Abstract
Objective
To investigate the effects of non-enzymatic glycation on the anti-inflammatory properties of apolipoprotein (apo) A-I.
Methods and Results
Rabbits were infused with saline, lipid-free apoA-I from normal subjects (apoA-IN), lipid-free apoA-I non-enzymatically glycated by incubation with methylglyoxal (apoA-IGlyc in vitro), non-enzymatically glycated lipid-free apoA-I from subjects with diabetes (apoA-IGlyc in vivo), discoidal reconstituted HDL containing phosphatidylcholine and apoA-IN, (A-IN)rHDL, or apoA-IGlyc in vitro, (A-IGlyc in vitro)rHDL. At 24 h post-infusion, acute vascular inflammation was induced by inserting a non-occlusive, periarterial carotid collar. The animals were sacrificed 24 h post-collar insertion. The collars caused intima/media neutrophil infiltration and increased endothelial expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). ApoA-IN infusion decreased neutrophil infiltration and VCAM-1 and ICAM-1 expression by 89, 90 and 66%, respectively. The apoA-IGlyc in vitro infusion decreased neutrophil infiltration by 53%, but did not reduce VCAM-1 or ICAM-1 expression. ApoA-IGlyc in vivo did not inhibit neutrophil infiltration or adhesion molecule expression. (A-IGlyc in vitro)rHDL also inhibited vascular inflammation less effectively than (A-IN)rHDL. The reduced anti-inflammatory properties of non-enzymatically glycated apoA-I were attributed to a reduced ability to inhibit nuclear factor-κB activation and reactive oxygen species formation.
Conclusion
Non-enzymatic glycation impairs the anti-inflammatory properties of apoA-I.
Keywords: apoA-I, inflammation, HDL, adhesion molecules, neutrophils, NF-κB, reactive oxygen species
The ability of high density lipoproteins (HDL) to inhibit inflammation in vitro is well recognized. Work from this and other laboratories has shown that HDL from human plasma, and discoidal reconstituted HDL containing phosphatidylcholine and apolipoprotein (apo) A-I, (A-I)rHDL, inhibit intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) expression in activated, cultured human umbilical vein endothelial cells1,2. Discoidal (A-I)rHDL also inhibit inflammation in vivo by reducing E-selectin expression in a porcine model of acute cutaneous inflammation3. Discoidal (A-I)rHDL also improve renal function, reduce renal injury, decrease renal leucocyte infiltration, and decrease ICAM-1 and P-selectin expression in a rat model of ischemia/reperfusion injury4.
Recent work from this laboratory has shown that discoidal (A-I)rHDL prevent the acute vascular inflammation that results from the placement of non-occlusive peri-arterial collars around rabbit carotid arteries5. In that study three daily infusions of either discoidal (A-I)rHDL, lipid-free apoA-I, or small phosphatidylcholine-containing unilamellar vesicles, markedly reduced inflammation in the collared arteries, as evidenced by decreased intima/media neutrophil infiltration and reduced endothelial expression of VCAM-1, ICAM-1 and monocyte chemoattractant protein-15. A follow-up study established that comparable anti-inflammatory effects were mediated by a single apoA-I infusion administered 24 h prior to, or at the time of, collar insertion6.
Both type 1 and type 2 diabetes are associated with subclinical inflammation and modestly elevated plasma levels of inflammatory markers such as C-reactive protein, soluble ICAM-1 and soluble VCAM-17–9. Pro-inflammatory cytokines, interleukin-6, interleukin-1β and tumour necrosis factor-α levels are also elevated in subjects with diabetes10. Under conditions of chronic hyperglycemia, as is frequently observed in poorly-controlled diabetes, plasma proteins and apolipoproteins, such as apoA-I, may become non-enzymatically glycated11,12. While these modifications are usually attributed to persistently elevated blood glucose levels, it is noteworthy that glucose mediates these changes very slowly13. However, highly reactive glucose-derived dicarbonyl compounds, such as methylglyoxal (MG), glycoaldehyde and 3-deoxyglucosone, non-enzymatically glycate plasma proteins and apolipoproteins at a rapid rate14. This causes extensive cross-linking and irreversible conversion of the modified proteins into advanced glycation end products (AGEs), which are ligands for the endothelial advanced glycation end product receptor, RAGE15,16. The binding of AGEs to RAGE activates the endothelium, increases VCAM-1, ICAM-1 and E-selectin expression17,18, and exacerbates diabetes-associated inflammation. These findings, together with the observation that hyperglycemia can alter HDL function19, raise the possibility that non-enzymatic glycation may reduce the anti-inflammatory properties of apoA-I.
We have addressed this question by comparing lipid-free apoA-I from normal subjects (apoA-IN), lipid-free apoA-IN that has been non-enzymatically glycated in vitro by incubation with methylglyoxal (MG) (apoA-IGlyc in vitro), and lipid-free apoA-I from subjects with type 2 diabetes and microvascular complications (apoA-IGlyc in vivo) in terms of their ability to inhibit acute vascular inflammation in collared carotid arteries of normocholesterolemic New Zealand White (NZW) rabbits. The results establish that apoA-IGlyc in vitro and apoA-IGlyc in vivo inhibit acute vascular inflammation less effectively than apoA-IN. In the case of apoA-IGlyc in vitro, these adverse effects were apparent irrespective of whether it was administered in a lipid-free form or as a component of discoidal rHDL. These reduced anti-inflammatory properties of non-enzymatically glycated apoA-I were associated with enhanced phosphorylation of the inhibitor of κB, IκBα, reduced inhibition of nuclear translocation of nuclear factor-κB (NF-κB) and a reduced ability to inhibit reactive oxygen species (ROS) formation.
