Abstract
Regulation of protein function via cracking, or local unfolding and refolding of substructures, is becoming a widely recognized mechanism of functional control. Oftentimes, cracking events are localized to secondary and tertiary structure interactions between domains that control the optimal position for catalysis and/or the formation of protein complexes. Small changes in free energy associated with ligand binding, phosphorylation, etc., can tip the balance and provide a regulatory functional switch. However, understanding the factors controlling function in single-domain proteins is still a significant challenge to structural biologists. We investigated the functional landscape of a single-domain plant-type ferredoxin protein and the effect of a distal loop on the electron-transfer center. We find the global stability and structure are minimally perturbed with mutation, whereas the functional properties are altered. Specifically, truncating the L1,2 loop does not lead to large-scale changes in the structure, determined via X-ray crystallography. Further, the overall thermal stability of the protein is only marginally perturbed by the mutation. However, even though the mutation is distal to the iron–sulfur cluster (∼20 Å), it leads to a significant change in the redox potential of the iron–sulfur cluster (57 mV). Structure-based all-atom simulations indicate correlated dynamical changes between the surface-exposed loop and the iron–sulfur cluster-binding region. Our results suggest intrinsic communication channels within the ferredoxin fold, composed of many short-range interactions, lead to the propagation of long-range signals. Accordingly, protein interface interactions that involve L1,2 could potentially signal functional changes in distal regions, similar to what is observed in other allosteric systems.
Keywords: electron transfer, functional energy landscape, iron–sulfur proteins, protein folding
Over the last several decades, our understanding of protein function has evolved from a rather static perspective, where signaling and function have been understood through surface complementarity arguments, such as the “lock and key” paradigm (1, 2), to a more dynamic view where protein conformational fluctuations are inextricably linked to function (3). As our understanding of protein dynamics expands, we are revealing many mechanisms by which proteins exploit conformational fluctuations to perform cellular function. In multidomain proteins, relative repositioning of domains is often linked to their levels of activity. For example, large-scale domain rearrangements in the four-domain Src kinase (4) and C-terminal Src kinase (5, 6) lead to these proteins being in so-called “on” or “off” states, and functional regulation may be obtained by adjusting the balance between these conformations (7). In proteins such as adenylate kinase, domain rearrangements can be rate limiting during each round of catalysis (8), where the enzyme cycles between ligand-competent and ligand-release conformations. Because the kinetics of these transitions control the activity level of the protein, substantial theoretical (9–13) and experimental (14, 15) work has aimed at revealing the details of the functionally relevant transition-state ensembles. These investigations have shown that rearrangements may involve a degree of cracking, or localized unfolding and refolding, which serves to reduce the associated free-energy barriers (16, 17). By modulating the balance of these order–disorder transitions that control domain–domain interactions/orientations, cells may tune the functional dynamics. Despite these conceptual advances in understanding enzyme regulation, these principles may not be applicable to single-domain proteins if they do not exhibit large-scale structural rearrangements. In those cases, alternative modes of functional control are likely employed by the cell.
In the present study, we investigate the relationship between structure, dynamics, and function for a single-domain plant-type ferredoxin (Fdx) protein. This Fdx folding motif is among the most abundant in nature, and it has been well studied previously biochemically (18–23), making Fdx an excellent candidate for quantitative, biophysical characterization. Moreover, the plant-type Fdx folding motif has been found in numerous redox proteins and enzymes, as well as in functionally unrelated proteins that do not contain the [2Fe-2S] cluster, such as ubiquitin and the immunoglobulin-binding domain of protein G (24, 25). Circular permutation of the secondary structure units helped identify previously unrecognized homologs, such as the mercury transporter protein MerP (26). This versatility suggests that a fundamental understanding of the fold’s dynamical properties may facilitate the design of proteins with tightly regulated, and unique, functional roles (27). The development of “modular” proteins that can be artificially assembled into large functional complexes is also at the heart of modern synthetic biology (28). When complexed with iron–sulfur clusters, Fdx proteins possess electron transport properties, which suggests they could be a component in artificial photosynthetic devices and other biologically motivated molecular “circuitry.”
The potential role of iron–sulfur (FeS) proteins in synthetic biology is inspired by their role inside the cell. These proteins are key players in the three life-essential processes: photosynthesis, nitrogen fixation, and respiration (29, 30). The plant-type Fdx proteins carry a single surface-exposed [2Fe-2S] iron–sulfur cluster (ISC) (19), and homologous proteins are found in all organisms, e.g., adrenodoxin in human mitochondria (20). The oxidation-reduction potentials (Em) of these Fdx proteins range from -325 mV to around -450 mV (21), making Fdx one of the strongest soluble reducing agents in nature (22). The plant-type Fdx functions primarily as the electron acceptor of photosystem I, and upon reduction Fdx functions as an electron donor in a myriad of key metabolic pathways (e.g., NADP+ reduction, carbon assimilation, nitrite and sulfite reduction, glutamate synthesis, and thioredoxin reduction, etc.) (22, 23, 31–35).
