Abstract
The radiotracer [11C]N-desmethyl-loperamide (dLop) images the in vivo function of P-glycoprotein (P-gp), a transporter that blocks the entry of drugs that are substrates into brain. When P-gp is inhibited, [11C]dLop, a potent opiate agonist, enters and becomes trapped in the brain. This trapping is beneficial from an imaging perspective, because it amplifies the PET signal, essentially by accumulating radioactivity over time. As we previously demonstrated that this trapping was not caused by binding to opiate receptors, we examined whether [11C]dLop, a weak base, is ionically trapped in acidic lysosomes. To test this hypothesis, we measured [3H]dLop accumulation in human cells by using lysosomotropics. Because the in vivo trapping of dLop was seen after P-gp inhibition, we also measured [3H]dLop uptake in P-gp–expressing cells treated with the P-gp inhibitor tariquidar. All lysosomotropics decreased [3H]dLop accumulation by at least 50%. In P-gp–expressing cells, tariquidar (and another P-gp inhibitor) surprisingly decreased [3H]dLop uptake. Consequently, we measured [11C]dLop uptake before and after tariquidar preadministration in lysosome-rich organs of P-gp KO mice and humans. After tariquidar pretreatment in both species, radioactivity uptake in these organs decreased by 35% to 40%. Our results indicate that dLop is trapped in lysosomes and that tariquidar competes with dLop for lysosomal accumulation in vitro and in vivo. Although tariquidar and dLop compete for lysosomal trapping in the periphery, such competition does not occur in brain because tariquidar has negligible entry into brain. In summary, tariquidar and [11C]dLop can be used in combination to selectively measure the function of P-gp at the blood–brain barrier.
Keywords: ATP-binding cassette, efflux
The efflux transporter P-glycoprotein (P-gp; encoded by ABCB1) blocks the entry of various compounds into cells, thereby protecting organs from exposure to potential toxins. At the blood–brain barrier, P-gp prevents the entry of substances from blood into neural tissue (1). Consequently, P-gp function can also impede the delivery of therapeutic drugs to the brain: overexpression of P-gp at the blood–brain barrier may contribute to drug resistance in epilepsy or to decreased drug effectiveness in HIV infection of the brain (2).
The radiotracer [11C]N-desmethyl-loperamide (dLop), used in PET, is a specific substrate for P-gp (3) and can measure the function of P-gp at the blood–brain barrier. Whereas brain uptake of [11C]dLop is minimal in monkeys and humans, its uptake is markedly higher after administration of the P-gp inhibitor tariquidar (4–6). Upon entry into brain, [11C]dLop is trapped and does not wash out from the brain, despite declining plasma concentrations (4, 6). Although dLop is an opiate agonist, its trapping is not a result of high-affinity binding to the opiate receptor, because brain uptake of [11C]dLop cannot be displaced by receptor-saturating doses of an opiate agonist or antagonist (5). This irreversible trapping of [11C]dLop is desirable from an imaging perspective because it amplifies the PET signal, essentially by accumulating radioactivity over time. This situation is similar to the irreversible trapping of [18F]fluorodeoxyglucose, which is phosphorylated by the enzyme glucose-6-kinase and thereby trapped intracellularly, at least for the duration of the PET scan (7). The signal from [18F]fluorodeoxyglucose is amplified in the sense that its ionically charged radiometabolite accumulates intracellularly, i.e., the signal is integrated over time. [11C]dLop is also trapped in brain, but the mechanism of its trapping is unknown.
One way that molecules can become trapped in cells is via weak base sequestration in intracellular acidic organelles, primarily in lysosomes but also in endocytic compartments (8). Weak bases with pKa values* greater than 7.0 can readily diffuse as neutral molecules across membranes into cells (cytosolic pH of approximately 7.0) and then into acidic lysosomes (pH of approximately 5.5), where the basic moiety becomes protonated (10, 11). As increasing amounts of weak base accumulate in the lysosomes, the free proton pool is depleted; consequently, the lysosomal pH increases and sequestration of drug within lysosomes reaches saturation (11, 12). Inhibitors of the vacuolar type H+-ATPase (v-ATPase) can also increase lysosomal pH (13).
Given that dLop has an experimentally determined pKa of 7.3 (5), we hypothesized that the trapping of [11C]dLop in the brain occurs by passive diffusion of this weak base into acidic organelles, primarily lysosomes, followed by protonation within the organelle. To test this hypothesis, we measured the accumulation of [3H]dLop in the presence of other weak bases in human cell lines and visualized the competition among these bases by using confocal microscopy. To confirm that the in vitro results occurred in vivo, we measured the uptake of [11C]dLop before and after injection of another weak base in lysosome-rich organs of P-gp KO mice and healthy humans.
Results
dLop Is Trapped in Lysosomes in Vitro.
