Abstract
The ATP-dependent chromatin-remodeling enzyme brahma-related gene 1 (BRG1) regulates transcription of specific target genes during embryonic and postnatal development. Deletion of Brg1 from embryonic blood vessels results in yolk sac vascular remodeling defects. We now report that misregulation of the canonical Wnt signaling pathway underlies many Brg1 mutant vascular phenotypes. Brg1 deletion resulted in down-regulation of several Wnt receptors of the frizzled family, degradation of the intracellular Wnt signaling molecule β-catenin, and an overall decrease in Wnt signaling in endothelial cells. Pharmacological stabilization of β-catenin significantly rescued Brg1 mutant vessel morphology and transcription of Wnt target genes. Our data demonstrate that BRG1 impacts the canonical Wnt pathway at two different levels in vascular endothelium: through transcriptional regulation of both Wnt receptor genes and Wnt target genes. These findings establish an epigenetic mechanism for the modulation of Wnt signaling during embryonic vascular development.
Keywords: SWItch/Sucrose NonFermentable, angiogenesis, lithium chloride, frizzled receptor
Eukaryotes fit a large quantity of DNA into the nuclei of their cells by packaging it into a compact structure called chromatin. This packaging presents a barrier to transcription factors that must access DNA to bring about changes in gene expression. ATP-dependent chromatin-remodeling complexes alleviate this problem by temporarily unraveling and reorganizing chromatin, thereby making DNA more accessible to proteins that are required for transcription (1, 2). Once considered to be ubiquitous mediators of transcription, chromatin-remodeling complexes are increasingly recognized for their specialized functions and specific genomic targets during development (3, 4). Much of this specificity has been shown to stem from variations in the subunit composition of these large, multiprotein complexes (5–8).
One family of ATP-dependent chromatin-remodeling complexes, the mammalian SWI/SNF (SWItch/Sucrose NonFermentable)-like complexes, promotes or represses transcription of genes by increasing or decreasing accessibility of DNA to large transcriptional machinery at specific loci (9, 10). Mammalian SWI/SNF complexes contain one of two central ATPase catalytic subunits: brahma (BRM) or brahma-related gene 1 (BRG1). Global deletion of Brg1 in mice leads to embryonic lethality at peri-implantation (11). Tissue-specific conditional mutations have elucidated numerous developmental roles for BRG1, including zygotic genome activation, erythropoiesis, T-cell, cardiac, and neuronal development (8, 12–15). Deletion of Brg1 in developing endothelial cells results in defective yolk sac angiogenesis, although the mechanism by which BRG1 affects vascular development is unclear (16, 17).
The canonical Wnt/wingless signaling pathway is one of several signaling pathways known to contribute to embryonic vascular development (18–20). Canonical Wnt signaling occurs when soluble Wnt ligands interact with a cell-surface receptor complex consisting of the lipoprotein receptor-related 5/6 (Lrp5/6) proteins and the seven-transmembrane domain frizzled (Fzd) family proteins (21). This ligand–receptor interaction stabilizes the intracellular-signaling molecule β-catenin by inactivating a cytoplasmic destruction complex that would otherwise target β-catenin for degradation. Stabilized β-catenin translocates to the nucleus where it coregulates transcription of Wnt target genes by interacting with transcription factors in the lymphoid enhancer (LEF)/T-cell factor (TCF) family. These target genes mediate multiple effects that are critical for vascular development such as proliferation, differentiation, and morphogenesis (21).
Wnt signaling occurs in numerous embryonic and yolk sac vessels during midgestation (22), and mutations in various Wnt signaling components result in vascular abnormalities reminiscent of those seen in embryos deleted for endothelial Brg1. Global deletion of Fzd5 causes lethality at embryonic day 10.75 (E10.75) due to defective yolk sac and placental angiogenesis (23). In addition, mice that are conditionally deleted of vascular β-catenin die in utero with defective vascular patterning and increased vascular fragility at E11.5–13.5 (24). Recently, a conditional gain-of-function mutation in β-catenin has been shown to result in aberrant vascular remodeling and arteriovenous specification as early as E9.5, demonstrating that early vascular development is equally sensitive to over- or underexpression of β-catenin (22). Although these phenotypes indicate the importance of Wnt signaling during embryonic vascular development, little is known about how the pathway is regulated in developing vessels. We now describe a unique epigenetic mechanism for modulation of Wnt signaling during early vascular development. This study demonstrates that BRG1 impacts Wnt signaling at two levels in endothelial cells: in addition to coregulating transcription of certain Wnt target genes, BRG1 stabilizes β-catenin by directly mediating expression of multiple frizzled receptors.
Results
Endothelial Deletion of Brg1 Results in Flattening and Thinning of Yolk Sac Blood Vessels.