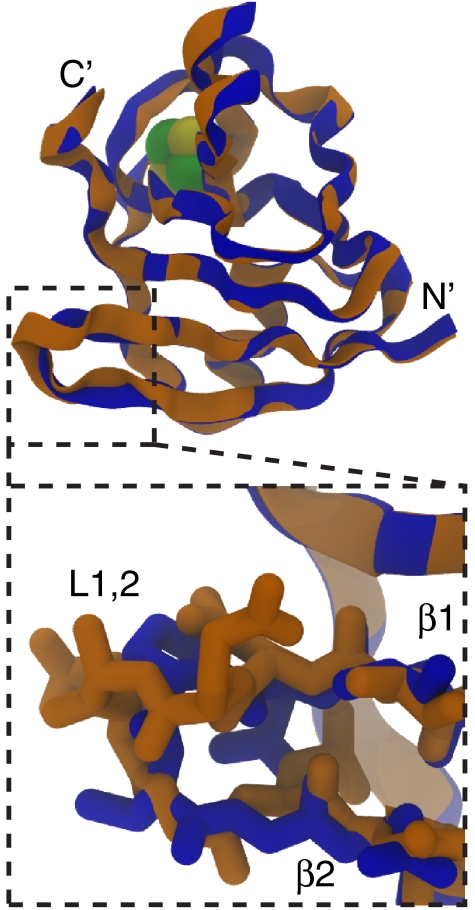
The Fdx fold (22, 23, 31, 36), also termed the β-grasp, or UB roll, is found throughout the cell. The minimum core elements consist of a four-stranded mixed β-sheet with an α-helix packed across one face. Loop insertions and additional strands/helices may be incorporated into this fold for functional purposes. A ribbon diagram of the tertiary fold of Mastigocladus laminosus Fdx (mFdx), is shown in Fig. 1 (19, 37).
Fig. 1.
Loop deletion does not have global effects on the structure of mFdx. (Top) Ribbon representation of M. laminosus Fdxwt (orange) and mFdxΔL1,2 deletion mutant (blue). The overall fold of the two molecules, as well as the position of the 2Fe-2S cluster (shown as spheres), is similar. (Bottom) The L1,2 region connecting β1 and β2 in mFdxΔL,1,2 is two residues shorter than the WT, resulting in a type-I β-turn conformation.
Substitution of the flexible L1,2 loop (residues 10–14) of wild-type Fdx (mFdxWT) with the rigid β-turn of a mesophilic Fdx (residues 10–12) (here we refer to this substituted mutant as mFdxΔL1,2),* yields a protein that is functional at 23 °C but loses electron-transfer (ET) activity at 55 °C (19), whereas mFdx is fully active. This loss of function is not due to dissociation of the [2Fe-2S] cluster, as this moiety is stable to 75 °C. The highly specific effects of this isolated mutation is a well-defined feature, making mFdx ideal for exploring the relationship between a protein’s functional and dynamical properties.
Through a combined experimental-theoretical investigation, we find that the network of short-range residue–residue interactions that define the Fdx fold directly facilitates long-range communication between L1,2 and the ISC pocket. Protein–film voltammetry (PFV) showed that the deletion of loop L1,2 alters the redox potential of the functional [2Fe-2S] center, ca. 20 Å removed from the site of mutation. X-ray crystallography and temperature-dependent CD studies revealed that, despite changes in the ET rates at high temperatures, the global structure and stability of the protein are robust. The only noticeable structural changes (i.e., larger than the uncertainties in the crystallographic coordinates) are in residues that directly interact with the mutated loop. Because these functional changes could not be attributed to changes in structure, or stability, we employed all-atom structure-based simulations (38) to characterize the native-basin fluctuations. These simulations revealed transdomain dynamic coupling (i.e., between two regions on opposing sides of a single domain) between the L1,2 loop and the [2Fe-2S] cluster. Taken together, these results demonstrate that the network of short-range native interactions in the Fdx fold possesses intrinsic communication channels that span the domain. These modes of communication connect functional centers to distal sites, which suggest previously undescribed modes of functional control in single-domain proteins within the cell.
Results
Minimal Structural Perturbation Observed upon Loop Deletion in mFdx.
Because conformational rearrangements are often coupled to changes in the activity of proteins, we first determined the crystal structure of mFdxΔL1,2 (2.3-Å resolution) to see if structural changes associated with loop deletion are sufficient to rationalize the large effect it has on the protein’s function. The structure was solved via molecular replacement methods using the AMoRe module of CCP4i (39), where mFdxWT (PDB ID code 1RFK) was used as a search model (excluding solvent). The solution resulted in an Rvalue of 34.4% and correlation coefficient of 56.5% in the resolution range of 10–4.0 Å. The initial Fobs-Fcalc and 2Fobs-Fcalc electron density maps, calculated after five cycles of restrained refinement using REFMAC5 (40), clearly indicated the presence of a β-turn between β1 and β2. The structure was further refined in the resolution range of 4.0–2.3 Å using REFMAC5. The structure was fitted into electron density maps using the graphics program O (41). The final model of mFdxΔL1,2 (Rvalue = 20.9; Rfree = 28.0) consists of residues 1–97 for both molecules and two [2Fe-2S] clusters (Table S1).
The structures of mFdxWT and mFdxΔL1,2 are shown in Fig. 1. The overall backbone fold of mFdxΔL1,2 is superimposable with that of mFdxWT, with a rmsd of 0.35 Å (excluding the L1,2 residues). The only major difference observed between the two structures is localized to the mutated L1,2 loop (Fig. 1), which is over 20 Å removed from the ISC and adopts a shorter type-I β-turn conformation in mFdxΔL1,2. The [2Fe-2S] moieties and the surrounding side chains (residues within 4 Å of the ISC) in the cluster-binding pocket are also superimposable, with a rmsd of 0.15 Å. Thus, the change in electron-transfer efficiency upon mutation cannot be attributed to any apparent shift in the protein’s configuration.
