Abstract
Steady-state development of plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs) requires the ligand for FMS-like tyrosine kinase 3 receptor (flt3L), but little is known about how other cytokines may also control this process. In this study, we show that IL-2 inhibits the development of both pDCs and cDCs from bone marrow cells under flt3L stimulation, by acting on lineage− flt3+ precursors. This inhibition of DC development by IL-2 requires IL-2Rα and IL2Rβ. IL-2Rα is specifically expressed in one stage of the DC precursor: the monocyte and DC progenitors (MDPs). Furthermore, more MDPs are found in flt3L-stimulated bone marrow cultures when IL-2 is present, suggesting that IL-2 may be inhibiting DC development at the MDP stage. Consistent with our in vitro findings, we observe that nonobese diabetic (NOD) mice, which express less IL-2 compared with diabetes-resistant NOD.Idd3/5 mice, have more splenic pDCs. Additionally, DCs developed in vitro in the presence of flt3L and IL-2 display reduced ability to stimulate T-cell proliferation compared with DCs developed in the presence of flt3L alone. Although the addition of IL-2 does not increase the apoptosis of DCs during their development, DCs developed in the presence of IL-2 are more prone to apoptosis upon interaction with T cells. Together our data show that IL-2 can inhibit both the development and the function of DCs. This pathway may have implications for the loss of immune tolerance: Reduced IL-2 signaling may lead to increased DC number and T-cell stimulatory capacity.
Dendritic cells (DCs) are potent antigen-presenting cells and are able to directly stimulate naive T cells for induction of either immunity or tolerance, depending on the context (1). Changes in either DC development or the number of DCs can alter both T-cell immunity and tolerance (2, 3). DCs can arise from both lymphoid and myeloid committed bone marrow (BM) precursors that lack markers of differentiated immune lineage (Lin) cells and express the FMS-like tyrosine kinase 3 receptor (flt3) (Lin−flt3+) (4). Accordingly, under steady-state conditions in vivo, the hematopoietic cytokine flt3 ligand (flt3L) is crucial for the development of the two main subsets of DCs: conventional DCs (cDCs) and plasmacytoid DCs (pDCs); the development of both populations can be modeled in vitro by stimulating BM with flt3L (4, 5). In peripheral lymphoid tissues, cDCs express high levels of CD11c, MHCII, and costimulatory molecules compared with pDCs. Both cDCs and pDCs can stimulate T cells, but pDCs are far less potent (6). Rather, pDCs are potent type I IFN producers (6). cDCs can be further divided into two subsets: CD8+ cDCs are specialized in cross-priming CD8+ T cells and polarizing Th1 responses, and CD11b+ cDCs preferentially induce both strong CD4 proliferation and Th2 responses (7).
DC development involves multiple stages of intermediate precursors, with each stage progressively more committed to becoming a DC (8). Myeloid progenitors (MPs), which can give rise to granulocytes, monocytes, and DCs, progress to monocyte and DC progenitors (MDPs) in the BM. MDPs can then become committed DC progenitors (CDPs) that can develop into both pDCs and cDCs. An intermediate step between CDPs and cDCs are the pre-cDCs that can be found in both the BM and the spleen.
Granulocyte macrophage colony-stimulating factor (GM-CSF) can also induce the differentiation of DCs from BM precursors, but mice lacking GM-CSF or its receptor had only a small decrease in DC numbers (9). Therefore, GM-CSF may be dispensable for steady-state DC maintenance and likely contributes mostly to DC generation from monocytes under inflammatory conditions (10). Furthermore, GM-CSF can block flt3L-driven development of pDC while promoting CD11b+ cDC growth from Lin−flt3+ precursors, by activating signal transducers and activation of transcription 5 (STAT5) that binds to the IFN regulatory factor 8 (Irf8) promoter, extinguishing its expression (11, 12). However, it is unclear whether other STAT5-activating cytokines can also control DC development, especially in a steady-state noninflammatory environment.
In this study, we have examined whether interleukin-2 (IL-2), another STAT5-activating cytokine, can affect DC development and/or function. IL-2 affects the function and development of many cell types including T cells and natural killer (NK) cells. Importantly, IL-2 and its high-affinity receptor, CD25/IL2Rα, are susceptibility genes for several human autoimmune diseases and related mouse models (13, 14). In autoimmune diabetes, decreased levels of IL-2 that lead to reduced Treg function and/or survival are thought to account for this susceptibility linkage (14, 15), but effects of IL-2 on other cell populations, including DCs, could also contribute to susceptibility. Therefore, knowledge of how IL-2 affects DCs is important for understanding the pathogenesis of autoimmune diseases such as type 1 diabetes. Here, we show that IL-2 can inhibit flt3L-dependent development of both pDCs and cDCs from Lin−flt3+ BM precursors. DC development appears to be blocked at the MDP stage. We also demonstrate that DCs developed in the presence of flt3L and IL-2 display markedly reduced T-cell stimulatory ability compared with DCs developed in the presence of flt3L alone. This decreased T-cell stimulation correlates with increased DC apoptosis upon interaction with T cells. Together our data suggest that IL-2 can regulate immune homeostasis via its effect on flt3L-dependent DC development and function.