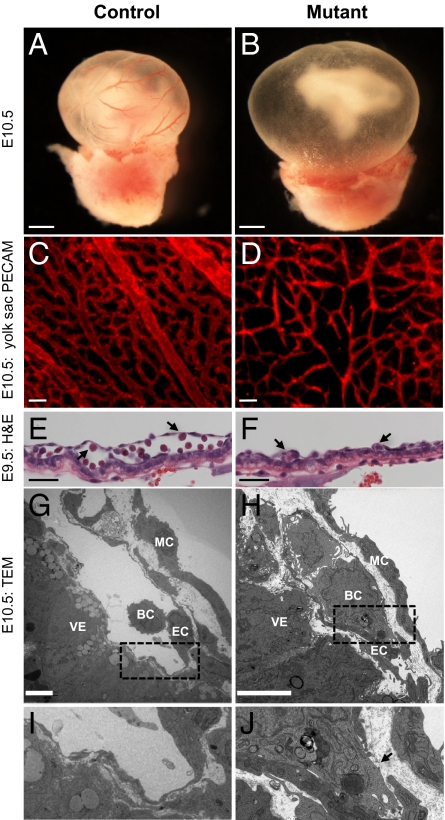
To determine the role of the SWI/SNF chromatin-remodeling enzyme BRG1 in vascular development, we previously deleted a floxed Brg1 allele from embryonic endothelial and hematopoietic cells using a Tie2-Cre transgene (16). These Brg1 mutant (Brg1fl/fl;Tie2-Cre+/0) embryos die at E10.5–11 with severe anemia due to failed transcription of embryonic β-globins and subsequent apoptosis of embryonic erythrocytes. In addition, Brg1 mutants display yolk sac-specific angiogenesis defects manifesting in vascular patterning abnormalities by E9.5. Subsequent analysis of Brg1-deleted yolk sac vessels revealed exaggerated abnormalities at E10.5 preceding embryonic lethality (Fig. 1). At this time, Brg1 mutant yolk sac vessels were uniformly thin, and many vessels failed to interconnect, resulting in a tapering, dead-end vasculature (Fig. 1D). Comparable vascular thinning and disconnectedness were not observed in the mutant embryonic head or intersomitic vessels (Fig. S1A), highlighting the specificity of these Brg1 mutant vascular phenotypes for the extraembryonic yolk sac.
Fig. 1.
Brg1 deletion results in vascular abnormalities in extraembryonic yolk sacs. (A and B) E10.5 littermate control Brg1fl/fl (A) and mutant Brg1fl/fl;Tie2-Cre+/0 embryos (B). (Scale bars: 1 mm.) (C and D) Anti-PECAM1 staining on histological sections of E10.5 yolk sacs reveal thinner vessels in mutants (D) compared with controls (C). (Scale bars: 100 μm.) (E and F) Hematoxylin and eosin (H&E)-stained paraffin sections of E9.5 littermate control Brg1fl/fl (E) and mutant Brg1fl/fl;Tie2-Cre+/0 (F) yolk sac vessels. Vessels are flattened in mutants (F) compared with controls (E). Arrows indicate embryonic blood cells. (Scale bars: 50 μm.) (G–J) Transmission electron micrographs (TEM) of control Brg1fl/fl (G and I) versus mutant Brg1fl/fl;Tie2-Cre+/0 (H and J) yolk sac vessels reveal discontinuities in the mutant endothelial lining (arrow in J). MC, mural cell; VE, visceral endoderm; BC, blood cell; EC, endothelial cell. (Scale bars: 5 μm.)
In addition to the thinning of yolk sac microvasculature, Brg1 mutants underwent thinning of vitelline vessels—the major vessels connecting the embryo to the yolk sac. Vitelline vessel diameters were measured at the first branch point as the vessels entered the yolk sac, and significant thinning was observed in Brg1 mutants starting at E9.5 (Fig. S1B). Pan-endothelial markers were expressed at comparable levels in primary endothelial cells isolated from E10.5 control and mutant yolk sacs (Fig. S1C). Likewise, proliferation rates and apoptosis were comparable in control and Brg1 mutant yolk sac endothelium (Fig. S1 D and E). However, Brg1 mutant yolk sac vessels were deflated by E9.5 when assessed histologically, whereas control yolk sac vessels had inflated luminal spaces with numerous circulating embryonic blood cells (Fig. 1 E and F). The flattening of Brg1 mutant yolk sac vessels was further verified by transmission electron microscopy. By E10.5, mutant blood vessels were tightly wrapped around circulating embryonic blood cells (Fig. 1 H and J), whereas control vessels maintained an abundant luminal space (Fig. 1 G and I). Importantly, discontinuities were observed in the endothelial cell lining of mutant yolk sac vessels (Fig. 1J, arrow). These discontinuities were too small for blood cells to hemorrhage from mutant vessels but were large enough for plasma leakage to occur. Such plasma leakage could account for the vascular flattening and microvascular thinning observed in Brg1 mutant yolk sac vessels (Fig. 1 F and D). Likewise, plasma leakage from yolk sac vessels may explain the hyper-inflated and distended yolk sac cavities that were consistently associated with mutant embryos (Fig. 1B).