Methods
For the in vivo arm of the study single infusions of apoA-IN or apoA-IGlyc in vitro in the lipid-free form or as a constituent of discoidal rHDL, or lipid-free apoA-IGlyc in vivo were administered to normocholesterolemic NZW rabbits 24 h prior to inserting a non-occlusive peri-arterial carotid collar. The animals were sacrificed 24 h post-collar insertion. Inflammation was assessed immunohistochemically as endothelial expression of ICAM-1 and VCAM-1 and intima/media neutrophil infiltration. For the in vitro studies, (A-IN)rHDL and (A-IGlyc in vitro) were incubated with cytokine-activated human coronary artery endothelial cells (HCAECs). VCAM-1 and ICAM-1 protein expression was quantified by flow cytometry. mRNA levels were determined by real time PCR. Phosphorylation of IκBα and NF-κB nuclear translocation were assessed by western blotting. ROS formation was assessed by incubation with dihydroethidium (DHE). Details are in the online Supplementary Material.
Results
Characterization of lipid-free apoA-IN, lipid-free apoA-IGlyc in vitro, lipid-free apoA-IGlyc in vivo, discoidal (A-IN)rHDL and discoidal (A-IGlyc in vitro)rHDL (Supplemental Table I)
The (A-IN)rHDL consisted of a major population of particles (diameter 12.5 nm) and two minor populations of particles, 14.3 and 8.5 nm in diameter. Incubation with MG did not affect discoidal (A-I)rHDL stoichiometry, but reduced their diameters to 14.0, 10.7 and 8.3 nm (not shown). As judged by SDS-PAGE, cross-linking was evident in the apoA-IGlyc in vitro (Supplemental Fig. I) and the discoidal (A-IGlyc in vitro)rHDL (not shown)20,21. The apoA-IGlyc in vivo from subjects with type 2 diabetes (HbA1c 7.0±0.4%, total cholesterol 3.1±0.5 mM, triglycerides 1.7±0.3 mM, HDL-C 0.9±0.1 mM) was not cross-linked and migrated to the same position as lipid-free apoA-IN (Supplemental Fig. I).
Compared to lipid-free A-IN and discoidal (A-IN)rHDL that were incubated in the absence of MG, incubation with MG modified approximately 40% of the arginine residues, 25% of the lysine residues, and 15% of the tryptophan residues in the lipid-free apoA-IGlyc in vitro and discoidal (A-IGlyc in vitro)rHDL (p<0.05) (Supplemental Table I). The arginine, lysine and tryptophan residues in the lipid-free apoA-IGlyc in vivo were not modified significantly.
Incubation with MG increased lipid-free apoA-I Nε-carboxymethyllysine (CML) levels from 6.0±0.7 to 11.0±2.8 pmol/mg protein (p<0.05) and Nε-carboxyethyllysine (CEL) levels from 2.9±0.7 to 51.7±15.4 pmol/mg protein (p<0.01). Lipid-free apoA-IGlyc in vivo CML and CEL levels were 10.0±1.4 pmol/mg protein (p<0.05 versus lipid-free apoA-IN) and 13.0±0.3 pmol/mg protein (p<0.0001 versus lipid-free apoA-IN), respectively. Lipid-free apoA-IN (2S)-2-amino-5-(2-amino-5-methyl-4-oxo-4,5-dihydro-imidazol-1-yl)-pentanoic acid (MG-H2) levels were 39.2±1.5 pmol/mg protein, compared to 7306.0±46.5 pmol/mg protein for lipid-free apoA-IGlyc in vitro (p<0.0001), and 50.2±1.6 pmol/mg protein for lipid-free apoA-IGlyc in vivo (p<0.01).
The effects of non-enzymatic glycation on the conformation of apoA-I were assessed by surface plasmon resonance. Non-enzymatic glycation altered the conformation of the epitope recognized by monoclonal antibody (mAb) AI-1.2 in the N-terminal domain of apoA-IGlyc in vivo (Supplemental Fig. II, white bars). The conformation of the central epitope recognized by mAb AI-115.1 was also altered in apoA-IGlyc in vitro (black bars) and apoA-IGlyc in vivo (white bars). The conformation of the epitopes in the central region of apoA-IGlyc in vitro, to which mAb AI-17 binds, and in the C-terminal domain, to which mAb AI-141.7 binds, were also significantly modified (Supplemental Fig. II).
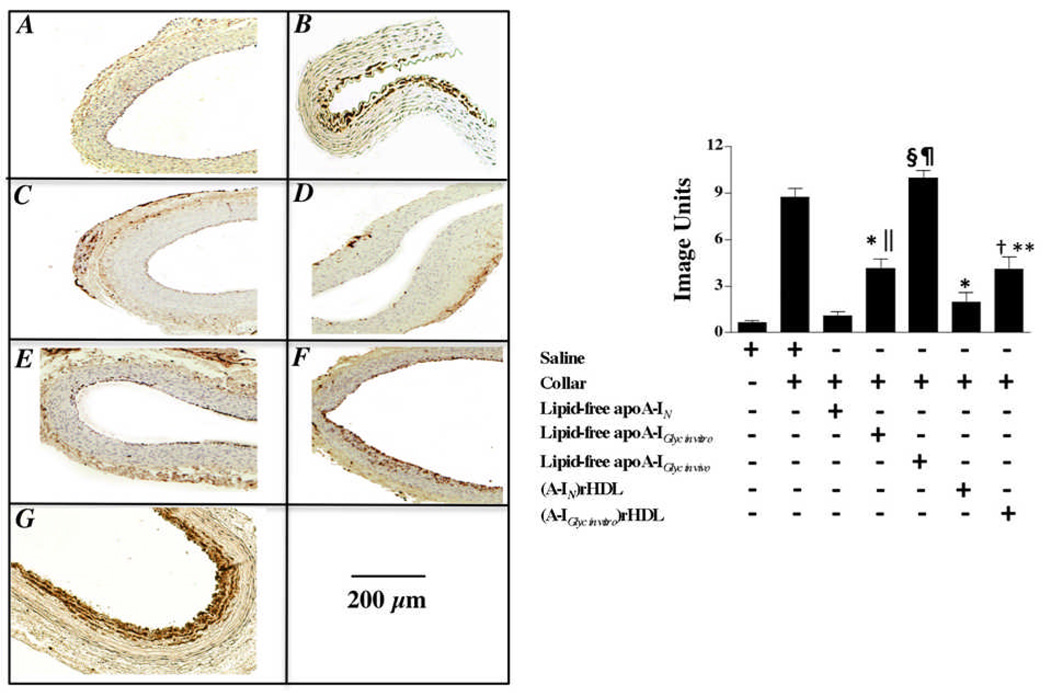
Effect of non-enzymatically glycated apoA-I on neutrophil infiltration into collared carotid arteries (Fig. 1)
Figure 1. Effect of non-enzymatic glycation on the ability of lipid-free apoA-I and discoidal (A-I)rHDL to inhibit neutrophil infiltration into the artery wall.
