Abstract
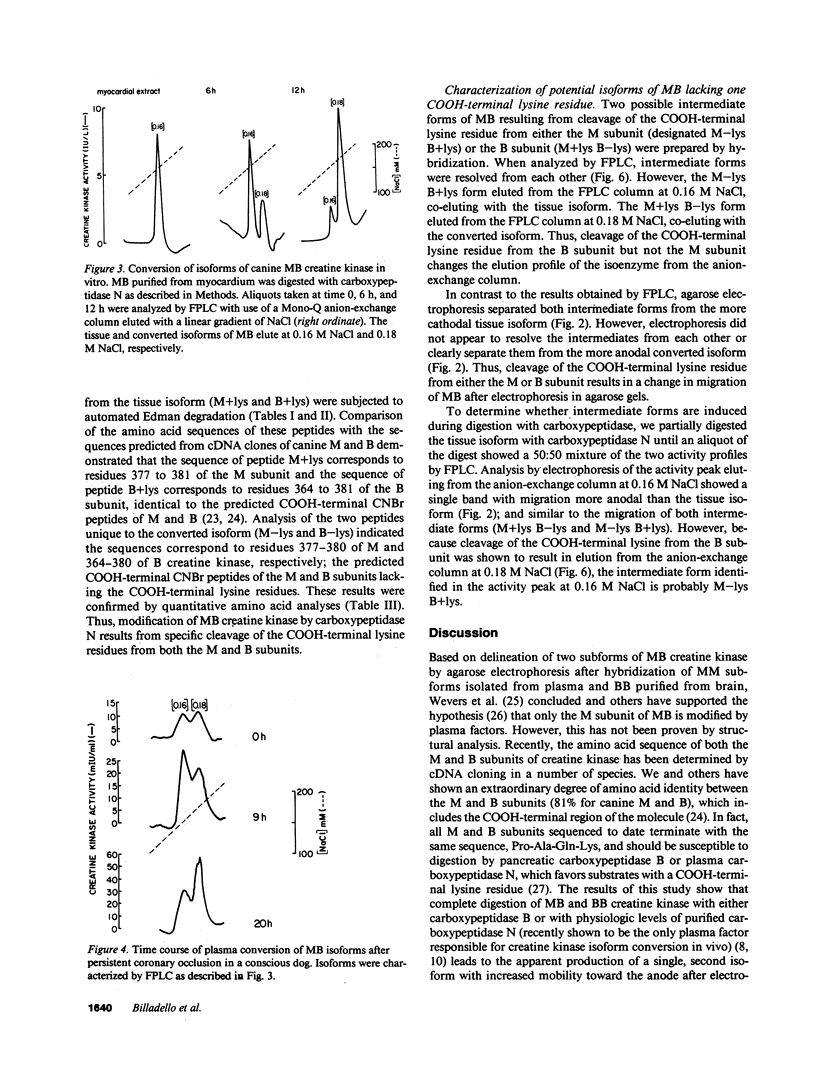
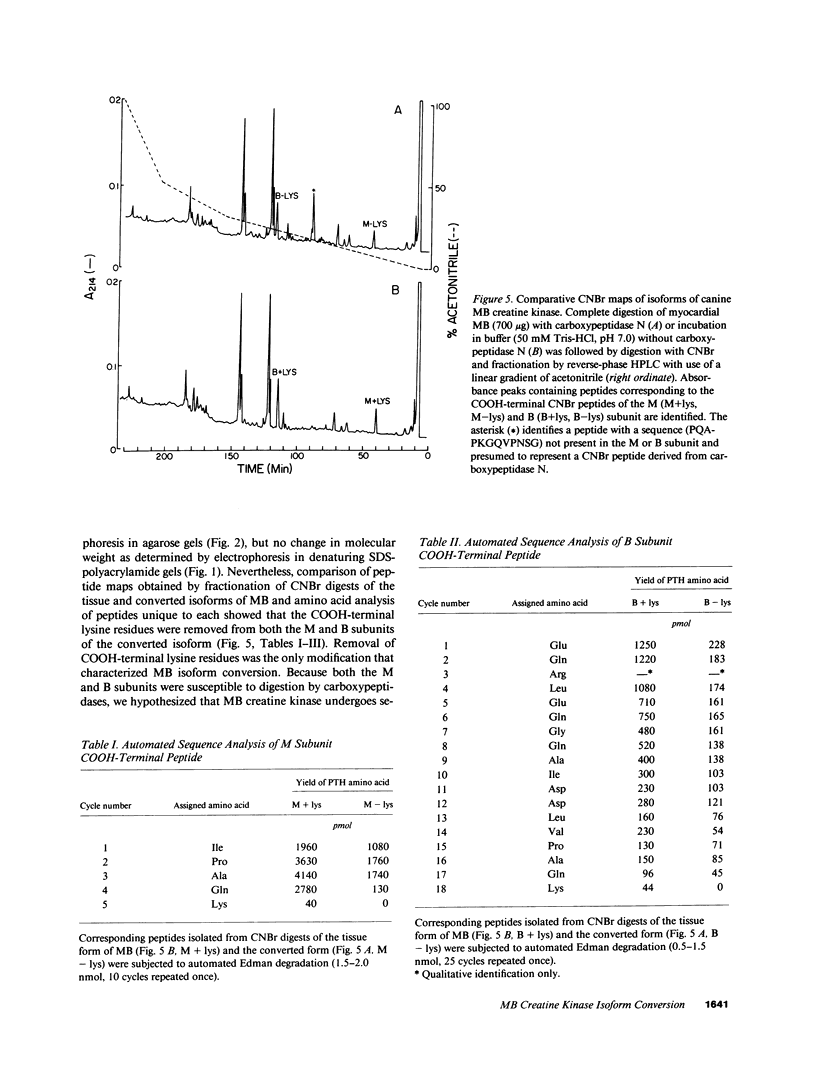
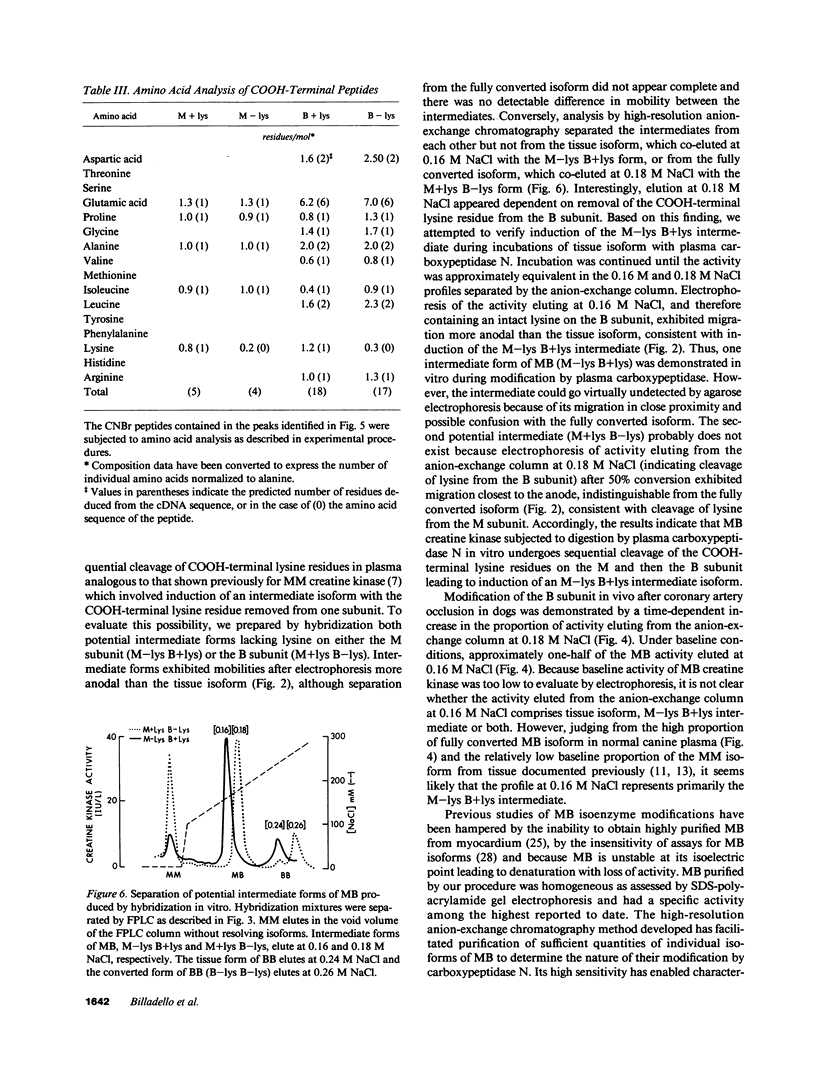
Time-dependent removal of the COOH-terminal lysine residue from each subunit of tissue MM creatine kinase by plasma carboxypeptidase N produces two additional isoforms that are readily separated, thereby permitting sensitive, early detection of acute myocardial infarction. Only two isoforms of MB creatine kinase have been detected in plasma leading to speculation that the COOH-terminal lysine on the B subunit is resistant to hydrolysis. To define the biochemical changes resulting in MB creatine kinase isoform conversion, we incubated highly purified MB creatine kinase from canine myocardium with plasma carboxypeptidase N. Quantitative anion-exchange chromatography of incubation mixtures and serial plasma samples from dogs subjected to coronary occlusion revealed a second, more acidic form evolved with time that was separated from the tissue isoform. Cyanogen bromide digestion of the two isoforms followed by amino acid sequencing of COOH-terminal peptides showed that MB creatine kinase undergoes removal of the COOH-terminal lysine residue from both M and B subunits. An intermediate form lacking lysine on the M subunit was delineated during incubations by the combined use of anion-exchange chromatography and conventional electrophoretic techniques. Thus, sequential cleavage of lysine from subunits of MB creatine kinase produces an intermediate isoform that has not been detected previously because of difficulties separating it from the tissue and fully converted isoforms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abendschein D. R., Serota H., Plummer T. H., Jr, Amiraian K., Strauss A. W., Sobel B. E., Jaffe A. S. Conversion of MM creatine kinase isoforms in human plasma by carboxypeptidase N. J Lab Clin Med. 1987 Dec;110(6):798–806. [PubMed] [Google Scholar]
