized with FMRP in neural granules and has been implicated in LTM formation 4. Thus, we asked whether Fmr1 and stau interact functionally, using a classic test for genetic interaction. Heterozygous stauD3/+ or Fmr13/+ flies displayed normal one-day memory after spaced or massed training; in contrast, double heterozygous stauD3/+;Fmr13/+ flies showed defective one-day memory after ST but not after MT (Fig. 2g,h). This result suggests that FMRP and Staufen interact genetically during LTM formation specifically.
FMRP also has been implicated as a molecular component of the RISC (RNAi) complex with Ago1. So we decided to evaluate whether Fmr1 and Ago1 interacted during memory formation. Heterozygous Fmr13/+ or Ago108121/+ flies displayed normal one-day memory after spaced or massed training; in contrast, the double heterozygote, Ago108121/+; Fmr13/+, showed defective one-day memory after ST but not after massed training (Fig. 2i, j). No such transdominant effect was observed in Ago2414/+;Fmr13/+ flies (Fig. S3c). We also were able to demonstrate an acute adult effect of Ago1 overexpression on LTM formation (Fig. S3d). Together with the transdominant interaction between FMRP and Ago1, this observation suggests that FMRP regulates LTM formation via the miRNA pathway.
Inhibition of protein synthesis ameliorates the LTM defect in Fmr1 mutants
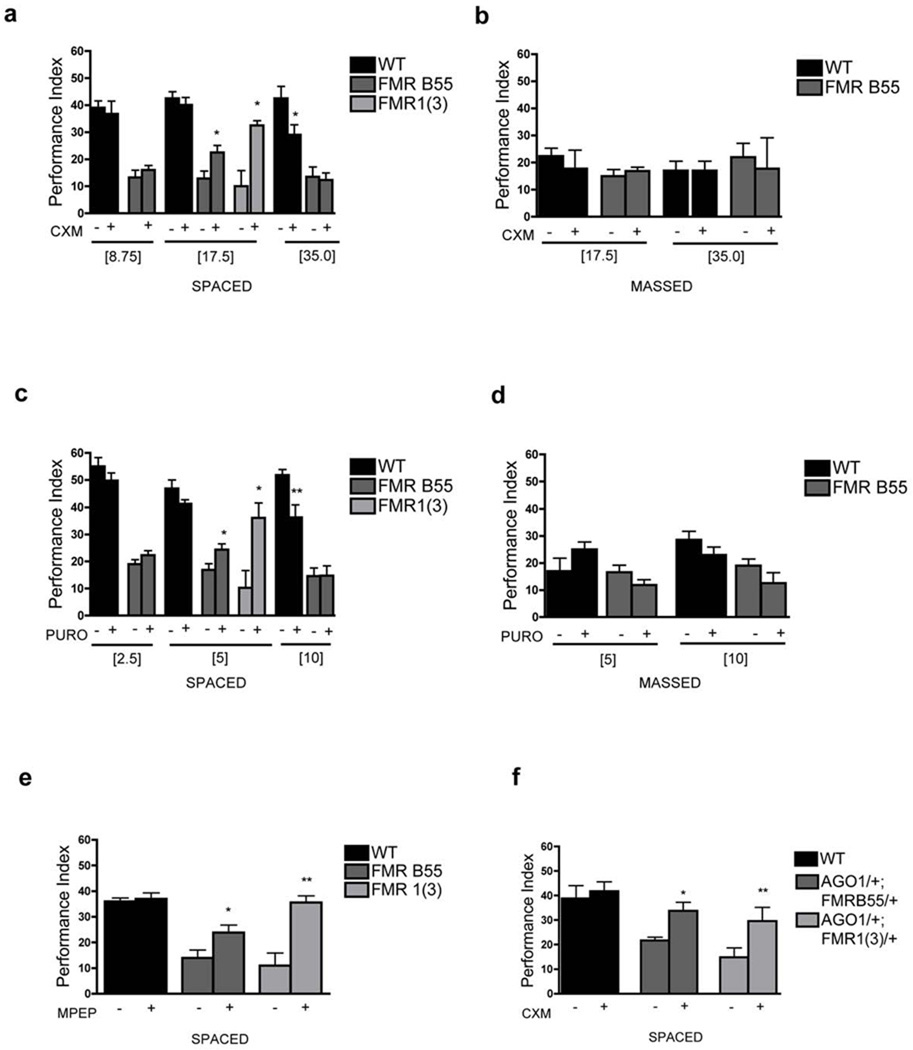
Several reports of increased global protein synthesis have led to the hypothesis that FMRP inhibits protein synthesis. So, could this FMRP-mediated misregulation of protein synthesis affect LTM formation? We tested this question by feeding wild-type and Fmr1 mutants flies protein synthesis inhibitors, cycloheximide (CXM) or puromycin (PURO). We used variations around concentration established for memory inhibition for CXM 7 and measurement of protein synthesis inhibition in Drosophila brain for Puromycin (Tully, unpublished). At relatively low concentrations of CXM or PURO, one-day memory after ST was not affected in wild-type flies but significantly ameliorated in the Fmr1B55 and Fmr13 mutants (Fig. 3a,c), while no effects on one-day memory after MT were observed (Fig. 3b,d). We also observed a significant improvement of LTM with metabotropic glutamate receptor antagonist, MPEP, for the Fmr1B55 and Fmr13 mutants (Fig. 3e; cf. 11).