Wnt Signaling Is Down-Regulated in Brg1 Mutant Yolk Sac Endothelium.
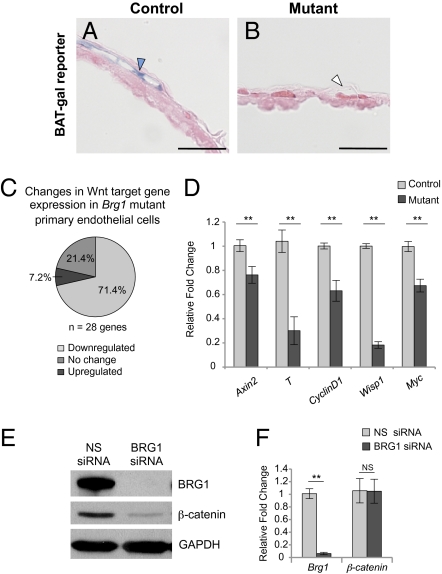
The Brg1 mutant phenotypes of vitelline vessel thinning, endothelial cell discontinuity, and plasma leakage are similar to phenotypes observed in embryos with deletion of vascular β-catenin, a key component of the canonical Wnt signaling pathway (24). Likewise, the yolk sac-specific vascular abnormalities in Brg1 mutants are reminiscent of yolk sac-specific vascular phenotypes observed in mutants for the Wnt receptor Fzd5 (23). To investigate the possibility that Brg1 mutant yolk sac vascular phenotypes result from a down-regulation of Wnt signaling in the absence of BRG1, we crossed a Wnt signaling reporter mouse with our Brg1 mutants. The β-catenin-activated transgene driving expression of the nuclear β-galactosidase reporter (BAT-gal) has been successfully used to visualize Wnt signaling in developing embryonic and postnatal vasculature (22, 25, 26). We found that endogenous Wnt signaling is evident at low levels in control yolk sac endothelium at E10.5 (Fig. 2A) but is undetectable in Brg1 mutant yolk sac endothelium (Fig. 2B), providing strong evidence that Brg1 depletion results in decreased Wnt signaling in vivo.
Fig. 2.
Wnt signaling is down-regulated, and β-catenin protein is degraded in Brg1 mutants. (A and B) Embryos were crossed onto the BAT-gal Wnt reporter line for detection of Wnt signaling activity. E10.5 X-gal–stained yolk sacs were cryosectioned and counterstained with eosin. Blue arrowhead, reporter-positive endothelium; white arrowhead, reporter-negative endothelium. (Scale bars: 50 μm.) (C and D) Endothelial cells from littermate control and mutant yolk sacs were isolated, RNA was purified, and cDNA was synthesized. (C) Samples were processed for qPCR using a custom-designed array containing 28 Wnt target genes (Fig. S5A). Data from four independent experiments were compiled using SABiosciences Excel-based data analysis. (D) qPCR was carried out for five Wnt target genes (Axin2, T, CyclinD1, Wisp1, and Myc). Errors represent ±SEM from six independent experiments, and significant differences between littermate controls and mutants were calculated using a two-tailed Student's t test (**P < 0.005). (E and F) C166 cells were transfected with nonspecific (NS) or Brg1-specific siRNA for 48 h. (E) Protein samples were subjected to Western blot analysis with antibodies that recognize BRG1, β-catenin, or GAPDH. (F) RNA was isolated, cDNA was synthesized, and qPCR for Brg1 or β-catenin was carried out. Errors represent ±SEM from four independent experiments, and significant differences between NS or Brg1-specific siRNA-transfected cells were calculated using a two-tailed Student's t test (**P < 0.005).
To further investigate the status of downstream Wnt signaling in Brg1 mutant yolk sac endothelium, we designed a custom real-time quantitative PCR (qPCR) array containing 28 direct Wnt target genes. We found 70% of these genes down-regulated in primary Brg1 mutant endothelial cells isolated from E10.5 yolk sacs (Fig. 2C). To support these array data, we selected five of these down-regulated Wnt target genes for validation by qPCR. Transcript levels of the Wnt targets Axin2, T, CyclinD1, Wisp1, and Myc were significantly reduced in mutant yolk sac endothelial cells (Fig. 2D). This decrease in Wnt target gene expression corresponds well with the loss of Wnt signaling observed using the BAT-gal reporter transgene and suggests that BRG1 influences Wnt signaling in the yolk sac during vascular development.
BRG1 Depletion Results in a Decrease in β-Catenin Protein Levels in Endothelial Cells.