- Billadello J. J., Kelly D. P., Roman D. G., Strauss A. W. The complete nucleotide sequence of canine brain B creatine kinase mRNA: homology in the coding and 3' noncoding regions among species. Biochem Biophys Res Commun. 1986 Jul 16;138(1):392–398. doi: 10.1016/0006-291x(86)90294-9. [DOI] [PubMed] [Google Scholar]
- Billadello J. J., Roman D. G., Grace A. M., Sobel B. E., Strauss A. W. The nature of post-translational formation of MM creatine kinase isoforms. J Biol Chem. 1985 Dec 5;260(28):14988–14992. [PubMed] [Google Scholar]
- Devries S. R., Sobel B. E., Abendschein D. R. Early detection of myocardial reperfusion by assay of plasma MM-creatine kinase isoforms in dogs. Circulation. 1986 Sep;74(3):567–572. doi: 10.1161/01.cir.74.3.567. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Grace A. M., Perryman M. B., Roberts R. Purification and characterization of human mitochondrial creatine kinase. A single enzyme form. J Biol Chem. 1983 Dec 25;258(24):15346–15354. [PubMed] [Google Scholar]
- Grace A., Roberts R. Improved procedures for purification of human and canine creatine kinase isoenzymes. Clin Chim Acta. 1982 Aug 4;123(1-2):59–71. doi: 10.1016/0009-8981(82)90114-0. [DOI] [PubMed] [Google Scholar]
- Hackel D. B., Reimer K. A., Ideker R. E., Mikat E. M., Hartwell T. D., Parker C. B., Braunwald E. B., Buja M., Gold H. K., Jaffe A. S. Comparison of enzymatic and anatomic estimates of myocardial infarct size in man. Circulation. 1984 Nov;70(5):824–835. doi: 10.1161/01.cir.70.5.824. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Abendschein D. R., Strauss A. W., Sobel B. E. Early detection of myocardial infarction in conscious dogs by analysis of plasma MM creatine kinase isoforms. Circulation. 1985 Feb;71(2):363–369. doi: 10.1161/01.cir.71.2.363. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Grace A. M., Billadello J. J., Gross R. W., Strauss A. W., Sobel B. E. Nondenaturing quantification of subforms of canine MM creatine kinase isoenzymes (isoforms) and their interconversion. J Lab Clin Med. 1984 Mar;103(3):470–484. [PubMed] [Google Scholar]