Carotid artery sections (n=5) from non-collared (Panel A) and collared normocholesterolemic NZW rabbits that received a single infusion of either saline (Panel B), lipid-free apoA-IN (Panel C), lipid-free apoA-IGlyc in vitro (Panel E), lipid-free apoA-IGlyc in vivo (Panel G), discoidal (A-IN)rHDL (Panel D), or discoidal (A-IGlyc in vitro)rHDL (Panel F) were immunostained for CD18+ cells. Representative stained sections are shown. Staining was quantified (Supplemental Material) and is presented as the mean±SEM.
*p<0.0001 versus saline; †p<0.001 versus saline; §p<0.0001 versus lipid-free apoA-IN; ║p<0.001 versus lipid-free apoA-IN; ¶p<0.01 versus lipid-free apoA-IGlyc in vitro; **p<0.05 versus discoidal (A-IN)rHDL.
Pre-infusion plasma concentrations of total cholesterol, HDL-cholesterol and rabbit apoA-I were 1.05±0.09 mM, 0.63±0.29 mM, and 0.78±0.04 mg/ml, respectively. At sacrifice the total cholesterol and HDL-cholesterol concentrations were 1.29±0.11 and 0.64±0.24 mM, respectively. ApoA-I levels could not be determined on the samples obtained at sacrifice because the anti-rabbit apoA-I antibody partly cross-reacted with human apoA-I. However, the results of an earlier study in which rabbits were infused with 8 mg/kg of rabbit apoA-I, established that this intervention leads to only a modest and transient increase in plasma apoA-I levels5.
Compared to what was observed for the non-collared arteries (Fig. 1A), extensive infiltration of CD18+ cells was apparent in the intima/media of the collared arteries from the saline-infused animals (Fig. 1B). These neutrophils were most likely recruited from the vessel lumen. However, the possibility that some neutrophils may also have been recruited via the adventitia cannot be excluded. The absence of cells staining positive for RAM11 and CD43 confirmed that these cells were not macrophages or lymphocytes (not shown). The lipid-free apoA-IN infusion decreased neutrophil infiltration into the artery wall from 8.7±0.4 to 1.0±0.2 image units (Fig. 1C) (p<0.0001). Infusion of lipid-free apoA-IGlyc in vitro (Fig 1E) decreased neutrophil infiltration from 8.7±0.4 to 4.1±0.1 image units (p<0.0001). This inhibition was less than that mediated by unmodified, lipid-free apoA-IN (p<0.001). Lipid-free apoA-IGlyc in vivo did not inhibit neutrophil infiltration into the collared arteries (Fig 1G).
The discoidal (A-IN)rHDL infusion decreased neutrophil infiltration into the collared arteries from 8.7±0.4 (saline infused animals) to 2.0±0.3 image units (p<0.0001). The discoidal (A-IGlyc in vitro)rHDL (Fig 1F) decreased neutrophil infiltration from 8.7±0.4 to 4.1±0.8 image units (p<0.001) and therefore inhibited neutrophil infiltration into the collared arteries less effectively than the discoidal (A-IN)rHDL (Fig 1D) (p<0.05).
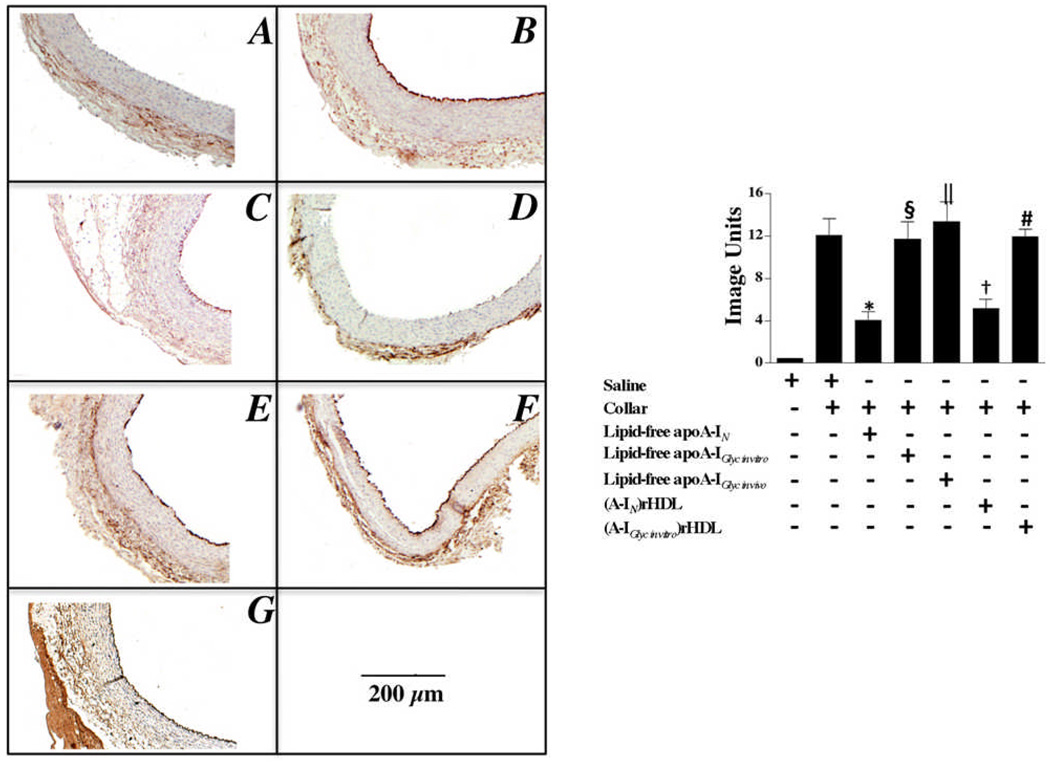
Effect of non-enzymatically glycated apoA-I on ICAM-1 expression in collared carotid arteries (Fig. 2)
Figure 2. Effect of non-enzymatic glycation on the ability of lipid-free apoA-I and discoidal (A-I)rHDL to inhibit ICAM-1 expression.