Given the phenotypic similarities between our vascular Brg1 mutants and the previously described vascular β-catenin mutants (24), we sought to determine whether β-catenin expression was altered upon loss of BRG1. We used Brg1-specific siRNA oligos to knock down BRG1 expression in the C166 murine yolk sac endothelial cell line (27). Immunoblots revealed a significant reduction in β-catenin protein levels following BRG1 knockdown (Fig. 2E and Fig. S2B). Furthermore, immunofluorescent staining of control and BRG1-depleted C166 endothelial cells and of primary endothelial cells isolated from control and mutant yolk sacs demonstrated a modest decrease in β-catenin protein (Fig. S2 A and C).
In addition to its role in nuclear Wnt signaling, β-catenin also plays an important role in stabilization of adherens junctions—multiprotein complexes that mediate homotypic endothelial cell adhesion and control endothelial cell permeability (28). Because we had observed discontinuities in Brg1 mutant yolk sac endothelial cells by electron microscopy (Fig. 1J), we investigated the expression of adherens junction proteins. We observed diminished β-catenin expression at cell junctions in BRG1-depleted C166 endothelial cells (Fig. S2A). However, we did not detect any changes in α-catenin, desmoplakin, or VE-cadherin (Fig. S2B), indicating that adherens junctions are not grossly disrupted upon BRG1 depletion.
Because β-catenin protein levels were reduced with BRG1 depletion, we investigated whether β-catenin is a direct transcriptional target of BRG1. We measured β-catenin transcript levels in BRG1-depleted C166 endothelial cells and in Brg1 mutant primary yolk sac endothelial cells, but we detected no down-regulation of β-catenin transcription (Fig. 2F and Fig. S2D). These results indicate that β-catenin is not a direct transcriptional target of BRG1 and suggest that the decreased β-catenin levels observed in the absence of BRG1 are due to protein degradation.
Pharmacological Rescue of β-Catenin Degradation Significantly Improves Brg1 Mutant Yolk Sac Vessel Morphology.
In the absence of Wnt signaling, cytoplasmic β-catenin is phosphorylated by glycogen synthase kinase-3β (GSK-3β) and targeted for degradation via the ubiquitin–proteasome pathway (21). Lithium chloride (LiCl) is a known inhibitor of GSK-3β, and because it prevents β-catenin degradation, LiCl has been used extensively to promote Wnt signaling in vitro, including in cultured endothelial cells (29, 30). Administration of LiCl to pregnant mice rescues embryos with cardiac defects due to mutation of Wnt2 (31). We attempted a similar in vivo rescue of Brg1 mutant yolk sac vasculature to determine the degree to which β-catenin degradation contributes to vascular abnormalities.
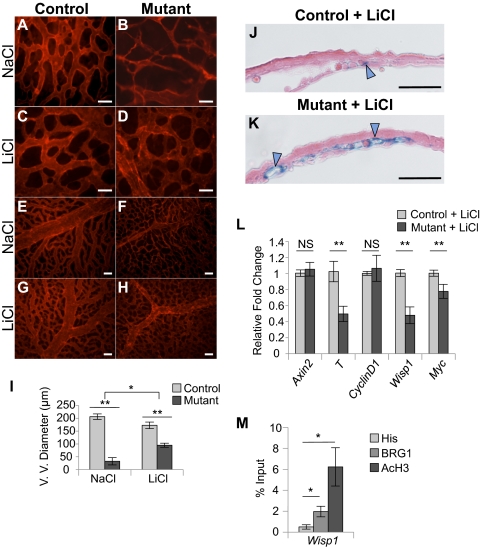
Following 2 d of injection with LiCl, we dissected and immunostained extraembryonic yolk sacs at E10.5 to assess the morphology and patterning of mutant blood vessels. We observed significant rescue of vessel morphology in Brg1 mutant yolk sacs that had been exposed to LiCl (Fig. 3 D, H, and I and Fig. S3D). LiCl-treated Brg1 mutant microvasculature was thicker and more interconnected than microvasculature from mutants treated with NaCl as a negative control (compare B and D in Fig. 3). Likewise, Brg1 mutant vitelline vessel diameters were partially rescued by LiCl treatment (Fig. 3 H and I). Mutant vitelline vessel diameters were 16% of control diameters with NaCl treatment and improved to 55% of control diameters with LiCl treatment (Fig. 3I). Furthermore, mutant yolk sacs displayed a modest rescue of vascular flattening following LiCl treatment, as assessed by histology and light microscopy (Fig. S3D).
Fig. 3.