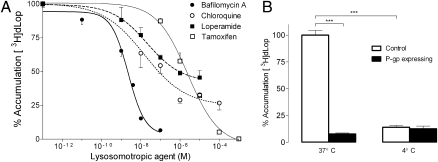
Pretreatment of KB-3–1 (human adenocarcinoma) cells with four compounds that raise lysosomal pH decreased [3H]dLop accumulation in a dose-dependent manner (Fig. 1A). Bafilomycin A (a v-ATPase inhibitor) (13) and tamoxifen (pKa of 8.5) (12) fully blocked [3H]dLop accumulation, whereas chloroquine (pKas of 8.4 and 10.8) (13) and loperamide (pKa of 7.3) (5) blocked as much as 50% of control levels of [3H]dLop accumulation. Bafilomycin A [IC50, 2.27 × 10−9 M; 95% confidence interval (CI), 1.66–3.11 × 10−9 M] was more potent at preventing [3H]dLop accumulation than tamoxifen (IC50, 2.47 × 10−6 M; 95% CI, 1.65–3.71 × 10−6 M), chloroquine (IC50, 1.53 × 10−8 M; 95% CI, 0.44–5.34 × 10−8 M), or loperamide (IC50, 2.07 × 10−8 M; 95% CI, 0.66–6.50 × 10−8 M). In contrast to these four lysosomotropic agents, tetraethylammonium chloride (TEA-HCl; negative control), which is not a weak base, did not alter [3H]dLop accumulation (Fig. S1).
Fig. 1.
The P-gp substrate dLop is trapped in lysosomes. (A) Reduction of [3H]dLop (1 nM) accumulation in control KB-3–1 cells by compounds that increase lysosomal pH (i.e., lysosomotropic agents). Data represent mean ± SD (n = 3 observations); error bars are unidirectional for figure clarity. (B) The effect of temperature on the accumulation of [3H]dLop (1 nM) in control (KB-3-1) and P-gp–expressing (KB-8-5-11) cells. Accumulation in control (□) cells is much lower at 4 °C than at 37 °C, and accumulation in P-gp–expressing (■) cells is not affected by temperature (***P < 0.001). Data represent mean ± SD (n = 3 observations).
To further support these results, we compared the amount of [3H]dLop in KB-3–1 (control) cells versus that in KB-8–5–11 (P-gp–expressing) cells at 37 °C and 4 °C because low temperatures abrogate ATPase activity (14) and thus should diminish cellular accumulation of a weak base in control cells. In KB-3–1 cells, accumulation was 86% lower (P < 0.001) at 4 °C than at 37 °C. In KB-8–5–11 cells, which accumulated 90% less [3H]dLop than KB-3–1 cells, as previously shown (3), the already low accumulation did not differ (P = 0.56) between temperatures (Fig. 1B).
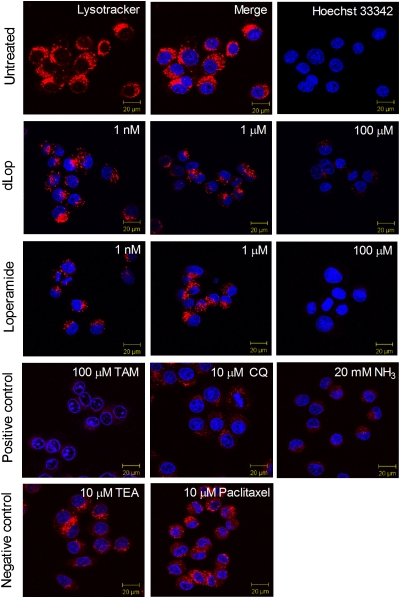
We also found that increasing concentrations of dLop decreased the lysosomal accumulation of the fluorescent weak base LysoTracker Red DND-99 (Fig. 2). KB-3–1 cells showed punctate staining of LysoTracker Red in lysosomes (red, Fig. 2) located in the cytoplasmic space and of Hoechst 33342 in nuclei (blue in first row, Fig. 2). When treated with 100 μM dLop or 100 μM loperamide, cells showed substantial loss of LysoTracker staining in lysosomes (second and third rows, Fig. 2). A similar decrease was observed in cells that were treated with the weak bases tamoxifen (100 μM), chloroquine (10 μM), and ammonia (20 mM; pKa 9.2; fourth row, Fig. 2) (13). In contrast, when treated with TEA-HCl (10 μM) and paclitaxel (10 μM), neither of which are weak bases, cells showed no loss of LysoTracker uptake (fifth row, Fig. 2).
Fig. 2.
Reduction in accumulation of a fluorescent weak base from lysosomes by increasing concentrations of dLop and loperamide as assessed by confocal microscopy. Images are merged to show the simultaneous staining of the lysosomes with LysoTracker Red DND-99 (10 nM) and of the nucleus with Hoechst 33342 (8 μM; row 1). Weak bases dLop, loperamide, tamoxifen (TAM), chloroquine (CQ), and ammonia (NH3) block the accumulation of the fluorescent base (rows 2–4); the cationic compound TEA-HCl and the nonbase paclitaxel do not (row 5).
