Abstract
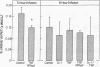
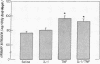
To improve our understanding of the metabolic role of cytokines in protein wasting, we estimated the rates of protein synthesis and degradation in muscle and liver tissues in intact rats treated with several doses of recombinant IL 1 and/or tumor necrosis factor (TNF)/cachectin. Protein breakdown in muscle and liver were derived in vivo from the relationship between [14C]leucine distribution and tissue dilution in reference to circulating leucine. Synthesis was derived from the relationship between [14C]leucine appearance in the protein-bound and free-tissue leucine pools. To specifically relate changes in leucine tracer metabolism to protein dynamics, we separately measured the effect of these cytokines on blood flow to different tissues. The increase in dilution of the tissue-free [14C]leucine by TNF and TNF/IL 1 mixture, but not by IL 1 alone, could not be explained by a hemodynamic effect of these cytokines. Rather, this finding indicated that muscle proteolysis is enhanced by TNF and synergistically augmented by the addition of IL 1. Compatible with these data was the finding that more prolonged infusions of recombinant TNF/cachectin and the combination with IL 1 increased urinary nitrogen excretion. Changes in [14C]leucine dilution in the liver were less pronounced than those in skeletal muscle and consistent with net anabolic effect of TNF on liver protein. We conclude that rats exposed systemically to sublethal doses of TNF respond with increasing muscle and decreasing liver proteolysis, similar to that observed in inflammation and in cancer.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Vidrich A., Khairallah E. A. Compartmentation of free amino acids for protein synthesis in rat liver. Biochem J. 1974 Jun;140(3):539–545. doi: 10.1042/bj1400539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Bessey P. Q., Watters J. M., Aoki T. T., Wilmore D. W. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984 Sep;200(3):264–281. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. Interleukin-1 has independent effects on deoxyribonucleic acid and collagen synthesis in cultures of rat calvariae. Endocrinology. 1986 Jan;118(1):74–81. doi: 10.1210/endo-118-1-74. [DOI] [PubMed] [Google Scholar]
- Cerami A., Ikeda Y., Le Trang N., Hotez P. J., Beutler B. Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol Lett. 1985;11(3-4):173–177. doi: 10.1016/0165-2478(85)90165-8. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1: amino acid sequences, multiple biological activities and comparison with tumor necrosis factor (cachectin). Year Immunol. 1986;2:68–89. [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Goldberg A. L., Kettelhut I. C., Furuno K., Fagan J. M., Baracos V. Activation of protein breakdown and prostaglandin E2 production in rat skeletal muscle in fever is signaled by a macrophage product distinct from interleukin 1 or other known monokines. J Clin Invest. 1988 May;81(5):1378–1383. doi: 10.1172/JCI113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren P. O., Jagenburg R., Karlström L., Pedersen P., Seeman T. Changes of protein metabolism in liver and skeletal muscle following trauma complicated by sepsis. J Trauma. 1984 Mar;24(3):224–228. doi: 10.1097/00005373-198403000-00007. [DOI] [PubMed] [Google Scholar]
- Hasselgren P. O., Talamini M., James J. H., Fischer J. E. Protein metabolism in different types of skeletal muscle during early and late sepsis in rats. Arch Surg. 1986 Aug;121(8):918–923. doi: 10.1001/archsurg.1986.01400080064011. [DOI] [PubMed] [Google Scholar]