- Ingwall J. S., Kramer M. F., Fifer M. A., Lorell B. H., Shemin R., Grossman W., Allen P. D. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985 Oct 24;313(17):1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- Jaffe A. S., Serota H., Grace A., Sobel B. E. Diagnostic changes in plasma creatine kinase isoforms early after the onset of acute myocardial infarction. Circulation. 1986 Jul;74(1):105–109. doi: 10.1161/01.cir.74.1.105. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michelutti L., Falter H., Certossi S., Marcotte B., Mazzuchin A. Isolation and purification of creatine kinase conversion factor from human serum and its identification as carboxypeptidase N. Clin Biochem. 1987 Feb;20(1):21–29. doi: 10.1016/s0009-9120(87)80093-0. [DOI] [PubMed] [Google Scholar]
- Oshima G., Kato J., Erdös E. G. Plasma carboxypeptidase N, subunits and characteristics. Arch Biochem Biophys. 1975 Sep;170(1):132–138. doi: 10.1016/0003-9861(75)90104-6. [DOI] [PubMed] [Google Scholar]
- Panteghini M., Cuccia C., Malchiodi A., Calarco M., Pagnoni N. Isoforms of creatine kinase MM and MB in acute myocardial infarction: a clinical evaluation. Clin Chim Acta. 1986 Feb 28;155(1):1–9. doi: 10.1016/0009-8981(86)90093-8. [DOI] [PubMed] [Google Scholar]
- Perryman M. B., Knell J. D., Roberts R. Carboxypeptidase-catalyzed hydrolysis of C-terminal lysine: mechanism for in vivo production of multiple forms of creatine kinase in plasma. Clin Chem. 1984 May;30(5):662–664. [PubMed] [Google Scholar]
- Plummer T. H., Jr, Hurwitz M. Y. Human plasma carboxypeptidase N. Isolation and characterization. J Biol Chem. 1978 Jun 10;253(11):3907–3912. [PubMed] [Google Scholar]
- Plummer T. H., Jr, Kimmel M. T. An improved spectrophotometric assay for human plasma carboxypeptidase N1. Anal Biochem. 1980 Nov 1;108(2):348–353. doi: 10.1016/0003-2697(80)90598-9. [DOI] [PubMed] [Google Scholar]