Carotid artery sections (n=5) from non-collared (Panel A) and collared normocholesterolemic NZW rabbits that received a single infusion of either saline (Panel B), lipid-free apoA-IN (Panel C), lipid-free apoA-IGlyc in vitro (Panel E), lipid-free apoA-IGlyc in vivo (Panel G), discoidal (A-IN)rHDL (Panel D), or discoidal (A-IGlyc in vitro)rHDL (Panel F) were immunostained for ICAM-1. Representative stained sections are shown. Staining was quantified (Supplemental Material) and is presented as the mean±SEM.
*p<0.0001 versus saline; †p<0.001 versus saline; §p<0.0001 versus lipid-free apoA-IN; ║p<0.001 versus lipid-free apoA-IN; #p<0.0001 versus discoidal (A-IN)rHDL.
The carotid collars markedly increased endothelial ICAM-1 expression in the saline-infused animals (Fig. 2A versus 2B). The lipid-free apoA-IN infusion decreased ICAM-1 expression from 12.1±0.3 to 4.1±0.4 image units (p<0.0001) (Fig. 2C). Neither lipid-free apoA-IGlyc in vitro (Fig. 2E) nor apoA-IGlyc in vivo (Fig. 2G) decreased ICAM-1 expression. Relative to the saline-infused animals, the discoidal (A-IN)rHDL decreased ICAM-1 expression from 12.0±0.3 to 5.2±0.7 image units (p<0.01) (Fig. 2D). The discoidal (A-IGlyc in vitro)rHDL did not inhibit ICAM-1 expression (Fig. 2F).
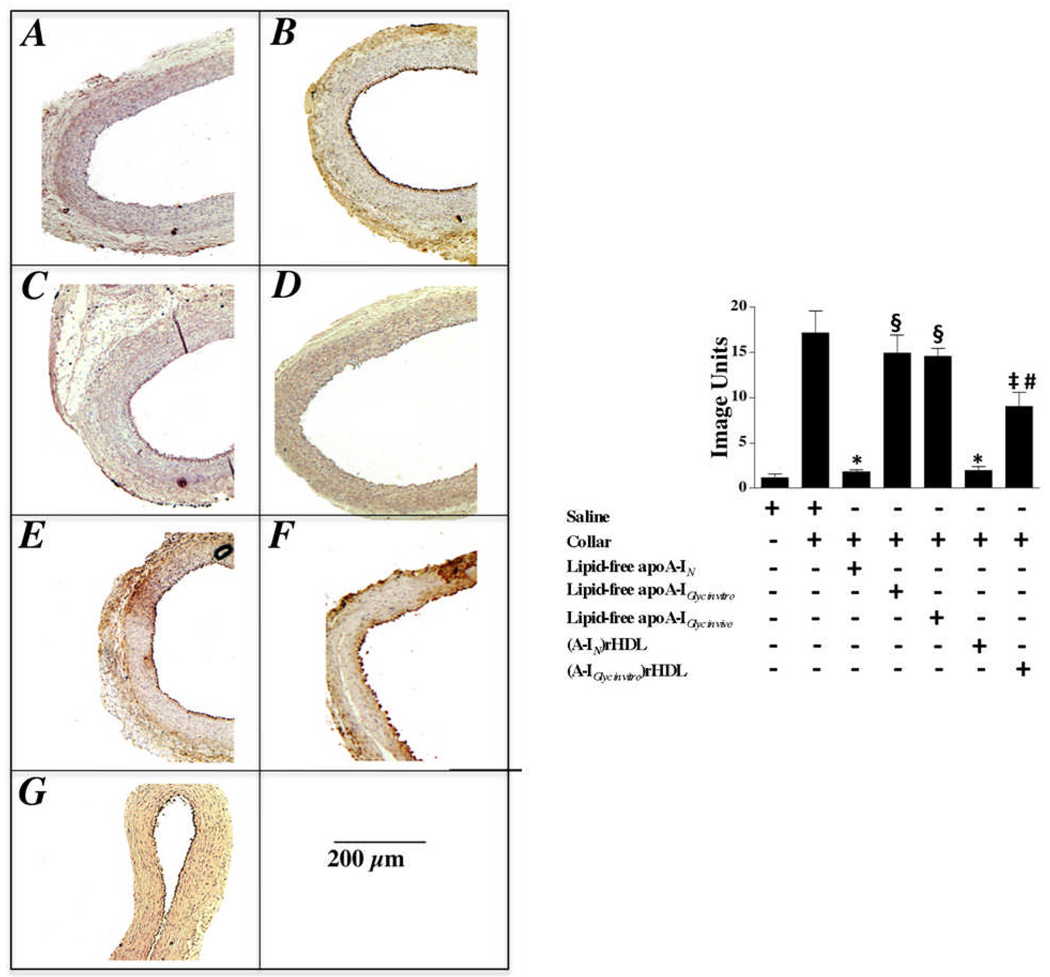
Effect of non-enzymatically glycated apoA-I on VCAM-1 expression in collared carotid arteries (Fig. 3)
Figure 3. Effect of non-enzymatic glycation on the ability of lipid-free apoA-I and discoidal (A-I)rHDL to inhibit VCAM-1 expression.
Carotid artery sections (n=5) from non-collared (Panel A) and collared normocholesterolemic NZW rabbits that received a single infusion of either saline (Panel B), lipid-free apoA-IN (Panel C), lipid-free apoA-IGlyc in vitro (Panel E), lipid-free apoA-IGlyc in vivo (Panel G), discoidal (A-IN)rHDL (Panel D), or discoidal (A-IGlyc in vitro)rHDL (Panel F) were immunostained for VCAM-1. Representative stained sections are shown. Staining was quantified (Supplemental Material) and is presented as the mean±SEM.