Loop Deletion Alters the Redox Potential in mFdx.
Despite the minimal effect that L1,2 deletion has on the tertiary structure, this deletion maintains activity well below the optimal temperature of the thermophilic organism but abolishes the electron transport properties of this protein in vitro at 55 °C (19). Electron transport efficiencies are the result of a combination of intra- and interprotein interactions and properties including distance, orientation, hydrogen bonding, van der Waals, electrostatic interactions, and folding characteristics. To determine if loop mutation has a specific effect on the individual Fdx domain, we measured the redox potential and global stabilities (see below) of the WT and mutant proteins.
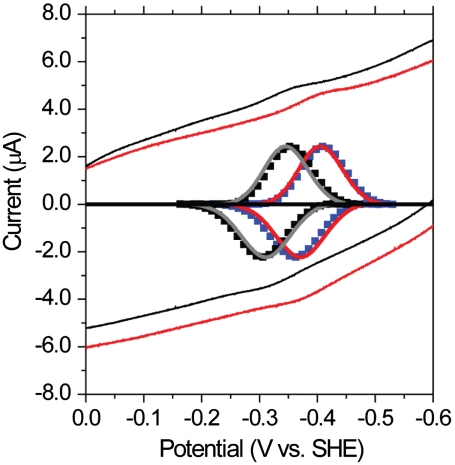
Using protein–film voltammetry (42). we measured the redox potential (Em) of the ISC at pH 7.0 for both mFdxWT and mFdxΔL1,2. Plots of applied potential versus the measured current for mFdxWT and mFdxΔL1,2 are given in Fig. 2. mFdxWT has a maximum cathodic peak at -345 mV and maximum anodic peak at -305 mV, yielding an Em of -325 mV. This value is in excellent agreement with previous reports achieved with solution titrations coupled with electron paramagnetic detection methods (21). It is important to note that while the addition of neomycin is extremely useful in the collection of protein–film voltammetry measurements, it has the potential to alter the observed redox potential of specific systems (43). However, in the case of mFdx the observed redox potential for mFdx is unchanged in the presence/absence of neomycin (21). mFdxΔL1,2 exhibits a cathodic peak at -400 mV and maximum anodic peak at -364 mV yielding an Em of -382 mV and a change in Em values of ∼57 mV. Thus mFdxΔL1,2 has a significantly altered Em value, though it is a suitable Em for accepting electrons from photosystem I (Em of -550 mV). We next asked whether alterations in the solution ionic strength, compatible with the typical ranges used in enzymatic assays (19, 44), would modify the observed potentials. We found that addition of 100 mM NaCl does not have a measurable effect on the respective redox potentials of the WT or mutant proteins (Fig. 2). This robustness of the redox potential, under physiological changes in ionic strength, may have evolved to allow Fdx to function under a wider range of cellular conditions. Much more dramatic changes in conditions (i.e., addition of molar quantities of salt and extremes in pH) will be necessary to identify the origins and limits of this kind of robust functional dynamics to changes in solution conditions, though this is beyond the scope of the present investigation.
Fig. 2.
Redox changes are observed for the ΔL1,2 mutant via protein–film voltammograms of mFdxWT (shown in black) and mFdxΔL1,2 (shown in red). Baseline-subtracted voltammograms of mFdxWT (shown in gray) and mFdxΔL1,2 mutant (shown in red) in 100 mM Bis-Tris 100 mM NaCl pH 7.0 (not shown to scale). Baseline-subtracted voltammograms in low salt (100 mM Bis-Tris pH 7.0) for mFdxWT (black squares) and mFdxΔL1,2 (blue squares) show no major effect of salt concentration on the redox potential. Midpoint potentials (Em) were found by determining the potential equidistant from the cathodic and anodic peak potentials of the oxidative and reductive curves. Em values for mFdxWT and mFdxΔL1,2 were determined to be -325 ± 10 mV and -382 ± 10 mV, respectively. Voltammograms were generated using highly oriented edge-plane graphite electrode containing neomycin as a coadsorbate. The reference electrode was 3 M Ag/AgCl. All values shown above are corrected to standard hydrogen electrode (SHE) values.
When studying electron transport processes, it is often difficult to partition the contributions of the donor and the acceptor because intermolecular interactions will also affect the transport properties. Here, by measuring the effects of distal loop swap on the redox potential of an isolated Fdx protein, we have identified a direct intramolecular effect that loop characteristics have on the active site.
Loop Deletion Has Minimal Effects on Global Protein Stability.
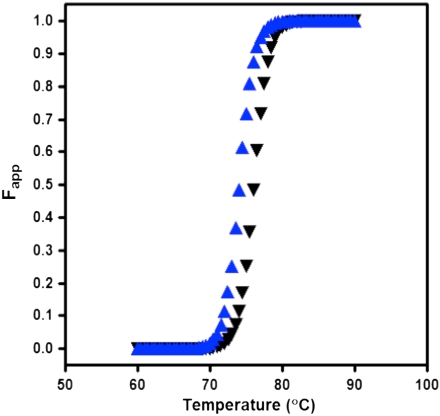
As deletion of L1,2 does not lead to substantial structural changes, an alternative explanation for the observed functional coupling between these distal sites is that mutation reduces the stability of the protein, which results in frequent excursions to denatured, or partially denatured, conformations. To address this potential effect, we measured the thermal stability of the WT and mutated mFdx proteins. The fraction of unfolded protein, Fapp, as a function of temperature for mFdxWT and mFdxΔL1,2 is shown in Fig. 3. Both proteins are thermally stable and fully folded at temperatures below 70 °C. They exhibit cooperative transitions between 70 °C and 80 °C and are fully unfolded at temperatures above 80 °C. The midpoints of the transitions are 76.1±0.2 °C and 74.0±0.3 °C for mFdxWT and mFdxΔL1,2, indicating minimal perturbation to the stability of the protein upon loop deletion. Because the physiological temperature (55 °C) is far below the folding temperatures, this change in stability should lead to a negligible increase in the probability of being unfolded in vivo. Thus, it is unlikely that the large changes in functional dynamics are the result of this modest change in stability.