P-gp Inhibitors Tariquidar and DCPQ Displace Lysosomal Accumulation of [3H]dLop in Vitro.
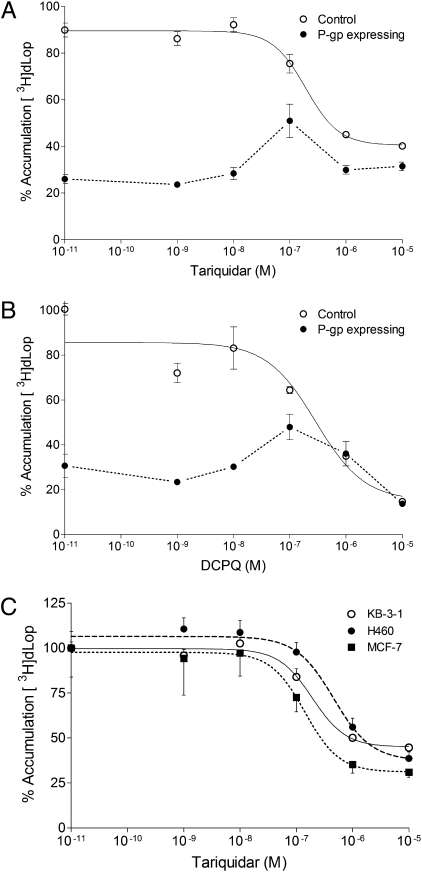
We found that the P-gp inhibitors tariquidar (Fig. 3A) and (2R)-anti-5-{3-[4-(10,11-dichloromethanodibenzo-suber-5-yl)piperazin-1-yl]-2-hydroxypropoxy}quinoline trihydrochloride (DCPQ) (identified as 14b in ref. 15) (Fig. 3B) had two effects on [3H]dLop accumulation in control and P-gp–expressing cells. The first effect was expected for a P-gp inhibitor: at concentrations lower than 100 nM, neither inhibitor affected [3H]dLop accumulation in control cells and both increased [3H]dLop accumulation in P-gp–expressing cells. However, the second effect was unexpected: at concentrations greater than 100 nM, tariquidar and DCPQ both decreased [3H]dLop accumulation in control and P-gp–expressing cells. This unexpected effect was not specific to KB cells, as tariquidar also decreased the accumulation of [3H]dLop in two other cell lines derived from different tumor backgrounds (MCF7 human breast cancer cells and H460 human large-cell lung cancer cells; Fig. 3C). As another control, we measured the accumulation of the nonbase P-gp substrate [3H]paclitaxel in the presence of P-gp inhibitors. Both tariquidar and DCPQ acted only as inhibitors of P-gp for [3H]paclitaxel. That is, at concentrations as high as 10 μM, tariquidar and DCPQ increased accumulation of [3H]paclitaxel in P-gp–expressing cells but had no effect on accumulation in control cells (Fig. S2).
Fig. 3.
Increasing concentrations of two P-gp inhibitors decrease the accumulation of [3H]dLop in various cell lines. (A) Tariquidar and (B) DCPQ have two effects on [3H]dLop accumulation in control KB-3–1 (○) and P-gp–expressing KB-8–5–11 (●) cells. First, at concentrations lower than 100 nM, both inhibitors increase the accumulation of [3H]dLop but have no effect in control cells. Second, at concentrations greater than 100 nM, both inhibitors decrease the accumulation of [3H]dLop in both cell types. (C) Tariquidar reduces [3H]dLop accumulation in two control cell lines [H460 (●) and MCF-7 (■)] derived from different tumors than KB-3–1 cells. Data represent mean ± SD (n = 3 observations).
P-gp Inhibitors Tariquidar and DCPQ Are Lysosomally Trapped.
Given that tariquidar and DCPQ decreased the accumulation of [3H]dLop, we used three methods to test our hypothesis that tariquidar and DCPQ are lysosomally trapped. First, in cellular radioaccumulation assays, preincubating cells with increasing concentrations of tamoxifen (IC50, 1.74 × 10−4 M; 95% CI, 5.32 × 10−7 to 5.67 × 10−2 M) and chloroquine (IC50, 2.35 × 10−6 M; 95% CI, 0.028–1.96 × 10−5 M) reduced [3H]tariquidar accumulation in KB-3–1 cells (Fig. 4A). Second, by using confocal microscopy, we found that cells treated with at least 500 nM tariquidar or those treated with at least 1 μM DCPQ showed substantially lower LysoTracker staining in lysosomes (Fig. 4B) than the positive controls. Third, analysis of cellular morphology with flow cytometry showed a dose-dependent increase in the granularity (measured by side light scatter) of KB-3–1 cells treated with tariquidar (Fig. S3), consistent with previous reports of increases in cellular granularity caused by the swelling of lysosomes (16).