*p<0.0001 versus saline; ‡p<0.01 versus saline; §p<0.0001 versus lipid-free apoA-IN; #p<0.0001 versus discoidal (A-IN)rHDL.
As reported previously and confirmed here, carotid collars increase endothelial expression of VCAM-1 (Fig. 3A versus 3B)5,6. Infusion of lipid-free apoA-IN reduced VCAM-1 expression from 17.2±0.6 to 1.8±0.1 image units (p<0.0001) (Fig. 3C). Lipid-free apoA-IGlyc in vitro (Fig. 3E) and lipid-free apoA-IGlyc in vivo (Fig. 3G) did not inhibit VCAM-1 expression.
The discoidal (A-IN)rHDL decreased VCAM-1 expression from 17.2±0.6 to 2.0±0.2 image units (p<0.0001), while the discoidal (A-IGlyc in vitro)rHDL reduced VCAM-1 expression to 9.0±0.6 image units (p<0.01). The discoidal (A-IGlyc in vitro)rHDL (Fig. 3F) therefore inhibited VCAM-1 expression less effectively than discoidal (A-IN)rHDL (Fig. 3D) (p<0.0001).
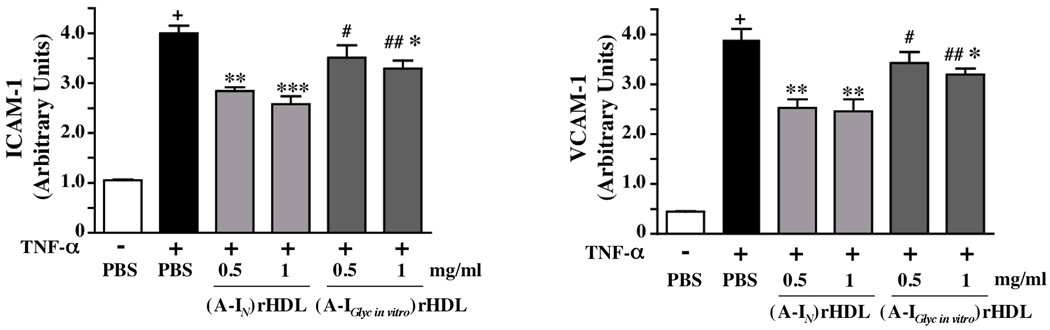
Effect of non-enzymatically glycated apoA-I on ICAM-1 and VCAM-1 expression in HCAECs (Fig 4)
Figure 4. Inhibition of ICAM-1 and VCAM-1 expression in HCAECs by (A-IN)rHDL and (A-IGlyc in vitro)rHDL.
HCAECs were incubated for 16 h with either PBS, (A-IN)rHDL, or (A-Iglyc in vitro)rHDL (final apoA-I concentration 0.5 or 1.0 mg/ml), then stimulated for 5 h with TNF-α (final concentration 0.2 ng/ml). Cell surface expression of ICAM-1 and VCAM-1 was quantified by flow cytometry and is shown as the mean±SEM of 3 independent experiments.
+p<0.001 vs PBS, *p<0.05 vs TNF-α, **p<0.01 vs TNF-α, ***p<0.001 vs TNF-α, #p<0.05 vs 0.5 mg/ml (A-IN)rHDL, ##p<0.05 vs 1.0 mg/ml (A-IN)rHDL
Experiments were carried out to determine if non-enzymatically glycated apoA-I inhibits inflammation less effectively than apoA-IN in cultured HCAECs. Discoidal rHDL were used for this study because lipid-free apoA-I does not inhibit inflammation in cultured endothelial cells1.
Stimulation of HCAECs with TNF-α significantly increased ICAM-1 and VCAM-1 protein expression (Fig. 4). Pre-incubation with discoidal (A-IN)rHDL (final apoA-I concentration 0.5 mg/ml) reduced ICAM-1 expression from 4.0±0.2 to 2.8±0.1 units (p<0.01) and VCAM-1 expression from 3.9±0.2 to 2.5±0.2 units (p<0.01). Comparable results were obtained at a final apoA-I concentration of 1.0 mg/ml, where pre-incubation with (A-IGlyc in vitro)rHDL reduced ICAM-1 expression from 4.0±0.2 to 3.3±0.2, and VCAM-1 expression from 3.9±0.2 to 3.2±0.1 arbitrary units (p<0.05 for both versus TNF-α only). The (A-IGlyc in vitro)rHDL did not significantly inhibit adhesion molecule expression at a final apoA-I concentration of 0.5 mg/ml. Overall, the (A-IGlyc in vitro)rHDL inhibited VCAM-1 and ICAM-1 expression less effectively than (A-IN)rHDL (p<0.05).
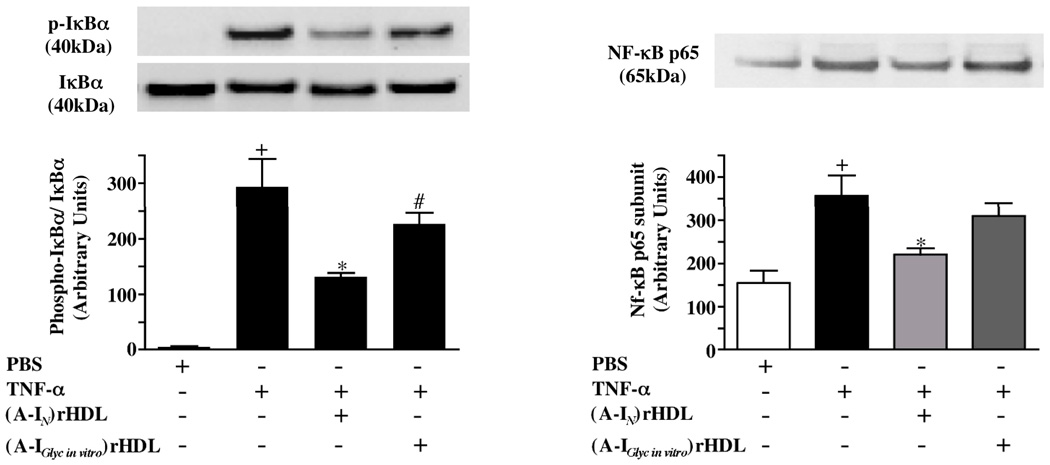
Effect of non-enzymatically glycated apoA-I on IκBα phosphorylation and NF-κB nuclear translocation (Fig. 5)
Figure 5. Effect of (A-IN)rHDL and (A-IGlyc in vitro)rHDL on IkBα phosphorylation and NF-kB p65 subunit nuclear translocation in HCAECs.