Fig. 3.
The thermostabilities of mFdxWT and mFdxΔL1,2 are comparable. Fraction of unfolded protein as a function of temperature for mFdxWT (black triangles) and mFdxΔL1,2 (blue triangles) as measured from the mean-residue ellipticity at 222 nm.
The lack of coupling between the thermal stability and functional dynamics in the single-domain mFdx protein contrasts with our understanding of enzymatic catalysis in many multidomain proteins, such as adenylate kinase. In adenylate kinase, the dynamics of the large-scale rearrangements that govern catalysis are often correlated with the thermal stability of the protein (8). It has been argued that the coupling of stability and functional dynamics allows the same protein fold to carry out similar functional roles in organisms with different physiological temperatures. Additionally, as a protein’s stability evolves, so does the propensity to crack (9, 10, 17). This physical-chemical coupling between stability and function enables a range of sequences to have similar functional properties, so long as sufficient stability is selected.
In contrast to this picture, for mFdx we find that substitution of the distal L1,2 loop from a mesophilic sequence into a thermophilic sequence has a negligible effect on its stability and conformation, whereas it has a pronounced effect on the functional properties of the thermophilic protein. These results suggest an alternative mechanism of regulation in mFdx. Specifically, it suggests that the Fdx fold has internal communication channels that allow it to “sense” signals on one side of the domain and translate them into functional changes in distal residues. By mutating the L1,2 loop, we have artificially “flipped the switch” in mFdx. Because each organism has a unique set of potential interaction partners for Fdx-like proteins, the precise chemical details of each may lead to highly specific effects on the functional residues.
Simulations Suggest a Mechanism of Communication in mFdx.
Because we cannot rationalize the functional impact of L1,2 deletion in terms of a conformational change or a change in thermal stability, we employed simulations to explore the possibility that the connectivity of short-ranged native interactions leads to the observed long-range functionally relevant transdomain communication. Specifically, we used simulations that employed a single energetic basin, where only interactions that are formed in the crystal structure are stabilizing, for the apo forms of both mFdxWT and mFdxΔL1,2†. These simulations enabled us to determine whether L1,2 deletion affects the ISC-binding pocket as part of a global change in the dynamics, or through a specific communication route. Because structure-based simulations (38, 45), which are grounded in energy landscape theory (46), accurately describe the native-ensemble structural fluctuations of biomolecular systems (10, 38, 47–51), we utilized an all-atom structure-based model (38) to explore the mechanism of L1,2-ISC-pocket communication. This model does not explicitly include long- or short-range electrostatic interactions, but it allows for the dissection of the geometric features that may impact function and folding. Therefore, the observed coupling between L1,2 and the binding site would be a consequence of the local connectivity of interactions, which is a property of the Fdx fold.
Our simulations reveal that deletion of L1,2 leads to temperature-dependent differences in the structural fluctuations of the WT and mutant proteins as shown in Fig. 4. Specifically, the spatial root-mean-squared fluctuations (rmsf) for mFdxWT and mFdxΔL1,2 are similar across the protein (with the exception of the site of mutation) in the folded state at lower temperature, though appreciable differences surface as the temperature is increased. Near the folding temperature, three regions distal to the L1,2 region exhibit large fluctuations in mFdxWT, which are attenuated in mFdxΔL1,2 (Fig. 4A). Changes are observed in the regions proximal to residue A43 and D68 (denoted here as r43 and r68) and the C-terminal residues. r43 and r68 are located near the ISC-binding cysteine residues (positions 41, 46, 49, and 79).
Fig. 4.
Dynamical effects of the ΔL1,2 mutation are observed in simulations. (A) Differences in fluctuations between mFdxΔL1,2 and the mFdxWT, at low (blue squares) and higher (open triangles) temperature. Red triangles indicate the changes at higher T when all non-L1,2 native contacts are exchanged between mFdxΔL1,2 and mFdxWT. The plot shows the average taken from the two sets of hybrid simulations. Yellow lines mark the cysteines that coordinate the [2Fe-2S] cluster. (B) Cartoon representation of mFdxWT, with the Fe-coordinating Cys shown in yellow. Translucent clouds indicate which residues have an rms fluctuation greater than 1.5 Å. The red shading is proportional to the residue’s reduction in rms upon mutation (for the hybrid force fields). The most strongly affected residues (positions 41–48, 63–71, 93–97, which decrease by more than 5%) are colored solid red. All contacts between the mutated residues 10 and 14 (colored sticks) with residues outside of the L1,2 (in blue) are shown as green lines. (C) Spatial cross-correlation matrix showing correlated motion between Cα atoms.