Fig. 4.
Weak base competition between two P-gp inhibitors and compounds that increase lysosomal pH in KB-3–1 cells. (A) Two weak bases, tamoxifen and chloroquine, decrease the cellular accumulation of [3H]tariquidar (1 nM) from cells. Data represent mean ± SD (n = 3 observations). (B) Reduction in accumulation of the fluorescent weak base LysoTracker Red in cellular lysosomes by increasing concentrations of tariquidar and DCPQ assessed by confocal microscopy. Fluorescent staining shows the lysosomes (red, LysoTracker Red 10 nM) and the nuclei (blue, Hoechst 33342 8 μM).
Weak Bases Decrease Accumulation of [11C]dLop in Lysosome-Rich Organs of Mice.
Preinjection of weak bases before injection of [11C]dLop in mice decreased accumulation of radioactivity in the kidneys and the spleen, two lysosome-rich organs (Table 1). To examine the effects of tariquidar other than as an inhibitor of P-gp, we used P-gp KO mice. Expressed relative to the radioactivity measured over a period of 60 min in the kidneys of saline solution-treated mice, the uptake of [11C]dLop decreased by 35% (P < 0.05) in tariquidar-treated mice and by 35% (P < 0.05) in chloroquine-treated mice, but not in paclitaxel-treated mice. Similar results were obtained for radioactivity measurements in the spleen: uptake decreased by 40% (P < 0.05) in tariquidar-treated mice. Although the uptake in chloroquine-treated mice decreased by 20%, the change was not significant, most likely because the low resolution of the PET images made it difficult to discern the spleen from the kidney. Uptake did not decrease significantly in paclitaxel-treated mice. Finally, the radioactivity measured in muscle (i.e., negative control) did not show any significant differences in uptake among any of the treatment groups (Table 1).
Table 1.
Uptake of radioactivity measured over 60 min in organs of P-gp KO mice after pretreatment with four drugs and injection of [11C]dLop
| Organ uptake, SUV · min |
|||||||
| Organ | Saline | Tariquidar | Change, % | Chloroquine | Change, % | Paclitaxel | Change, % |
| Brain | 20 ± 5 | 18 ± 5 | −10 | 24 ± 7 | 16 | 20 ± 3 | −1 |
| Kidney | 184 ± 22 | 119 ± 25 | −35* | 119 ± 5 | −35* | 202 ± 27 | 10 |
| Spleen | 144 ± 24 | 85 ± 17 | −40* | 116 ± 13 | −20 | 138 ± 27 | −4 |
| Muscle | 10 ± 3 | 14 ± 3 | 41 | 15 ± 6 | 56 | 15 ± 4 | 56 |
Mice were pretreated with drugs 30 min before injection of [11C]dLop. Data represent mean ± SD; n = 3 mice per treatment group. Percent change represents difference in means between treatment and saline solution-treated groups.
*P < 0.05 using one-way analysis of variance followed by t test.
Lysosomal competition in the brains of P-gp KO mice was not detected with the use of PET, as brain radioactivity did not significantly change in any treatment condition (Table 1). However, we confirmed that competition occurs in isolated neurons in which the blood–brain barrier is not functional (Fig. S4).
Tariquidar Decreases Accumulation of [11C]dLop in Lysosome-Rich Organs of Humans.
Preinjection of tariquidar (2 mg/kg, i.v.) before [11C]dLop injection decreased radioactivity accumulation measured from 5 to 120 min in the kidneys and spleen of humans (Table 2) compared with that measured at baseline conditions. In the kidneys, radioactivity measured over a period of 60 min (4) decreased by 41% (P < 0.05), and in the spleen, it decreased by 38% (P < 0.05; Fig. S5). Although tariquidar behaves as a lysosomotropic agent in these organs, it still acts as an inhibitor of P-gp, as demonstrated by the significant reduction of radioactivity excretion into the bladder and gallbladder (Table 2).
Table 2.
Uptake of radioactivity measured from 5 to 120 min in organs of healthy humans after pretreatment with tariquidar and injection of [11C]dLop
| Uptake, SUV · min | |||
| Organ | Baseline | Tariquidar 2 mg/kg | Change, % |
| Brain | 13 ± 3 | 15 ± 2 | 21 |
| Lung | 208 ± 77 | 167 ± 51 | −17 |
| Spleen | 369 ± 89 | 217 ± 37 | −38† |
| Kidney | 481 ± 105 | 280 ± 68 | −41† |
| Liver | 446 ± 123 | 541 ± 141 | 23 |
| Gallbladder* | 947 ± 256 | 254 ± 93 | −71† |
| Bladder* | 428 ± 256 | 146 ± 71 | −66† |
The area under the time-activity curve (organ uptake) was measured starting 5 min after injection of [11C]dLop. Data represent mean ± SD; n = 6 subjects.