HCAECs were incubated for 16 h with either PBS, (A-IN)rHDL, or (A-Iglyc in vitro)rHDL (final apoA-I concentration 1.0 mg/ml), stimulated for 10 min with TNF-α (2 ng/ml) and subjected to immunoblot analysis for cytosolic phosphorylated-IkBα, IkBα and nuclear NF-kB p65 subunit content. Phosphorylated-IkBα/IkBα ratios were normalized to total IkB. Results are expressed as mean±SEM of 3 independent experiments.
+p<0.01 vs PBS, *p<0.05 vs TNF-α, #p<0.05 vs (A-IN)rHDL
Phosphorylation of IκBα disrupts the inactive cytosolic NF-κB/IκB complex, causing NF-kB to translocate to the nucleus, where it binds to promoter regions in the ICAM-1 and VCAM-1 genes and increases their expression. As discoidal (A-IN)rHDL inhibit IκBα phosphorylation and NF-kB nuclear translocation22, we examined whether the attenuated anti-inflammatory properties of (A-IGlyc in vitro)rHDL could be due to reduced inhibition of IκBα phosphorylation and NF-kB nuclear translocation.
Stimulation of HCAECs with TNF-α increased the phosphorylated-IκBα/IκBα ratio and nuclear NF-κB p65 subunit levels. Pre-incubation with (A-IN)rHDL reduced the phosphorylated-IκBα/IκBα ratio by 55% (from 293.6±50.5 to 132.1±6.4 units) and nuclear NF-κB p65 subunit levels by 38% (from 356.9±47.2 to 220.7±14.5 units) (p<0.05 for both). Pre-incubation with (A-IGlyc in vitro)rHDL did not significantly reduce IκBα phosphorylation or nuclear translocation of the NF-κB p65 subunit (p<0.05 versus (A-IN)rHDL).
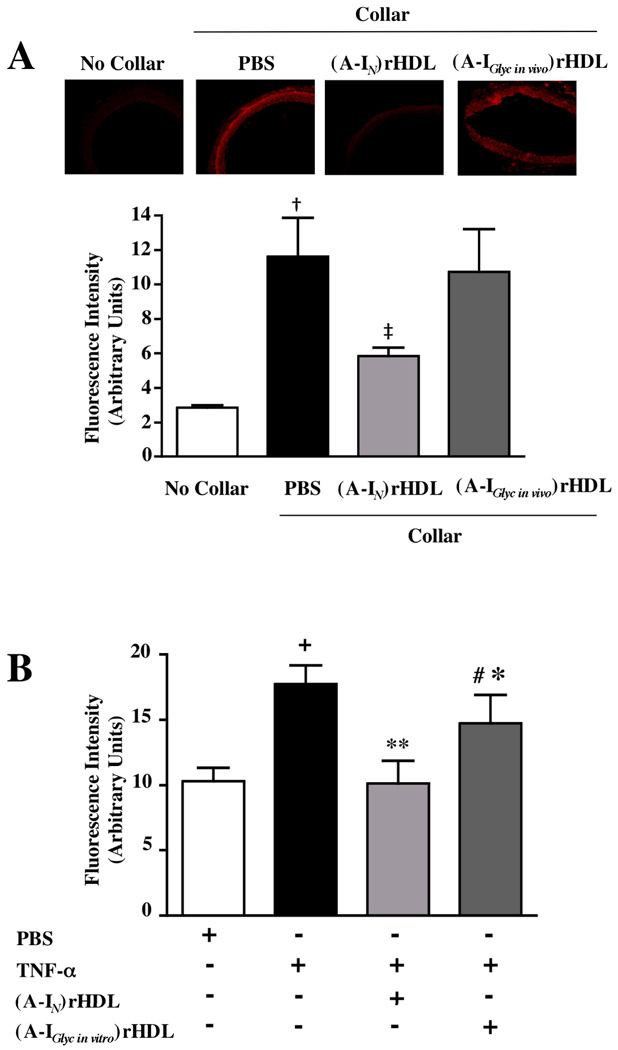
Effect of non-enzymatically glycated apoA-I on ROS production in vitro and in vivo (Fig. 6)
Figure 6. Effect of non-enzymatic glycation on the ability of apoA-I to inhibit ROS generation in NZW rabbit collared carotid arteries and HCAECs.
ROS generation was detected as DHE-derived fluorescent oxidation products in non-collared and collared carotid artery sections from NZW rabbits following administration of a single infusion of saline, lipid-free apoA-IN or lipid-free apoA-IGlyc in vivo (Panel A). TNF-α-induced ROS production was assessed as DHE-derived fluorescent oxidation product formation in HCAECs after incubation for 16 h with PBS, (A-IN)rHDL, or (A-IGlyc in vitro)rHDL (final apoA-I concentration 1 mg/mL) (Panel B). Results are expressed as mean±SEM of 3 independent experiments performed in triplicate.
†p<0.05 vs no collar; ‡p<0.05 vs PBS; +p<0.05 vs PBS; *p<0.05 vs TNF-α; **p<0.01 vs TNF-α; #p<0.05 vs (A-IN)rHDL
We have reported previously that (A-IN)rHDL infusions inhibit ROS production in collared NZW rabbit carotid arteries5,22. To ascertain if (A-IGlyc in vivo)rHDL inhibits ROS production less effectively than (A-IN)rHDL, collard carotid artery sections were incubated with DHE. In the presence of superoxide, DHE is oxidized to products that fluoresce when they intercalate into DNA. The carotid collars mediated robust formation of DHE-derived oxidation products (Fig 6A). A single (A-IN)rHDL infusion reduced the collar-mediated fluorescent oxidation product formation by 51%, from 11.9±0.9 to 5.8±0.3 units (p<0.05). (A-IGlyc in vivo)rHDL did not decrease collar-mediated formation of DHE-derived fluorescent oxidation products (p<0.05 versus (A-IN)rHDL), (Fig. 6A).