Inhibition of β-catenin degradation with LiCl treatment rescues vascular anomalies and Wnt signaling in Brg1 mutant yolk sacs. (A–H) Pregnant mice were injected with 400 mg/kg NaCl (A and B, E and F) or LiCl (C and D, G and H) on E8.5 and E9.5. Embryos were dissected at E10.5, and littermate control Brg1fl/fl (A, C, E, G) and mutant Brg1fl/fl;Tie2-Cre+/0 (B, D, F, H) yolk sacs were whole-mount immunostained with antibodies against PECAM1. (A–D) (Scale bars: 50 μm.) (E–H) (Scale bars: 100 μm.) (I) Mean vitelline vessel (V. V.) diameter measurements from 10 control and 3 mutant yolk sacs treated with NaCl or from 12 control and 4 mutant yolk sacs treated with LiCl. Errors were calculated as ±SEM, and a two-tailed Student's t test was used to detect statistical differences in controls versus mutants or between NaCl- and LiCl-treated mutants (*P < 0.05; **P < 0.005). (J and K) Embryos carrying the BAT-gal Wnt reporter transgene were treated in utero with LiCl for 2 d before dissection at E10.5. X-gal–stained yolk sacs were cryosectioned and counterstained with eosin. Blue arrowheads, reporter-positive endothelium. (Scale bars: 50 μm.) (L) Endothelial cells from LiCl-treated littermate control and mutant yolk sacs were isolated, RNA was purified, cDNA was synthesized, and qPCR was carried out for Wnt target genes (Axin2, T, CyclinD1, Wisp1, and Myc). Errors represent ±SEM from six independent experiments, and significant differences between littermate controls and mutants were calculated using a two-tailed Student's t test (**P < 0.005). (M) Chromatin immunoprecipitation assays were carried out using isotype-matched antibodies against BRG1, acetylated histone H3 (AcH3) as a positive control, or a polyhistidine epitope tag (His) as a negative control. DNA was isolated and amplified by qPCR to determine whether BRG1 and AcH3 were associated with the promoter region of Wisp1. Data from four independent experiments were combined and are presented as the percentage (%) of input ±SEM; significant differences were calculated using a two-tailed Student's t test (*P < 0.05).
Importantly, LiCl treatment did not rescue the severe anemia that we previously described in Brg1 mutants (16). Brg1 mutant embryos and yolk sacs treated with LiCl or NaCl displayed a comparable pallor by gross observation and deficit of embryonic blood cells (Fig. S3 E–I). This finding settles a point of debate as to the impact of blood loss on vascular patterning in Brg1 mutants. Although alterations in blood flow biomechanics may contribute partially to Brg1 vascular phenotypes, our LiCl-mediated rescue of vessel patterning and morphology clearly indicates that a paucity of blood is not solely responsible for the mutant phenotypes. Altogether, these in vivo experiments provide evidence that deficient levels of β-catenin contribute to Brg1 mutant yolk sac vascular phenotypes.
Pharmacological Rescue of β-Catenin Degradation Restores Transcription of Selected Wnt Target Genes.
To determine whether Wnt signaling is rescued in Brg1 mutant yolk sac vasculature upon LiCl treatment, we analyzed embryos carrying the BAT-gal Wnt signaling reporter transgene. LiCl dramatically rescued reporter activity in Brg1 mutant yolk sac endothelial cells (Figs. 3K and Fig. S4H), which is consistent with the rescue of vascular morphological abnormalities in Brg1 mutant yolk sacs. Interestingly, LiCl also rescued reporter activity in Brg1 mutant placental endothelial cells, which underwent a striking loss of reporter activity in the absence of treatment (Fig. S4 I–N). However, we did not observe either placental abnormalities in Brg1 mutants or gross changes to placental vasculature with LiCl treatment.
We also isolated endothelial cells from control versus mutant yolk sacs for quantitative assessment of Wnt target genes that were down-regulated in untreated Brg1 mutants (Fig. 2D). Interestingly, of the five Wnt target genes that we assessed, Axin2 and CyclinD1 were rescued to normal transcript levels with LiCl treatment, whereas T, Wisp1, and Myc remained down-regulated (Fig. 3L). We hypothesized that the genes remaining down-regulated following LiCl treatment might require BRG1 for coregulation of their transcription. In support of this hypothesis, chromatin immunoprecipitation (ChIP) assays indicated that BRG1 associates with the promoter region of Wisp1 and that it is a direct target of BRG1 in endothelial cells (Fig. 3M). Previous studies have shown that BRG1 and β-catenin coregulate Wnt signaling in nonvascular cells by mediating chromatin remodeling and transcriptional activation of Wnt target genes (32, 33). Our data demonstrate that BRG1 similarly coregulates transcription of a subset of Wnt target genes in vascular endothelial cells.
BRG1 Mediates Transcription of Fzd Receptors.