*n = 5 because organ was not visualized in one subject.
†P < 0.05 using paired t test.
Discussion
Lysosomal Trapping of a P-gp Substrate.
Our in vitro results support the hypothesis that the trapping of dLop in tissue is a result of accumulation of dLop as a protonated weak base within acidic organelles, primarily lysosomes. We demonstrated the mechanism of trapping in three ways. First, we found that preincubating cells with three weak bases or an inhibitor of the v-ATPase decreased the cellular accumulation of [3H]dLop. Second, accumulation in KB-3–1 cells was significantly lower at 4 °C than at 37 °C, which suggests that energy-dependent acidification of the lysosome is necessary for dLop sequestration. Third, we found that dLop displaced the lysosomal dye LysoTracker Red DND-99 from lysosomes. Our findings are consistent with previous observations of weak-base P-gp substrates such as doxorubicin (17), daunomycin (18), and vinblastine (19) being trapped in lysosomes.
These results preclude the possibility of dLop accumulation in mitochondria or cellular accumulation through uptake transporters. The net negative membrane potential of the mitochondria typically drives the accumulation of permanently cationic molecules (10). Given that the cation TEA-H+ did not compete for dLop accumulation, it is unlikely that dLop accumulates in the mitochondria or that a cationic uptake transporter is involved (20).
Lysosomal Trapping of Two P-gp Inhibitors.
An unexpected finding was that the P-gp inhibitors tariquidar and DCPQ are also trapped in lysosomes. This behavior was demonstrated in vitro in four ways. First, we found that preblocking with the inhibitors (>100 nM) decreased [3H]dLop accumulation. Second, preincubating cells with two weak bases reduced [3H]tariquidar accumulation, suggesting that tariquidar accumulates within lysosomes. Third, we found that treatment of cells with more than 500 nM tariquidar and 10 μM DCPQ prevented the accumulation of the fluorescent weak base LysoTracker Red DND-99 in lysosomes. Finally, KB-3–1 cells treated with tariquidar showed a dose-dependent increase in granularity (i.e., swelling of intracellular vesicles), providing further evidence of tariquidar accumulating within the lysosomes (16). Although the lysosomal trapping of tariquidar and DCPQ was not previously known, other clinically used P-gp inhibitors, such as cyclosporin A (21) and verapamil (22), have been shown to interfere with the lysosomal sequestration of drugs. We expect that other P-gp inhibitors, such as elacridar and zosuquidar (an analogue of DCPQ), would also be lysosomotropic because they possess tertiary amines that are weakly basic.
By using PET imaging, we also demonstrated that weak base competition occurs in an acute time frame (≤120 min) in lysosome-rich organs of mice and humans. In P-gp KO mice, pretreatment with tariquidar (32 mg/kg i.v.) decreased the uptake of [11C]dLop in the kidneys and the spleen. In humans, tariquidar (2 mg/kg i.v.) had a similar effect on the uptake of [11C]dLop in the kidneys and the spleen. This decrease was not likely caused by a lower input function of [11C]dLop, because a previous study that used higher doses of tariquidar (4 mg/kg and 6 mg/kg i.v.) did not show a change in plasma concentration or protein binding of the parent radiotracer (4). Our findings correlate with those of Ndolo and colleagues (23), who demonstrated that lysosomal pH changes occur in mice when weak bases are administered chronically (i.e., for weeks) (23). However, we show that lysosomal competition between two weak bases can occur in vivo in an acute time frame, a finding that, to our knowledge, has not been demonstrated previously.
In Vivo Interactions of P-gp Substrate and Inhibitor if both Are Lysosomotropic.
How would the competing phenomena of efflux via transport and lysosomal trapping affect in vivo studies? We demonstrated that tariquidar can act as a lysosomotropic agent in mice and humans because it blocks the accumulation of [11C]dLop in peripheral organs that are rich in lysosomes. Although this interaction complicates the interpretation of results with [11C]dLop in the periphery, other properties of tariquidar allow it to be used with [11C]dLop to selectively measure the function of P-gp at the blood–brain barrier. We recently demonstrated that tariquidar is not only an inhibitor of P-gp but also a substrate for another efflux transporter, breast cancer resistance protein (BCRP), thus preventing the entry of tariquidar into brain (24); others have shown that [11C]tariquidar does not enter the rodent brain (25, 26). Thus, tariquidar cannot enter the brain and confound the PET signal by blocking the lysosomal trapping of [11C]dLop. Additionally, high concentrations of a substrate can effectively inhibit a transporter. However, even if tariquidar achieves an adequate concentration in vivo to be an inhibitor of BCRP, this property would not affect the signal from [11C]dLop, which is a selective substrate for human P-gp (3). In conclusion, the three pharmacological properties of tariquidar (inhibitor of P-gp, substrate for BCRP, and lysosomotropic agent) allow it to be used with [11C]dLop to selectively measure the function of P-gp at the blood–brain barrier.