This result was recapitulated in cultured HCAECs, where pre-incubation with (A-IN) rHDL and (A-IGlyc in vivo)rHDL reduced the TNF-α-mediated formation of fluorescent products from 17.8±0.8 to 11.4±1.0 (p<0.01) and 14.8±1.2 (p<0.05) arbitrary units, respectively (Fig. 6B). (A-IN)rHDL inhibited DHE-derived fluorescent oxidation product formation more effectively than (A-IGlyc in vivo)rHDL (p<0.05) (Fig 6B).
Discussion
We have previously reported that implantation of non-occlusive silastic collars around carotid arteries in normocholesterolemic rabbits induces an acute inflammatory response that causes infiltration of neutrophils into the intima/media and increases endothelial expression of VCAM-1 and ICAM-15,6. Both the neutrophil infiltration and adhesion molecule expression are markedly decreased when small amounts of apoA-I, either in the lipid-free form or as a constituent of discoidal (A-I)rHDL, are infused into the animals prior to collar insertion. The present study shows that these anti-inflammatory properties of lipid-free apoA-I and discoidal (A-I)rHDL are markedly reduced if the animals are infused with apoA-I that has been non-enzymatically glycated by incubation in vitro with MG, or modified in vivo as a consequence of the persistent hyperglycemia that can occur in type 2 diabetes.
Although the modifications that were sustained when apoA-I was non-enzymatically glycated by incubation with MG differed from those observed for apoA-I from subjects with type 2 diabetes (Supplemental Table I), both preparations displayed similar reductions in their anti-inflammatory properties. This is consistent with the proposition that in vivo glycation of apoA-I in people with diabetes may compromise HDL functionality and increase cardiovascular risk.
There are several possible explanations for the increased cardiovascular risk in people with type 2 diabetes. One relates to the prevalence of diabetic dyslipidemia, which is characterized by elevated plasma triglycerides, an LDL fraction containing potentially pro-atherogenic small, dense particles, and low HDL cholesterol levels. The HDL in these individuals also tend to be smaller, triglyceride-enriched and more dense than normal. Recent reports have established that triglyceride-enrichment can compromise the functionality of HDL23–25. The current study extends these observations by showing that the non-enzymatic glycation of apoA-I, which is known to occur in diabetes, may further compromise HDL functionality.
A possible explanation for the reduced anti-inflammatory properties of non-enzymatically glycated apoA-I may be that it is cleared from the circulation more rapidly than normal apoA-I. However, a recent study carried out in this laboratory, in which normocholesterolemic NZW rabbits received a single 8 mg/kg infusion of 125I-labelled lipid-free apoA-IN, indicated that this is unlikely to be the case. Those results showed that <10% of the radiolabel remained in the circulation at 3 h post-infusion (Patel and Rye, unpublished, 2009). Thus, even if non-enzymatically glycated apoA-I was catabolized more rapidly than apoA-IN, both preparations would have been cleared from the circulation long before carotid collar insertion. This suggests that, rather than having a direct, physical effect on the artery wall, the anti-inflammatory properties of apoA-I may reflect altered gene transcription and the inhibition of one or more key intracellular inflammation signalling pathways.
The reduction in VCAM-1 expression following administration of apoA-IN is consistent with reduced activation of NF-κB, a key inflammatory mediator, and the primary regulator of VCAM-1 gene transcription22,26–29. ApoA-IN, by contrast, inhibited ICAM-1 gene expression to a lesser extent that VCAM-1. This is most likely because ICAM-1 is regulated by several signalling pathways, only one of which involves NF-kB30. The present results are therefore consistent with non-enzymatic glycation significantly compromising the ability of apoA-I to inhibit inflammation by directly inhibiting the NF-κB pathway (Fig. 5).
The inhibition of VCAM-1 and ICAM-1 gene expression by lipid-free or lipid-associated apoA-I is most likely initiated by the binding of apoA-I to specific receptors or domains, such as lipid rafts, on the endothelial surface. The reduced anti-inflammatory properties of non-enzymatically glycated apoA-I may therefore be a consequence of structural changes that prevent it from accessing these domains. Evidence that this could be the case comes from our current (Supplemental Fig II) and earlier work showing that non-enzymatic glycation alters the conformation of the central and C-terminal domains of apoA-I20,21. This may mask specific apoA-I binding sites and inhibit interactions with endothelial receptors and/or membrane domains that downregulate inflammatory signalling pathways.
The structural and conformational changes that occur when apoA-I is non-enzymatically glycated by MG in vitro are similar to what has been reported in vivo for AGE formation14. It is also well established that AGEs that are generated in vivo, as well as proteins that are non-enzymatically glycated in vitro, are ligands for RAGE31, and that the binding of AGE to RAGE upregulates VCAM-1, and possibly ICAM-1 expression, via activation of NF-kB14,18,32,33. When taken together these observations suggest that the structural changes that occur when apoA-I is non-enzymatically glycated may maintain VCAM-1 and ICAM-1 expression via enhanced binding to RAGE.
In summary, this study shows that non-enzymatic glycation adversely affects the anti-inflammatory properties of apoA-I, irrespective of whether the modifications occur in vitro or in vivo. This finding is of considerable physiological significance given that subjects with type 2 diabetes, especially those with micro- and macro-vascular complications, tend to be in a pro-inflammatory state. The current results highlight the importance of maintaining good glycemic control in such individuals, and indicate that therapeutic intervention with cross-link breakers, which reportedly prevent protein modifications, have the potential to decrease the risk of the microvascular, and possibly the macrovascular complications that accompany this increasingly prevalent disorder.
Supplementary Material
Acknowledgements
Sources of Funding: National Health and Medical Research Council of Australia Grant 222722.