To probe whether the dynamic changes in the ISC-binding pocket are the result of communication between it and the L1,2 loop, as opposed to being the result of many small perturbations to the structure, additional “control” simulations were performed. That is, due to minor differences in the atomic coordinates of mFdxWT and mFdxΔL1,2, the native atomic contacts between non-L1,2 residues are 81% identical. To test whether these disparities in the contact maps contribute significantly to the differences in fluctuations, we also calculated the fluctuations for models that employed “hybrid” contact maps. The two hybrid force fields were constructed by defining the native contacts as (i) all the non-L1,2 contacts of mFdxWT and the L1,2 contacts of mFdxΔL1,2 or (ii) including the L1,2 contacts of mFdxWT and the non-L1,2 contacts of mFdxΔL1,2. Regardless of which set of non-L1,2 contacts was employed, simulations that employed the L1,2 contacts from mFdxΔL1,2 displayed reduced fluctuations in regions r43 and r68.
Careful examination of the native contacts of the L1,2 regions suggests two mechanisms by which loop deletion may alter the structural fluctuation in the ISC-binding site. The most mobile regions, which are also the most affected by ΔL1,2 mutation, are r43, r68, and the C terminus (Fig. 4 A and B). In mFdxWT, the L1,2 residues (excluding those in strands β1 and β2) form 14 contacts with residues 35, 37, 54, and 91–94. In mFdxΔL1,2, L1,2 has only a total of five contacts, all of which are with residues 91 and 92. Because loop deletion results in the loss of all contacts between L1,2 and residues near r43 (positions 35, 37, and 54), the observed changes in r43 are reasonable to expect. Similarly, L1,2 deletion changes the contacts between L1,2 and residues near the C terminus (positions 91 and 92), which explains the changes in the dynamics of the C-terminal residues. Although there is no obvious, direct, structural connection between changes in L1,2 and r68, both the C terminus and r43 are located proximal to it. Thus, the changes in dynamics of the C terminus and r43 likely contribute to the changes in r68 dynamics. Inspection of the positional covariance matrix for the Cα atoms (Fig. 4C) reveals correlated motion of these three regions. Because these regions contribute to the electronic environment surrounding the iron–sulfur cluster, these results provide a clear mode of communication between L1,2 and the system’s electrochemical activity. This type of communication may signal changes across a domain, which might then affect the electrostatic environment at distal sites and influence protein–protein and protein–ligand interactions and is the subject of ongoing investigations (52).
Discussion
This study demonstrates that within a single protein domain fold the network of short-range native interactions can give rise to long-range modes of communication. Transdomain “cross-talk” between a catalytic center and a distal loop is an emerging theme by which single domains can transmit cellular signals (7, 53–55). In the single-domain protein interleukin-1β, point mutations that are distal to the receptor binding interface have no effect on stability, yet they have significant effects on the binding properties (55). In multidomain proteins, there are also examples of transdomain communication across individual domains, including in cAMP-dependent protein kinase (56) and in COOH-terminal Src kinase (Csk) (7). It was demonstrated (57) that chemical perturbations are linked to activity regulation through a “communication pathway” between a distal SH2 domain and the active site. In contrast to the present study, NMR experiments revealed that the SH2 domain of Csk populates two structurally similar conformations and selection of one also contributes to the functional changes. In mFdx, we found no evidence of a conformational change within the domain, yet changes in the fluctuations of the distal L1,2 loop are coupled to the functionally relevant dynamics of the ISC pocket.
In light of these results, it is worthwhile to consider the notion of allosteric communication in proteins. The term allostery describes the observation of regulation of activity within a protein upon ligand binding at one site, and transmission of information to a second, distal site. The conventional view holds that conformational perturbations or rearrangements convey the regulatory effect. However, it now appears that the mechanism of allosteric regulation may be more universal than the classical interpretation (58). In this updated view, allosteric signals can be transmitted through multiple, preexisting pathways and which pathway is chosen depends on topologies, binding events, and environmental conditions. Hence, long-range coupling via local interactions, as we observe here, can relay effects without protein structure being affected.
Because the physical origin of transdomain communication in mFdx can be traced back to the network of short-range, native, residue–residue interactions, it is reasonable to infer that the primary requirement for a protein to possess this dynamical communication channel is for it to obtain a Fdx-like fold. Here, a common feature of multidomain functional dynamics and mFdx may be seen. That is, similar to adenylate kinase, the global dynamics are encoded in the tertiary fold, and the scale of the motions is coupled to the global stability. For proteins that take on a Fdx-like fold, the network of residue–residue interactions will necessarily be very similar, because the finite size of protein side chains will limit the possible residue–residue connectivity. If the native interactions are similar between Fdx-like folded proteins, these communication avenues should also be preserved.
From a theoretical perspective, the role of local connectivity demonstrates how high-level functional dynamics may be understood in terms of simple considerations. If local connectivity governs the functional modes of communication, one must account only for the native interactions in order to reveal these dynamical properties. For single-domain proteins that do not undergo large conformational rearrangements, then one must employ only a model that accurately describes the fluctuations about a particular energetic minimum, such as the structure-based model used here. Although this approach captures the dynamical coupling between distal regions, future methodological advances may allow one to predict the degree of functional (in this case, the redox potential) changes from this type of dynamic information.