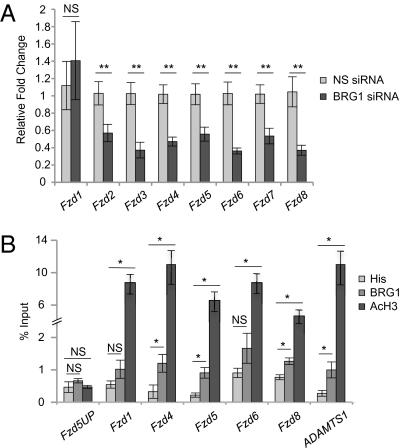
Because we found that β-catenin protein levels, but not transcript levels, were reduced upon depletion of BRG1, and because β-catenin is degraded in the absence of Wnt signaling (21), we decided to investigate the relationship between BRG1 and the Wnt signaling pathway upstream of β-catenin. Using a Wnt pathway qPCR array, we identified several Wnt receptor Fzd family members that were significantly down-regulated when BRG1 was knocked down in C166 yolk sac endothelial cells (Fig. S5B). We verified these data using qPCR with gene-specific primers and found that the transcript levels of Fzd2, -3, -4, -5, -6, -7, and -8 were significantly reduced upon BRG1 knockdown (Fig. 4A). Importantly, we performed qPCR on primary yolk sac endothelial cells isolated from control and mutant embryos and confirmed that the transcript levels of Fzd2, -3, -4, -7, and -8 were down-regulated in mutant cells (Fig. S5C). Fzd5 and Fzd6 were not significantly altered in the mutant primary endothelial cells, although it is possible to attribute this discrepancy to a lack of preparation purity compared with the yolk sac-derived C166 stable endothelial cell line. Nevertheless, we conclude that the significant down-regulation of several Fzd receptors upon deletion of Brg1 may contribute to the decrease in Wnt signaling observed in our mutant yolk sac vasculature.
Fig. 4.
BRG1 mediates expression of multiple Fzd receptors. (A) C166 cells were transfected with nonspecific (NS) or Brg1-specific siRNA for 48 h. RNA was isolated, cDNA was synthesized, and qPCR for Fzd1-8 was performed. Errors represent SEM, and significant differences between NS or Brg1-specific siRNA-transfected cells were calculated using a two-tailed Student's t test (**P < 0.005). (B) Endogenous chromatin from C166 cells was immunoprecipitated using isotype-matched antibodies against BRG1, acetylated histone H3 (AcH3) as a positive control or a polyhistidine epitope tag (His) as a negative control. DNA was isolated and amplified by qPCR to determine whether BRG1 and AcH3 were associated with the promoter regions of Fzd1, -4, -5, -6, or -8. A region upstream of the Fzd5 promoter was used as a negative control, and the ADAMTS1 promoter acted as a positive control gene region (17). Data from four independent experiments were combined and are presented as the percentage (%) input ±SEM; significant differences were calculated using a two-tailed Student's t test (*P < 0.05).
Because the transcript levels of several Fzd receptors were reduced with BRG1 deletion, we were interested in determining whether these Fzd genes are direct targets of BRG1. ChIP assays indicate that BRG1 associates with the promoter region of numerous Fzd receptor genes in C166 endothelial cells (Fig. 4B). We conclude that BRG1 epigenetically modulates vascular Wnt signaling through direct association and subsequent transcriptional activation of the Fzd family of Wnt receptors. Our data demonstrate a unique mechanism by which BRG1 influences the canonical Wnt pathway upstream of β-catenin.
Discussion
Wnt signaling has been gathering prominence in the field of embryonic vascular development for its recently defined roles in blood vessel patterning, specification, and angiogenesis (22, 25, 34, 35). However, a mechanistic understanding of Wnt signaling regulation in developing vasculature has been elusive. We now present evidence for epigenetic modulation of the Wnt pathway during blood vessel development by the chromatin-remodeling enzyme BRG1. Importantly, we demonstrate that BRG1 impacts the Wnt pathway at two different levels: through transcriptional regulation of multiple Fzd receptors and of Wnt target genes. Moreover, we show that vascular deletion of Brg1 results in aberrant yolk sac angiogenesis, which is partially rescued by stabilization of β-catenin (Fig. 5).
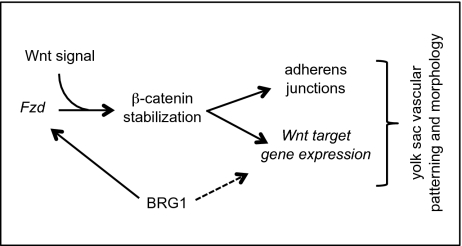
Fig. 5.
Model for how BRG1 impacts Wnt signaling during yolk sac vascular development. BRG1 epigenetically modifies Wnt signaling at two levels—through transcriptional regulation of multiple Fzd receptor genes and through subsequent association with β-catenin for regulation of certain downstream Wnt target genes. The dashed line indicates that BRG1 is not required for coregulation of all Wnt target genes in endothelial cells.
β-Catenin has two cellular roles: nuclear β-catenin is a key transcriptional activator of Wnt target genes, and cell-surface β-catenin helps anchor the transmembrane adhesion protein VE-cadherin to the actin cytoskeleton for adherens junction stabilization (36). We predict that Brg1 mutant yolk sac phenotypes are predominantly influenced by down-regulated β-catenin signaling rather than by junctional defects. We detect a loss of Wnt reporter activity in Brg1 mutant yolk sac endothelium in vivo, suggesting that nuclear β-catenin signaling is compromised in the absence of BRG1. We also demonstrate that a number of Wnt target genes are down-regulated upon Brg1 deletion in primary yolk sac endothelial cells. Taken together, these data indicate that BRG1 modulates nuclear Wnt signaling in yolk sac vasculature. Nevertheless, we do observe a reduction of β-catenin at the cell surface of cultured endothelial cells depleted of BRG1. Although we do not see changes in expression of other adherens junction proteins in these cells, we cannot exclude the possibility that down-regulated junctional β-catenin might contribute to Brg1 mutant yolk sac phenotypes.