- Puleo P. R., Perryman M. B., Bresser M. A., Rokey R., Pratt C. M., Roberts R. Creatine kinase isoform analysis in the detection and assessment of thrombolysis in man. Circulation. 1987 Jun;75(6):1162–1169. doi: 10.1161/01.cir.75.6.1162. [DOI] [PubMed] [Google Scholar]
- Roberts R., Gowda K. S., Ludbrook P. A., Sobel B. E. Specificity of elevated serum MB creatine phosphokinase activity in the diagnosis of acute myocardial infarction. Am J Cardiol. 1975 Oct 6;36(4):433–437. doi: 10.1016/0002-9149(75)90890-5. [DOI] [PubMed] [Google Scholar]
- Roberts R. Reperfusion and the plasma isoforms of creatine kinase isoenzymes: a clinical perspective. J Am Coll Cardiol. 1987 Feb;9(2):464–466. doi: 10.1016/s0735-1097(87)80406-0. [DOI] [PubMed] [Google Scholar]
- Roman D., Billadello J., Gordon J., Grace A., Sobel B., Strauss A. Complete nucleotide sequence of dog heart creatine kinase mRNA: conservation of amino acid sequence within and among species. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8394–8398. doi: 10.1073/pnas.82.24.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Vaidya H. C., Maynard Y., Dietzler D. N., Ladenson J. H. Direct measurement of creatine kinase-MB activity in serum after extraction with a monoclonal antibody specific to the MB isoenzyme. Clin Chem. 1986 Apr;32(4):657–663. [PubMed] [Google Scholar]
- Wevers R. A., Olthuis H. P., Van Niel J. C., Van Wilgenburg M. G., Soons J. B. A study on the dimeric structure of creatine kinase (EC 2.7.3.2). Clin Chim Acta. 1977 Mar 15;75(3):377–385. doi: 10.1016/0009-8981(77)90356-4. [DOI] [PubMed] [Google Scholar]
- Wevers R. A., Wolters R. J., Soons J. B. Isoelectric focusing and hybridisation experiments on creatine kinase (EC 2.7.3.2). Clin Chim Acta. 1977 Jul 15;78(2):271–276. doi: 10.1016/0009-8981(77)90316-3. [DOI] [PubMed] [Google Scholar]