Although the trapping property of a substrate radiotracer, such as [11C]dLop, is beneficial for measuring P-gp function in the brain, the trapping property of an inhibitor radiotracer intended to measure P-gp density, such as [11C]tariquidar, is not desirable. Provided the inhibitor penetrates the blood–brain barrier, we would expect that the competing phenomena of binding to transporter and trapping would limit the use of weak base inhibitors. Although [11C]tariquidar does not enter brains of WT mice, its uptake is reported to be high, with no evidence of washout in brains of triple P-gp and BCRP KO [mdr1a(−/−), mdr1b(−/−), bcrp(−/−)] mice (25, 26). In a situation in which a weak base [11C]inhibitor could penetrate the blood–brain barrier under physiological conditions, it would become trapped, resulting in a higher signal. As such, a radiolabeled inhibitor that is also a weak base may overestimate P-gp density in the brain.
The combined use of lysosomotropic substrates and inhibitors of P-gp for the treatment of clinical disorders may be problematic. For example, because P-gp has been shown to play a role in multidrug-resistant cancer (1), treatment regimens for these cancers have often included the use of a weak base P-gp inhibitor (elacridar, tariquidar, or zosuquidar) coadministered as an adjuvant with a weak base chemotherapeutic agent (27, 28). These chemotherapeutic agents, such as doxorubicin, daunorubicin, and vinblastine, are P-gp substrates known to be lysosomally trapped (17–19). Our in vivo findings in this study indicate that just 2 mg/kg (i.v.) tariquidar is sufficient to decrease the accumulation of tracer doses of [11C]dLop in peripheral organs by approximately 40%. Thus, lysosomal competition between the P-gp inhibitor and P-gp substrate/chemotherapeutic may have altered pharmacokinetics and diminished cellular uptake of cytotoxin, thus contributing to the poor results of these trials. Our study highlights the importance of assessing in vivo lysosomal drug–drug interactions.
Comparison of [11C]dLop with Other PET Radiotracers Used to Measure P-Gp Function.
How does [11C]dLop compare with other PET radiotracers, such as [11C]verapamil, [94mTc]sestamibi, and [68Ga]ethylenediamine-N-N′-bis[propyl(2-hydroxy-(R)-benzylimino)] (ENBPI), that have been developed for imaging of P-gp function? Our results from previous studies strongly suggest that [11C]dLop is a selective substrate for human P-gp (3) and that it generates minimal radiometabolites that enter the brain (4, 6). In contrast, [11C]verapamil undergoes extensive metabolism, likely generating radiometabolites that are themselves substrates for P-gp (29). [94mTc]Sestamibi and [68Ga]ENBPI are two metal complex radiotracers that carry a permanent positive charge and become trapped in mitochondria, thereby amplifying the signal (30). Consequently, these two tracers have potential for imaging P-gp function, especially in the periphery and in multidrug-resistant cancer, because they would not compete for lysosomal accumulation with a weak base P-gp inhibitor. However, both these tracers also show substrate activity in vitro for human multidrug resistance protein 1 (31, 32), and this cross-reactivity may confound the selective quantification of P-gp at the human blood–brain barrier.
[11C]dLop is a promising radiotracer to selectively image the function of P-gp at the human blood–brain barrier, but its application must appropriately control for its being an extraordinarily avid substrate for P-gp (33). Based on imaging studies in monkeys given high doses of tariquidar, we estimated that the single pass extraction of [11C]dLop into brain is greater than 40% (6). That is, during the brief (∼2 s) passage through the capillary bed, more than 40% of [11C]dLop enters brain when P-gp is inhibited. Such rapid uptake makes it dependent on regional blood flow. We have successfully corrected brain uptake of [11C]dLop for regional blood flow measured with [15O]H2O and have shown, for example, that the function of P-gp appears fairly uniformly distributed in monkey brain, at least at the resolution of PET imaging (6). The high single-pass extraction of [11C]dLop also means that P-gp, when not inhibited, acts rapidly and with high capacity to block entry of this substrate radioligand. That is, at baseline conditions, almost no [11C]dLop enters the brain. Because of this “floor effect,” the uptake of [11C]dLop is not sensitive to pathologic conditions of increased function of P-gp. This property is a deficiency of [11C]dLop, and we are currently examining the feasibility in human subjects to detect increased P-gp function by injecting both [11C]dLop and tariquidar. In comparison with normal areas of brain or with control subjects, pathological conditions of increased P-gp function (e.g., drug-resistant epilepsy) would be predicted to have a blunted effect of tariquidar to increase the brain uptake of [11C]dLop. However, for brain disorders in which P-gp function may be reduced, such as Alzheimer disease (34), [11C]dLop would be advantageous because it would enter the brain and have a strong signal, as it is irreversibly trapped in lysosomes.