β-Catenin was previously shown to recruit BRG1 for coregulation of Wnt target gene transcription in nonvascular cells (32, 33). Our studies demonstrate that a subset of Wnt target genes are similarly coregulated by BRG1 in endothelial cells. The Wnt target genes Wisp1, T, and Myc were down-regulated in Brg1 mutant endothelium—even after stabilization of β-catenin with LiCl treatment—implying that both β-catenin and BRG1 are necessary for their transcription. However, BRG1 is not required for coregulation of all Wnt target genes in endothelial cells because LiCl treatment rescued expression of Axin2 and CyclinD1 in Brg1 mutants. Perhaps these and other Wnt targets that do not require BRG1 for transcriptional regulation account for the partial but significant rescue of Brg1 mutant vascular phenotypes following β-catenin stabilization with LiCl.
The yolk sac specificity of the Brg1 mutant phenotypes that we have described is intriguing, given that Wnt signaling occurs concurrently in both embryonic and yolk sac vessels at midgestation (22) and that β-catenin vascular mutants display both embryonic and yolk sac vascular abnormalities (24). One explanation for this phenotypic specificity may lie in the Fzd genes that are targeted by BRG1. Global deletion of Fzd5 results in yolk sac-specific vascular phenotypes that are highly reminiscent of those seen in Brg1 mutants (23). We saw Fzd5 down-regulated following BRG1 depletion in endothelial cells, although its expression was not completely eliminated. Because Fzd5+/− embryos are phenotypically normal (23), our data indicate that partial down-regulation of Fzd5 is not solely responsible for Brg1 mutant yolk sac phenotypes. However, much functional redundancy is assumed to exist between the 10 mammalian Fzd receptors (21). We speculate that partial down-regulation of several Fzd receptors in the yolk sac creates a Wnt-signaling environment comparable to complete deletion of Fzd5. Little is known about the regulation of Fzd signaling, but our data provide evidence for the coordinated regulation of multiple Fzd receptors by a single chromatin-remodeling enzyme.
Another explanation for the yolk sac specificity of our Brg1 mutant phenotypes may lie in the functional redundancy between various chromatin-remodeling complexes. We previously assessed embryos deficient for both vascular Brg1 and the alternative SWI/SNF ATPase Brm, but the double mutants show no exacerbation of vascular phenotypes over those seen in the single Brg1 mutants (16). Therefore, other chromatin-remodeling complexes outside the SWI/SNF family may compensate for loss of Brg1 in embryonic vasculature and in non-Wnt signaling pathways in the yolk sac.
The significant rescue of Brg1 vascular mutant phenotypes by pharmacological stimulation of the Wnt signaling pathway provides convincing evidence that BRG1 epigenetically modifies Wnt signaling during yolk sac vascular development. One outstanding question is whether BRG1 plays a similar role at later developmental time points and during pathological angiogenesis. Wnt signaling regulates angiogenesis in the central nervous system during development of the blood–brain barrier (25, 34, 35) and in the retina during diabetic retinopathy (37). Induction of Brg1 vascular deletion at later time points and following pathological challenge will be required for a more comprehensive understanding of how BRG1 spatially and temporally influences vascular Wnt signaling under various angiogenic conditions.
Materials and Methods
Mice.
Brg1-floxed mice (Brg1fl/fl) (14), Tie2-Cre transgenic mice (38), and BAT-gal transgenic mice (39) were maintained on a mixed genetic background at the Oklahoma Medical Research Foundation animal facility. All animal use protocols were approved by the Institutional Animal Care and Use Committee. See SI Materials and Methods for genotyping information.
Cell Culture and Transfections.
C166 murine yolk sac endothelial cells (ATCC, no.CRL-2581) were maintained on Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS. Cells were transfected with 100 nM Brg1 siGENOME SMARTpool or nontargeting control siRNA (Thermo Scientific, catalog nos. M-041135–01 and D-001210–01, respectively) using Lipofectamine 2000 (Invitrogen) in serum-free OptiMEM (Invitrogen). After 48 h, cells were harvested in Laemmli buffer (62.5 mM Tris, pH 6.8, 10% glycerol, 5% SDS, 0.01% bromophenol blue) for Western blot analysis or TRIzol (Invitrogen) for transcript analysis.
Western Blot.