- Istfan N. W., Ling P. R., Bistrian B. R., Blackburn G. L. Systemic exchangeability of enteral leucine: relationship to plasma flux. Am J Physiol. 1988 Apr;254(4 Pt 2):R688–R698. doi: 10.1152/ajpregu.1988.254.4.R688. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Goldberg A. L. Tumor necrosis factor can induce fever in rats without activating protein breakdown in muscle or lipolysis in adipose tissue. J Clin Invest. 1988 May;81(5):1384–1389. doi: 10.1172/JCI113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Laughlin M. H., Mohrman S. J., Armstrong R. B. Muscular blood flow distribution patterns in the hindlimb of swimming rats. Am J Physiol. 1984 Mar;246(3 Pt 2):H398–H403. doi: 10.1152/ajpheart.1984.246.3.H398. [DOI] [PubMed] [Google Scholar]
- Ling P. R., Bistrian B. R., Blackburn G. L., Istfan N. Effect of fetal growth on maternal protein metabolism in postabsorptive rat. Am J Physiol. 1987 Mar;252(3 Pt 1):E380–E390. doi: 10.1152/ajpendo.1987.252.3.E380. [DOI] [PubMed] [Google Scholar]
- Malik A. B., Kaplan J. E., Saba T. M. Reference sample method for cardiac output and regional blood flow determinations in the rat. J Appl Physiol. 1976 Mar;40(3):472–475. doi: 10.1152/jappl.1976.40.3.472. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., O'Keefe S. J., Bothe A., Jr, Bistrian B. R., Blackburn G. L. In vivo demonstration of nitrogen-sparing mechanisms for glucose and amino acids in the injured rat. Metabolism. 1980 Feb;29(2):173–180. doi: 10.1016/0026-0495(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Svaninger G., Gelin J., Lundholm K. G. Interleukin 1 and tumor necrosis factor do not regulate protein balance in skeletal muscle. Am J Physiol. 1987 Dec;253(6 Pt 1):C766–C773. doi: 10.1152/ajpcell.1987.253.6.C766. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons P. S., Miles J. M., Gerich J. E., Haymond M. W. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984 Feb;73(2):412–420. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrado J., Moldawer L. L., Bistrian B. R., Dinarello C. A., Blackburn G. L. Effect of ibuprofen on fever and metabolic changes induced by continuous infusion of leukocytic pyrogen (interleukin 1) or endotoxin. Infect Immun. 1983 Dec;42(3):997–1005. doi: 10.1128/iai.42.3.997-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinas G. A., Mandrup-Poulsen T., Mølvig J., Baek L., Bendtzen K., Dinarello C. A., Nerup J. Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of Langerhans. Acta Endocrinol (Copenh) 1986 Dec;113(4):551–558. doi: 10.1530/acta.0.1130551. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Tocco-Bradley R., Moldawer L. L., Jones C. T., Gerson B., Blackburn G. L., Bistrian B. R. The biological activity in vivo of recombinant murine interleukin 1 in the rat. Proc Soc Exp Biol Med. 1986 Jun;182(2):263–271. doi: 10.3181/00379727-182-42338. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrich A., Airhart J., Bruno M. K., Khairallah E. A. Compartmentation of free amino acids for protein biosynthesis. Influence of diurnal changes in hepatic amino acid concentrations of the composition of the precursor pool charging aminoacyl-transfer ribonucleic acid. Biochem J. 1977 Feb 15;162(2):257–266. doi: 10.1042/bj1620257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Gabrilove J. L., Oettgen H. F., Brennan M. F. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg. 1987 Dec;122(12):1396–1400. doi: 10.1001/archsurg.1987.01400240042007. [DOI] [PubMed] [Google Scholar]
- Watters J. M., Bessey P. Q., Dinarello C. A., Wolff S. M., Wilmore D. W. Both inflammatory and endocrine mediators stimulate host responses to sepsis. Arch Surg. 1986 Feb;121(2):179–190. doi: 10.1001/archsurg.1986.01400020065008. [DOI] [PubMed] [Google Scholar]
- Yang R. D., Moldawer L. L., Sakamoto A., Keenan R. A., Matthews D. E., Young V. R., Wannemacher R. W., Jr, Blackburn G. L., Bistrian B. R. Leukocyte endogenous mediator alters protein dynamics in rats. Metabolism. 1983 Jul;32(7):654–660. doi: 10.1016/0026-0495(83)90120-8. [DOI] [PubMed] [Google Scholar]