Footnotes
Disclosures: No relationships to disclose
References
- 1.Baker PW, Rye KA, Gamble JR, Vadas MA, Barter PJ. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res. 1999;40:345–353. [PubMed] [Google Scholar]
- 2.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 3.Cockerill GW, Huehns TY, Weerasinghe A, Stocker C, Lerch PG, Miller NE, Haskard DO. Elevation of plasma high-density lipoprotein concentration reduces interleukin-1-induced expression of E-selectin in an in vivo model of acute inflammation. Circulation. 2001;103:108–112. doi: 10.1161/01.cir.103.1.108. [DOI] [PubMed] [Google Scholar]
- 4.Thiemermann C, Patel NS, Kvale EO, Cockerill GW, Brown PA, Stewart KN, Cuzzocrea S, Britti D, Mota-Filipe H, Chatterjee PK. High density lipoprotein (HDL) reduces renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1833–1843. doi: 10.1097/01.asn.0000075552.97794.8c. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 6.Puranik R, Bao S, Nobecourt E, Nicholls SJ, Dusting GJ, Barter PJ, Celermajer DS, Rye KA. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis. 2008;196:240–247. doi: 10.1016/j.atherosclerosis.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, Ridker PM, Nathan DM. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation. 2005;111:2446–2453. doi: 10.1161/01.CIR.0000165064.31505.3B. [DOI] [PubMed] [Google Scholar]
- 8.Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, Paolisso G, Giugliano D. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–2251. doi: 10.1161/01.cir.101.19.2247. [DOI] [PubMed] [Google Scholar]
- 9.Otsuki M, Hashimoto K, Morimoto Y, Kishimoto T, Kasayama S. Circulating vascular cell adhesion molecule-1 (VCAM-1) in atherosclerotic NIDDM patients. Diabetes. 1997;46:2096–2101. doi: 10.2337/diab.46.12.2096. [DOI] [PubMed] [Google Scholar]
- 10.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 11.Curtiss LK, Witztum JL. Plasma apolipoproteins AI, AII, B, CI, and E are glucosylated in hyperglycemic diabetic subjects. Diabetes. 1985;34:452–461. doi: 10.2337/diab.34.5.452. [DOI] [PubMed] [Google Scholar]
- 12.Shishino K, Murase M, Makino H, Saheki S. Glycated apolipoprotein A-I assay by combination of affinity chromatography and latex immunoagglutination. Ann Clin Biochem. 2000;37:498–506. doi: 10.1177/000456320003700411. [DOI] [PubMed] [Google Scholar]
- 13.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- 14.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 15.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 17.Kunt T, Forst T, Harzer O, Buchert G, Pfutzner A, Lobig M, Zschabitz A, Stofft E, Engelbach M, Beyer J. The influence of advanced glycation endproducts (AGE) on the expression of human endothelial adhesion molecules. Exp Clin Endocrinol Diabetes. 1998;106:183–188. doi: 10.1055/s-0029-1211974. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedrick CC, Thorpe SR, Fu MX, Harper CM, Yoo J, Kim SM, Wong H, Peters AL. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43:312–320. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- 20.Nobecourt E, Davies MJ, Brown BE, Curtiss LK, Bonnet DJ, Charlton F, Januszewski AS, Jenkins AJ, Barter PJ, Rye KA. The impact of glycation on apolipoprotein A-I structure and its ability to activate lecithin:cholesterol acyltransferase. Diabetologia. 2007;50:643–653. doi: 10.1007/s00125-006-0574-z. [DOI] [PubMed] [Google Scholar]
- 21.Nobecourt E, Zeng J, Davies MJ, Brown BE, Yadav S, Barter PJ, Rye KA. Effects of cross-link breakers, glycation inhibitors and insulin sensitisers on HDL function and the non-enzymatic glycation of apolipoprotein A-I. Diabetologia. 2008;51:1008–1017. doi: 10.1007/s00125-008-0986-z. [DOI] [PubMed] [Google Scholar]
- 22.Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G. The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.200196. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel S, Puranik R, Nakhla S, Lundman P, Stocker R, Wang XS, Lambert G, Rye KA, Barter PJ, Nicholls SJ, Celermajer DS. Acute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteins. Atherosclerosis. 2008;204:424–428. doi: 10.1016/j.atherosclerosis.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 24.de Souza JA, Vindis C, Hansel B, Negre-Salvayre A, Therond P, Serrano CV, Jr, Chantepie S, Salvayre R, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis. 2008;197:84–94. doi: 10.1016/j.atherosclerosis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Kontush A, Chapman MJ. Why is HDL functionally deficient in type 2 diabetes? Curr Diab Rep. 2008;8:51–59. doi: 10.1007/s11892-008-0010-5. [DOI] [PubMed] [Google Scholar]
- 26.Shu HB, Agranoff AB, Nabel EG, Leung K, Duckett CS, Neish AS, Collins T, Nabel GJ. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993;13:6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denk A, Goebeler M, Schmid S, Berberich I, Ritz O, Lindemann D, Ludwig S, Wirth T. Activation of NF-kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J Biol Chem. 2001;276:28451–28458. doi: 10.1074/jbc.M102698200. [DOI] [PubMed] [Google Scholar]
- 28.Weber C, Erl W, Pietsch A, Strobel M, Ziegler-Heitbrock HW, Weber PC. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb. 1994;14:1665–1673. doi: 10.1161/01.atv.14.10.1665. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Park JH, Kang JS, Kang YH. Involvement of transcription factors in plasma HDL protection against TNF-alpha-induced vascular cell adhesion molecule-1 expression. Int J Biochem Cell Biol. 2003;35:168–182. doi: 10.1016/s1357-2725(02)00173-5. [DOI] [PubMed] [Google Scholar]
- 30.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 31.Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, Stern DM, Al-Mohanna FA. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs) J Leukoc Biol. 2002;71:433–444. [PubMed] [Google Scholar]
- 32.Vlassara H, Fuh H, Donnelly T, Cybulsky M. Advanced glycation endproducts promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol Med. 1995;1:447–456. [PMC free article] [PubMed] [Google Scholar]
- 33.Boulanger E, Wautier MP, Wautier JL, Boval B, Panis Y, Wernert N, Danze PM, Dequiedt P. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 2002;61:148–156. doi: 10.1046/j.1523-1755.2002.00115.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.