In conclusion, to understand the mechanism whereby the selective P-gp substrate [11C]dLop is trapped in the brain, we used a broad translational approach that included in vitro studies in human cells as well as in vivo imaging of transgenic mice and healthy human subjects. Our data strongly support the hypothesis that both [11C]dLop and the P-gp inhibitor tariquidar are ionically trapped in acidic lysosomes. The competitive interaction of [11C]dLop and tariquidar in the lysosome is problematic in the periphery but not in the brain, because tariquidar has negligible entry into the brain. Thus, the use of [11C]dLop by itself or in combination with tariquidar provides a selective measure of the function of P-gp at the blood–brain barrier.
Materials and Methods
Details on the preparation of 3H- and 11C-labeled compounds, drug formulations, cell culture, assays that used [3H]dLop and [3H]tariquidar, confocal microscopy, in vivo imaging, and image analysis are provided in SI Materials and Methods.
Measurement of Radioactivity in Organs from PET Studies.
Reconstructed images from PET studies in animals and humans were used to measure the concentration of radioactivity (i.e., decay-corrected till injection time) in each organ. We measured radioactivity for 60 min in brain, kidney, spleen, and muscle of mice and from 5 to 120 min (4) in brain, lung, kidney, spleen, gallbladder, and urinary bladder of humans. The concentration of radioactivity was then expressed as the standardized uptake value (SUV), which normalizes for injected activity and body weight:
Time–activity curves were created for each organ by plotting SUV versus time, and the area under the time–activity curve (i.e., SUV · min) of each organ was calculated by using the trapezoidal method of integration.
Statistical Analysis.
For in vitro studies, concentration–response curves were fitted and analyzed by nonlinear regression by using a log-sigmoidal model with variable slope (Prism 5.0, GraphPad Software). Values of drug potency are reported as the concentration at which the drug decreased the accumulation of the radioactive compound by 50% (i.e., IC50), along with the 95% CI of the IC50. Statistical significance was evaluated for radioaccumulation assays by the Student t test (unpaired, two-tailed, α value of 0.05) after the data were tested for homogeneity of variance.
For mouse imaging studies, differences in mean area under the curve (SUV · min) were compared by using one-way analysis of variance followed by the Bonferroni posttest for multiple comparisons (α value of 0.05). For human studies, differences in the area under the time–activity curve (SUV · min) of each organ were compared between the baseline and tariquidar-treated condition by paired t test using SPSS Statistics software, version 17.0. Correction for multiple comparisons was performed by using the false discovery rate (35), with the threshold set at 0.05.
Supplementary Material
Acknowledgments
We thank Ms. Wenjie Xiao and Dr. Ellen Sidransky for assistance with hippocampal neuron cell culture, Eli Lilly and Co. for providing DCPQ, and Mr. George Leiman for editorial assistance. This research was supported by the Intramural Research Programs of the National Institute of Mental Health Projects Z01-MH-002795-07 and Z01-MH-002852-07 and National Cancer Institute Project Z01-BC-005598.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014641108/-/DCSupplemental.
*Although pKa is a measure of the acid strength of the conjugate Bronsted acid, we report the strength of bases by using pKa because this terminology has become common (9).
References
- 1.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Kannan P, et al. N-desmethyl-loperamide is selective for P-glycoprotein among three ATP-binding cassette transporters at the blood-brain barrier. Drug Metab Dispos. 2010;38:917–922. doi: 10.1124/dmd.109.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreisl WC, et al. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med. 2010;51:559–566. doi: 10.2967/jnumed.109.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarova N, et al. Synthesis and evaluation of [N-methyl-11C]N-desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem. 2008;51:6034–6043. doi: 10.1021/jm800510m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liow JS, et al. P-glycoprotein function at the blood-brain barrier imaged using 11C-N-desmethyl-loperamide in monkeys. J Nucl Med. 2009;50:108–115. doi: 10.2967/jnumed.108.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castell F, Cook GJ. Quantitative techniques in 18FDG PET scanning in oncology. Br J Cancer. 2008;98:1597–1601. doi: 10.1038/sj.bjc.6604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Duve C, et al. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 9.Sykes P, editor. A Guidebook to Mechanism in Organic Chemistry. 6th ed. London: Longman; 1988. [Google Scholar]