This study has revealed a mode of transdomain communication between the distal ISC-binding pocket and the L1,2 loop. Further, this communication does not appear to require long-range nonspecific interactions, but rather a network of short-range native interactions. Although these findings shed light on the relationship among protein structure, dynamics, and function, the standing challenge will be to identify these functional channels a priori. Here, we have explained the basis for an experimentally suggested communication channel between distal sites, but the communication was not predicted without explicitly including a particular perturbation (mutation of the L1,2 loop). These results suggest that analyzing interaction networks in proteins is a viable approach to identify “hidden” modes of dynamic coupling in proteins. Finally, a complete understanding of how short-ranged interactions give rise to long-range communication will provide guiding principles for designing molecular devices that are capable of transmitting and converting chemical signals into mechanical activity.
Methods
Crystals of mFdxΔL1,2 were obtained using the hanging-drop vapor diffusion method. Crystallographic data were collected at the European Synchrotron Radiation Facility beamline ID29 (λ = 0.976 Å) on an Area Detector Systems Corporation Quantum 210 CCD detector and were integrated and scaled using the HKL2000 suite (59). Protein–film voltammograms were generated using a CHI730C Electrochemical Workstation (CH Instruments Inc.). The electrode was prepared as reported earlier (60). All PFV measurements were taken at 4 °C to enhance signal to noise (42). The SOAS software package (61) (courtesy of Christophe Léger, Centre National de la Recherche Scientifique, Paris, France) was used to perform the baseline subtraction of voltammetry data. All-atom structure-based force fields (38) for the Apo forms of mFdxWT and mFdxΔL1,2 were prepared from the X-ray structures using the SMOG@ctbp Web tool (http://smog.ucsd.edu) (62), with tertiary contacts represented by Gaussian-based potentials (63). Simulations were performed with the Gromacs software package (64). Structural figures were prepared with VMD (65). Further details are provided in SI Text.
Supplementary Material
Acknowledgments.
We are thankful to Sichun Yang for many scientific discussions in the early stages of this research. This work was supported by the Center for Theoretical Biological Physics sponsored by the National Science Foundation (NSF) (Grant PHY-0822283) and NSF Grant NSF-MCB-1051438, National Institutes of Health Grant GM-54038, and the Los Alamos National Laboratory Laboratory Directed Research and Development program. R.N. acknowledges Israel Science Foundation ISF-863/09. P.C.W. is funded by a Los Alamos National Laboratory Director’s Postdoctoral Fellowship.
Footnotes
The authors declare no conflict of interest.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3P63).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019502108/-/DCSupplemental.
*Starting at E10, the sequence EAEGL of mFdx is replaced with PTG- -.
†Although the crystal structures have bound ISCs, the simulations were performed for Fdx without the ISC. Because communication of dynamics between L1,2 and the ISC-binding site is the result of the network of interactions within the Fdx fold, and these interactions are present in the simulation (because the ISC-bound configuration was used), it is appropriate to probe the degree of coupling from simulations in which the ISC is not explicitly represented.
References
- 1.Haldane JBS. Enzymes. New York: Longmans Green; 1930. [Google Scholar]
- 2.Pauling L. Nature of forces between large molecules of biological interest. Nature. 1948;161:707–709. doi: 10.1038/161707a0. [DOI] [PubMed] [Google Scholar]
- 3.Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes of motions of proteins. Science. 1991;254:1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- 4.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa A, et al. Structure of the carboxyl-terminal Src Kinase, Csk. J Biol Chem. 2002;277:14351–14354. doi: 10.1074/jbc.C200086200. [DOI] [PubMed] [Google Scholar]
- 6.Jamros MA, et al. Proteins at work: A combined small angle x-ray scattering and theoretical determination of the multiple structures involved on the protein kinase functional landscape. J Biol Chem. 2010;285:36121–36128. doi: 10.1074/jbc.M110.116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mill JE, et al. A novel disulfide bond in the SH2 domain of the C-terminal Src kinase controls catalytic activity. J Mol Biol. 2007;365:1460–1468. doi: 10.1016/j.jmb.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf-Watz M, et al. Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat Struct Biol. 2004;11:945–949. doi: 10.1038/nsmb821. [DOI] [PubMed] [Google Scholar]
- 9.Miyashita O, Onuchic JN, Wolynes PG. Nonlinear elasticity, proteinquakes, and the energy landscapes of functional transitions in proteins. Proc Natl Acad Sci USA. 2003;100:12570–12575. doi: 10.1073/pnas.2135471100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitford PC, Miyashita O, Levy Y, Onuchic JN. Conformational transitions of adenylate kinase: Switching by cracking. J Mol Biol. 2007;366:1661–1667. doi: 10.1016/j.jmb.2006.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Q, Wang J. Kinetics and statistical distributions of single-molecule conformational dynamics. J Phys Chem B. 2009;113:1517–1521. doi: 10.1021/jp808923a. [DOI] [PubMed] [Google Scholar]