Total protein was harvested from siRNA-transfected C166 cells, fractionated in a 9% SDS polyacrylamide gel, and transferred to a PVDF membrane for Western blot analysis with antibodies to BRG1 (Santa Cruz Biotechnology, sc-17796), active β-catenin (Millipore, 05-665), α-catenin (BD Transduction Laboratories, 610193), desmoplakin (Abcam, ab16434), VE-cadherin (BD Pharmingen, 550548), and GAPDH (Sigma, G9545). Band intensity was determined for each protein using ImageJ software (National Institutes of Health).
Primary Endothelial Cell Isolation.
Yolk sacs from E10.5 embryos were digested with collagenase-DNase solution (1.5 mg/mL collagenase, 25 mg/mL DNase, 25 mM Hepes in DMEM) for 30 min at 37 °C. Cells were washed once with PBS/0.1% BSA and resuspended in DMEM. Dynabeads conjugated to PECAM1 antibody (BD Pharmingen, 557355) were added, and samples were incubated for 30 min at 4 °C with rotation. Immunoprecipitated cells were washed once with PBS/0.1% BSA and eluted in TRIzol (Invitrogen).
qPCR.
To analyze transcript levels, total RNA from siRNA-transfected C166 cells or from primary yolk sac endothelial cells was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. The DNA-free kit (Ambion) was used to digest any contaminating DNA. cDNA was prepared using the MultiScribe Reverse Transcriptase kit (Applied Biosystems), and real-time quantitative PCR was performed using SYBR Green PCR master mix (Applied Biosystems) and the ABI7000 thermocycler (ABI) with gene-specific primers.
ChIP.
ChIP was performed as described (16), with modifications (SI Materials and Methods).
Yolk Sac Staining.
Whole-mount yolk sac immunostaining and X-gal staining for β-galactosidase activity were performed as described (16), with modifications (SI Materials and Methods).
LiCl Injections.
LiCl or NaCl (400 mg/kg, dissolved in water) was injected intraperitoneally into pregnant females at E8.5 and E9.5. Embryos were harvested for yolk sac vascular analysis or endothelial cell isolation at E10.5.
Supplementary Material
Acknowledgments
We thank James Riddle and Kimberly Bowles for their genotyping assistance and helpful discussions; Elisabetta Dejana for advice on embryonic endothelial cell isolation; Florea Lupu (Oklahoma Medical Research Foundation) and Victoria Madden (University of North Carolina) for assistance with TEM; Colin Lickwar for participation in the early stages of this project; and Rodger McEver, Lijun Xia, and Scott Bultman for critical reading of this manuscript. This work was funded by National Institutes of Health grants (to C.T.G. and T.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013751108/-/DCSupplemental.
References
- 1.Becker PB, Hörz W. ATP-dependent nucleosome remodeling. Annu Rev Biochem. 2002;71:247–273. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- 2.Smith CL, Peterson CL. ATP-dependent chromatin remodeling. Curr Top Dev Biol. 2005;65:115–148. doi: 10.1016/S0070-2153(04)65004-6. [DOI] [PubMed] [Google Scholar]
- 3.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon CS, Wagner D. Unwinding chromatin for development and growth: A few genes at a time. Trends Genet. 2007;23:403–412. doi: 10.1016/j.tig.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Ho L, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci USA. 2009;106:5181–5186. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessard J, et al. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JI, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Mohrmann L, Verrijzer CP. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim Biophys Acta. 2005;1681:59–73. doi: 10.1016/j.bbaexp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Sudarsanam P, Winston F. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 2000;16:345–351. doi: 10.1016/s0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- 11.Bultman S, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 12.Bultman SJ, et al. Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev. 2006;20:1744–1754. doi: 10.1101/gad.1435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bultman SJ, Gebuhr TC, Magnuson T. A Brg1 mutation that uncouples ATPase activity from chromatin remodeling reveals an essential role for SWI/SNF-related complexes in beta-globin expression and erythroid development. Genes Dev. 2005;19:2849–2861. doi: 10.1101/gad.1364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebuhr TC, et al. The role of Brg1, a catalytic subunit of mammalian chromatin-remodeling complexes, in T cell development. J Exp Med. 2003;198:1937–1949. doi: 10.1084/jem.20030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hang CT, et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008;135:493–500. doi: 10.1242/dev.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankunas K, et al. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: An essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin AM, D'Amore PA. Wnt signaling in the vasculature. Angiogenesis. 2002;5:1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- 20.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corada M, et al. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–949. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa T, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Cattelino A, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebner S, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phng LK, et al. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SJ, Greer P, Auerbach R. Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell Dev Biol Anim. 1996;32:292–299. doi: 10.1007/BF02723062. [DOI] [PubMed] [Google Scholar]
- 28.Rudini N, Dejana E. Adherens junctions. Curr Biol. 2008;18:R1080–R1082. doi: 10.1016/j.cub.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 29.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 31.Tian Y, et al. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker N, et al. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JI, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daneman R, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenman JM, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 36.Dejana E. Endothelial cell-cell junctions: Happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009;175:2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 39.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.