- 10.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J Pharm Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 11.MacIntyre AC, Cutler DJ. The potential role of lysosomes in tissue distribution of weak bases. Biopharm Drug Dispos. 1988;9:513–526. doi: 10.1002/bod.2510090602. [DOI] [PubMed] [Google Scholar]
- 12.Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agostinelli E, Seiler N. Lysosomotropic compounds and spermine enzymatic oxidation products in cancer therapy (review) Int J Oncol. 2007;31:473–484. [PubMed] [Google Scholar]
- 14.Sauna ZE, Nandigama K, Ambudkar SV. Exploiting reaction intermediates of the ATPase reaction to elucidate the mechanism of transport by P-glycoprotein (ABCB1) J Biol Chem. 2006;281:26501–26511. doi: 10.1074/jbc.M601917200. [DOI] [PubMed] [Google Scholar]
- 15.Pfister JR, et al. Methanodibenzosuberylpiperazines as potent multidrug resistance reversal agents. Bioorg Med Chem Lett. 1995;5:2473–2476. [Google Scholar]
- 16.Barranco WT, Eckhert CD. Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br J Cancer. 2006;94:884–890. doi: 10.1038/sj.bjc.6603009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler M, Grabski S, Hoff E, Simon SM. Defective pH regulation of acidic compartments in human breast cancer cells (MCF-7) is normalized in adriamycin-resistant cells (MCF-7adr) Biochemistry. 1996;35:2811–2817. doi: 10.1021/bi952234e. [DOI] [PubMed] [Google Scholar]
- 18.Willingham MC, Cornwell MM, Cardarelli CO, Gottesman MM, Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986;46:5941–5946. [PubMed] [Google Scholar]
- 19.Moriyama Y, Manabe T, Yoshimori T, Tashiro Y, Futai M. ATP-dependent uptake of anti-neoplastic agents by acidic organelles. J Biochem. 1994;115:213–218. doi: 10.1093/oxfordjournals.jbchem.a124320. [DOI] [PubMed] [Google Scholar]
- 20.Okabe M, et al. Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther. 2008;7:3081–3091. doi: 10.1158/1535-7163.MCT-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koponen M, Grieder A, Hauser R, Loor F. Interference of cyclosporin with lymphocyte proliferation: Effects on mitochondria and lysosomes of cyclosporin-sensitive or -resistant cell clones. Cell Immunol. 1985;93:486–496. doi: 10.1016/0008-8749(85)90153-4. [DOI] [PubMed] [Google Scholar]
- 22.Millot C, Millot JM, Morjani H, Desplaces A, Manfait M. Characterization of acidic vesicles in multidrug-resistant and sensitive cancer cells by acridine orange staining and confocal microspectrofluorometry. J Histochem Cytochem. 1997;45:1255–1264. doi: 10.1177/002215549704500909. [DOI] [PubMed] [Google Scholar]
- 23.Ndolo RA, Forrest ML, Krise JP. The role of lysosomes in limiting drug toxicity in mice. J Pharmacol Exp Ther. 2010;333:120–128. doi: 10.1124/jpet.109.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kannan P, et al. The “specific” P-glycoprotein inhibitor tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2) ACS Chem Neurosci. 2010 doi: 10.1021/cn100078a. 10.1021/cn100078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer F, et al. Synthesis and in vivo evaluation of [11C]tariquidar, a positron emission tomography radiotracer based on a third-generation P-glycoprotein inhibitor. Bioorg Med Chem. 2010;18:5489–5497. doi: 10.1016/j.bmc.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamura K, et al. Synthesis and evaluation of [11C]XR9576 to assess the function of drug efflux transporters using PET. Ann Nucl Med. 2010;24:403–412. doi: 10.1007/s12149-010-0373-y. [DOI] [PubMed] [Google Scholar]
- 27.Fox E, Bates SE. Tariquidar (XR9576): A P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447–459. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- 28.Kannan P, et al. Imaging the function of P-glycoprotein with radiotracers: Pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86:368–377. doi: 10.1038/clpt.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikoma Y, et al. Quantitative analysis of 11C-verapamil transfer at the human blood-brain barrier for evaluation of P-glycoprotein function. J Nucl Med. 2006;47:1531–1537. [PubMed] [Google Scholar]
- 30.Piwnica-Worms D, Sharma V. Probing multidrug resistance P-glycoprotein transporter activity with SPECT radiopharmaceuticals. Curr Top Med Chem. 2010;10:1834–1845. doi: 10.2174/156802610792928086. [DOI] [PubMed] [Google Scholar]
- 31.Hendrikse NH, et al. 99mTc-sestamibi is a substrate for P-glycoprotein and the multidrug resistance-associated protein. Br J Cancer. 1998;77:353–358. doi: 10.1038/bjc.1998.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma V, Prior JL, Belinsky MG, Kruh GD, Piwnica-Worms D. Characterization of a 67Ga/68Ga radiopharmaceutical for SPECT and PET of MDR1 P-glycoprotein transport activity in vivo: Validation in multidrug-resistant tumors and at the blood-brain barrier. J Nucl Med. 2005;46:354–364. [PubMed] [Google Scholar]
- 33.Zoghbi SS, et al. 11C-loperamide and its N-desmethyl radiometabolite are avid substrates for brain permeability-glycoprotein efflux. J Nucl Med. 2008;49:649–656. doi: 10.2967/jnumed.107.047308. [DOI] [PubMed] [Google Scholar]
- 34.Vogelgesang S, et al. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.