- 12.Brokaw JB, Chu JW. On the role of substrate binding and hinge unfolding in conformational changes of adenylate kinase. Biophys J. 2010;99:3420–3429. doi: 10.1016/j.bpj.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora K, Brooks CL. Large-scale allosteric conformational transitions of adenylate kinase appear to involve a population shift mechanism. Proc Natl Acad Sci USA. 2007;104:18496–18501. doi: 10.1073/pnas.0706443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae E, Phillips GN. Roles of static and dynamic domains in stability and catalysis of adenylate kinase. Proc Natl Acad Sci USA. 2006;103:2132–2137. doi: 10.1073/pnas.0507527103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson JA, et al. Illuminating the mechanistic roles of enzyme conformational dynamics. Proc Natl Acad Sci USA. 2007;104:18055–18060. doi: 10.1073/pnas.0708600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitford PC, Onuchic JN, Wolynes PG. Energy landscape along an enzymatic reaction trajectory: Hinges or cracks? HFSP J. 2008;2:61–64. doi: 10.2976/1.2894846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsson U, Wolf-Watz M. Overlap between folding and functional energy landscapes for adenylate kinase conformational change. Nat Commun. 2010 doi: 10.1038/ncomms1106. 10.1038/ncomms1106. [DOI] [PubMed] [Google Scholar]
- 18.Beinert H, Holm RH, Münck E. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 19.Fish A, Danielli T, Ohad I, Nechushtai R, Livnah O. Structural basis for the thermostability of ferredoxin from cyanobacterium Mastigocladus laminosus. J Mol Biol. 2005;350:599–608. doi: 10.1016/j.jmb.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 20.Grinberg AV, et al. Adrenodoxin: Structure, stability and electron transfer properties. Proteins. 2000;40:590–612. doi: 10.1002/1097-0134(20000901)40:4<590::aid-prot50>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Cammack R, et al. Midpoint redox potentials of plant and algal ferredoxin. Biochem J. 1977;168:205–209. doi: 10.1042/bj1680205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuyama K. Structure and function of plant-type ferredoxin. Photosynth Res. 2004;81:289–301. doi: 10.1023/B:PRES.0000036882.19322.0a. [DOI] [PubMed] [Google Scholar]
- 23.Rypniewski WR, et al. Crystallization and structure determination of 2.5-.ANG. resolution of the oxidized iron-sulfur [2Fe-2S] ferredoxin isolated from Anabaena. Biochemistry. 1991;30:4126–4131. doi: 10.1021/bi00231a003. [DOI] [PubMed] [Google Scholar]
- 24.Vriend G. Detection of common three-dimensional substructures in proteins. Proteins. 1991;11:52–58. doi: 10.1002/prot.340110107. [DOI] [PubMed] [Google Scholar]
- 25.Overington J. Structural constraints on residue substitution. Genet Eng. 1992;14:231–249. doi: 10.1007/978-1-4615-3424-2_13. [DOI] [PubMed] [Google Scholar]
- 26.Steele RA, Opella SJ. Structures of the reduced and mercury-bound forms of MerP, the periplasmic. Biochemistry. 1997;36:6885–6895. doi: 10.1021/bi9631632. [DOI] [PubMed] [Google Scholar]
- 27.Frolow F, Harel M, Sussman JL, Mevarech M, Shoham M. Insights into protein adaptation to a saturated salt environment from the crystal structure of a halophilic 2Fe-2S ferredoxin. Nat Struct Biol. 1996;3:452–458. doi: 10.1038/nsb0596-452. [DOI] [PubMed] [Google Scholar]
- 28.Purnick PEM, Weiss R. The second wave of synthetic biology: From modules to systems. Nat Rev Mol Cell Bio. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 29.Sheftel AD, et al. Humans possess two mitochondrial ferredoxin, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme and Fe/D cluster biosynthesis. Proc Natl Acad Sci USA. 2010;107:11775–11780. doi: 10.1073/pnas.1004250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rees DC, Howard JB. The interface between the biological and inorganic worlds: Iron-sulfur metalloclusters. Science. 2003;300:929–931. doi: 10.1126/science.1083075. [DOI] [PubMed] [Google Scholar]
- 31.Fukuyama K, Ueki N, Nakamura H, Tsukihara T, Matsubara H. Tertiary structure of [2Fe-2S] ferredoxin from Spirulina platensis refined at 2.5 A resolution: Structural comparisons of plant-type ferredoxin and an electrostatic potential analysis. J Biochem-Tokyo. 1995;117:1017–1023. doi: 10.1093/oxfordjournals.jbchem.a124800. [DOI] [PubMed] [Google Scholar]
- 32.Crossnoe CR, Germanas JP, LeMagueres P, Mustata G, Krause KL. The crystal structure of trichomonas vaginalis ferredoxin provides insight into metronidazole activation. J Mol Biol. 2002;318:503–518. doi: 10.1016/S0022-2836(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 33.Bes MT, et al. Crystal structure determination at 1.4 Å resolution of ferredoxin from the green alga Chlorella fusca. Structure. 1999;7:1201–1211. doi: 10.1016/s0969-2126(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, et al. Ternary protein complex of ferredoxin, ferredoxin:thioredoxin reductase, and thioredoxin studied by paramagnetic NMR spectroscopy. J Am Chem Soc. 2009;131:17576–17582. doi: 10.1021/ja904205k. [DOI] [PubMed] [Google Scholar]
- 35.Muraki N, Seo D, Shiba T, Sakurai T, Kurisu G. Asymmetric dimeric structure of ferredoxin-NAD(P)+ oxidoreductase from the green sulfur bacterium Chlorobaculum tepidum: Implications for binding ferredoxin and NADP+ J Mol Biol. 2010;401:403–414. doi: 10.1016/j.jmb.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Overington JP. Comparison of three-dimensional structures of homologous proteins. Curr Opin Struct Biol. 1992;2:394–401. [Google Scholar]
- 37.Fish A, Lebendiker M, Nechushtai R, Livnah O. Purification, crystallization and preliminary X-ray analysis of ferredoxin isolated from thermophilic cyanobacterium Mastigocladus laminosus. Acta Crystallogr D. 2003;59:734–736. doi: 10.1107/s0907444903002245. [DOI] [PubMed] [Google Scholar]