Figure 3. Inhibition of protein synthesis ameliorates the LTM defect of Fmr1 mutants.
A) At the low cycloheximide dose (8.75mM or 17.5mM), one day memory after ST in wild-type flies was not affected (P=0.682, P = 0.977). FMR B55 performance was not modified at 8.75mM (P=0.4229) but was ameliorated at 17.5mM (P = 0.0244), as was FMR1(3) (P=0.0196). At the 35mM, performance in wild-type flies was reduced (P = 0.018), while FMR B55 was unaffected (P = 0.806). N = 8 PIs per group. B) One-day memory after MT was unaffected. N = 8 PIs per group. C) Puromycin at low dose (2.5mM and 5mM) had no effect in wild-type (P = 0.278, P = 0.133). No effect for FMR B55 with 2.5mM (P = 0.174) but 5mM Puromycin ameliorated performance of both FMR B55 (P = 0.0233) and FMR1(3) (P = 0.0173), while puromycin 10mM disrupted memory in wild-type (P = 0.0059) but had no effect on FMR B55 (P = 0.979). N = 8 – 12 PIs per group. D) One-day memory after MT was unaffected by puromycin in wild-type or FMR B55. N = 8 PIs per group. E) MPEP, ameliorated one-day memory after ST in Fmr1 mutants (Fmr1B55: P =0.0355; Fmr13: P =0.0024). N = 8 PIs per group. F) CXM (17.5mM) ameliorated the deficit in one-day memory after ST in AGO1/+; FMR1B55/+ (P=0.0452) and AGO1/+;FMR1(3)/+ (P = 0.0058). N = 8 PIs per group. All graphs depict mean +/− S.E.M.
The genetic interaction of Fmr1 with staufen and Ago1 (above) led us to consider that excess protein synthesis may occur via a deregulation of the miRNA pathway in Fragile X mutants. To test this hypothesis, we assessed the effects of CXM on the Ago108121/+;Fmr13/+ and Ago108121/+;Fmr1B55/+ double heterozygotes. The memory defect one day after ST of the double heterozygote was significantly ameliorated in the drug-treated group compared to the vehicle-treated group (Fig. 3f). Together, these data reveal an ongoing requirement for FMRP, protein synthesis and miRNA processing during LTM formation.
DISCUSSION
Our behavioral study is the first to show an acute requirement for FMRP during long-term memory formation. Our results suggest that LTM dysfunction in Fmr1 mutants occurs via a miRNA-dependent misregulation of protein synthesis involving Ago1 and staufen. A general excess of protein synthesis possibly occludes the synthesis of subset of proteins required for LTM. This molecular mechanism may ramify more generally, as inhibition of protein synthesis reverses epileptic activity in the Fragile X knockout mouse 12. Our data also extend to the behavioral level the observation that dendritic elongation in response to the metabotropic glutamate receptor agonist, DHPG, could be blocked by the protein synthesis inhibitor, puromycin 13. In addition, the occlusion hypothesis could explain why the Fragile X knockout mouse failed to assemble polyribosomes and synthesize PSD95 and AMPAR in response to DHPG treatment 14, 15. More generally, the therapeutic use of inhibitors of protein synthesis may extend to other disorders, such as tuberous sclerosis, where deregulation of baseline protein synthesis also occurs or dampen other behavioral defects associated with Fragile X disease.
Supplementary Material
Supplementary Figure 1 Neurogenetic characterizations of disruptions of Fmr1
Supplementary Figure 2 Characterization of the acute role of FMRP
Supplementary Figure 3 FMRP is acutely involved in LTM formation
Footnotes
AUTHOR CONTRIBUTION
FB conceptualized the project,conducted experiments, wrote the manuscript; KB performed behavior experiments;HC performed imaging experiments; KB help with conceptualization; TT conceptualized and supervised the project and wrote the manuscript.
REFERENCES
- 1.Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Barbee SA, et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubnau J, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 5.Jin P, et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 6.Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 8.Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didelot G, et al. Tequila, a neurotrypsin ortholog, regulates long-term memory formation in Drosophila. Science. 2006;313:851–853. doi: 10.1126/science.1127215. [DOI] [PubMed] [Google Scholar]
- 10.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 11.McBride SM, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Chuang SC, et al. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiler IJ, et al. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Neurogenetic characterizations of disruptions of Fmr1
Supplementary Figure 2 Characterization of the acute role of FMRP
Supplementary Figure 3 FMRP is acutely involved in LTM formation