- 38.Whitford PC, et al. An all-atom structure-based potential for proteins: Bridging minimal models with empirical forcefields. Proteins. 2009;75:430–441. doi: 10.1002/prot.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anonymous. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 40.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 41.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 42.Armstrong FA, Butt JN, Sucheta A. Voltammetric studies of redox-active centers in metalloproteins adsorbed on electrodes. Methods Enzymol. 1993;227:479–500. doi: 10.1016/0076-6879(93)27020-h. [DOI] [PubMed] [Google Scholar]
- 43.Zu Y, et al. Reduction potentials of rieske clusters: Importance of the coupling between oxidation state and histidine protonation state. Biochemistry. 2003;42:12400–12408. doi: 10.1021/bi0350957. [DOI] [PubMed] [Google Scholar]
- 44.Fish A, Lebendiker M, Nechushtai R, Livnah O. Purification, crystallization and preliminary X-ray analysis of ferredoxin isolated from thermophilic cyanobacterium Mastigocladus laminosus. Acta Crystallogr D. 2003;59:734–736. doi: 10.1107/s0907444903002245. [DOI] [PubMed] [Google Scholar]
- 45.Clementi C, Nymeyer H, Onuchic JN. Topological and energetic factors: What determines the structural details of the transition state ensemble and “en-route” intermediates for protein folding? An investigation for small globular proteins. J Mol Biol. 2000;298:937–953. doi: 10.1006/jmbi.2000.3693. [DOI] [PubMed] [Google Scholar]
- 46.Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Funnels, pathways, and the energy landscape of protein-folding—A synthesis. Proteins. 1995;21:167–195. doi: 10.1002/prot.340210302. [DOI] [PubMed] [Google Scholar]
- 47.Roy M, et al. The native energy landscape for interleukin-1b. Modulation of the population ensemble through native-state topology. J Mol Biol. 2005;348:335–347. doi: 10.1016/j.jmb.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 48.Hyeon C, Jennings PA, Adams JA, Onuchic JN. Ligand-induced global transitions in the catalytic domain of protein kinase A. Proc Natl Acad Sci USA. 2009;106:3023–3028. doi: 10.1073/pnas.0813266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitford PC, et al. Accommodation of aminoacyl-tRNA into the ribosome involves reversible excursions along multiple pathways. RNA. 2010;16:1196–1204. doi: 10.1261/rna.2035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratje AH, et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468:713–716. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haliloglu T, Bahar I. Coarse-grained simulations of conformational dynamics of proteins: Application to apomyoglobin. Proteins. 1998;31:271–281. [PubMed] [Google Scholar]
- 52.Pyaram K, Kieslich CA, Yadav VN, Morikis D, Sahu A. Influence of electrostatics on the complement regulatory functions of kaposica, the complement inhibitor of Kaposi’s sarcoma-associated herpesvirus. J Immunol. 2010;184:1956–1967. doi: 10.4049/jimmunol.0903261. [DOI] [PubMed] [Google Scholar]
- 53.Tsai C-J, del Sol A, Nussinov R. Allostery: Absence of a change in shape does not imply that allostery is not at play. J Mol Biol. 2008;378:1–11. doi: 10.1016/j.jmb.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bahar I, Chennubhotla C, Tobi D. Intrinsic dynamics of enzymes in the unbound state and relation to allosteric regulation. Curr Opin Struct Biol. 2007;17:633–640. doi: 10.1016/j.sbi.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heidary DK, Roy M, Daumy GO, Cong Y, Jennings PA. Long-range coupling between separate docking sites in interleukin-1β. J Mol Biol. 2005;353:1187–1198. doi: 10.1016/j.jmb.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 56.Andersen MD, Shaffer J, Jennings PA, Adams JA. Structural characterization of protein kinase A as a function of nucleotide binding. J Biol Chem. 2001;276:14204–14211. doi: 10.1074/jbc.M011543200. [DOI] [PubMed] [Google Scholar]
- 57.Hamuro Y, et al. Phosphorylation driven motions in the COOH-terminal Src kinase, Csk, revealed through enhanced hydrogen-deuterium exchange and mass spectrometry (DXMS) J Mol Biol. 2002;323:871–881. doi: 10.1016/s0022-2836(02)01003-3. [DOI] [PubMed] [Google Scholar]
- 58.del Sol A, Tsai C-J, Ma B, Nussinov R. The origin of allosteric functional modulation: Multiple pre-existing pathways. Structure. 2009;17:1042–1050. doi: 10.1016/j.str.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 60.Bak DW, Zuris JA, Paddock ML, Jennings PA, Elliott SJ. Redox characterization of the FeS protein MitoNEET and impact of thiazolidinedione drug binding. Biochemistry. 2009;48:10193–10195. doi: 10.1021/bi9016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fourmond V, et al. SOAS: A free software to analyse electrochemical data and other one-dimensional signals. Bioelectrochemistry. 2009;76:141–147. doi: 10.1016/j.bioelechem.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Noel JK, Whitford PC, Sanbonmatsu KY, Onuchic JN. SMOG@ctbp: Simplified deployment of structure-based models in GROMACS. Nucleic Acids Res. 2010;38:W657–W661. doi: 10.1093/nar/gkq498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lammert H, Schug A, Onuchic JN. Robustness and generalization of structure-based models for protein folding and function. Proteins. 2009;77:881–891. doi: 10.1002/prot.22511. [DOI] [PubMed] [Google Scholar]
- 64.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 65.Humphrey W, Dalke A, Schulten K. VMD—Visual Molecular Dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.