Results
IL-2 Inhibits flt3L-Dependent pDC and cDC Development.
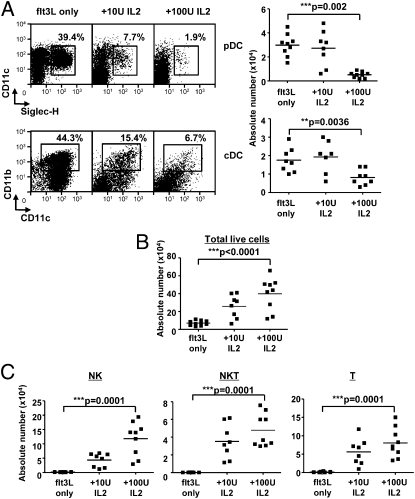
To examine the effect of IL-2 on DC development, we cultured total mouse BM cells in the presence of mflt3L (FL-BM) with or without IL-2 for 7 d and measured the percentage and number of pDCs and cDCs that develop. Here, we defined cDCs as CD11c+ CD11b+ and pDCs as CD11c+ sialic acid-binding Ig-like lectin H (Siglec-H)+. Other pDC markers such as B220 and CD317/BST2 are also expressed on other cell types (Fig. S1 and refs. 16 and 17), whereas Siglec-H is found specifically on pDCs both in vivo and in vitro in BM-derived cells (18). We observed that IL-2 can inhibit the development of both pDCs and cDCs in FL-BM cultures in a dose-dependent manner (Fig. 1A). This effect is distinct from the addition of GM-CSF to FL-BM cultures previously reported, which blocks pDC development but promotes CD11b+ cDC growth (11). In addition, we observed a dose-dependent increase in total cell numbers upon addition of IL-2 to FL-BM cultures (Fig. 1B). To determine what other cell types were increased with addition of IL-2, non-DC populations were characterized by staining for NK cells, B cells, NKT cells, and T cells. As expected from previous studies (19), we observed that IL-2 increased populations of NK, T, and NKT cells but not B cells in the FL-BM cultures (Fig. 1C).
Fig. 1.
IL-2 inhibits flt3L-dependent DC development and expands NK, NKT, and T cells. Total bone marrow (BM) cells were cultured with flt3L ± IL-2 and harvested on day 7 for flow cytometry. (A) Percentage (Left) and absolute number (Right) of pDCs (Upper) and cDCs (Lower). (B) Total number of live cells and (C) absolute numbers of NK (Left), NKT (Center), and T (Right) cells were measured. Absolute numbers are for 2 × 105 cells plated.
IL-2 Receptor Is Required for the Effect of IL-2 on DC Development.
The IL-2 receptor consists of the α-, β-, and γ-chains; and a family of cytokine receptors shares one or more of these chains, namely β and γ. We tested if the IL-2Rα chain, which is unique to IL-2, and the IL-2Rβ chain, which is shared with the IL-15 receptor, are required for inhibition of DC development. We found that anti-IL2Rα partially blocked IL-2–mediated inhibition of DC development (Fig. 2A). This result is in line with previous T-cell studies showing that IL-2 can signal without the IL2Rα chain at high doses (20). Blocking IL2Rβ chain, which is crucial for IL-2 signaling, can block the IL-2–mediated inhibition of DC development even at a high dose of IL-2 (Fig. 2B).
Fig. 2.
IL-2 receptor signaling is required for IL-2–mediated inhibition of DC development. Total BM cells were cultured with flt3L ± IL-2. αIL2Rα/CD25 (A) or αIL2Rβ/CD122 (B) (or their isotype controls) was added as indicated. Cells were harvested on day 7 for flow cytometry analyses. Absolute numbers are for 2 × 105 cells plated.
IL-2 Controls flt3L-Dependent DC Development by Acting on Lineage− flt3+ DC Precursors.
To determine if the effect of IL-2 on DCs occurs indirectly via its effect on other lineages, we sorted Lin−flt3+ cells, a population enriched for DC precursors (4) (Fig. S2A). We observed that flt3L induced expansion of these precursors that give rise to both pDCs and cDCs, which were inhibited upon addition of IL-2 (Fig. 3A). In addition, NK, NKT, and T cells were almost completely absent in these cultures (Fig. 3B), suggesting that the effect of IL-2 is not indirectly via an effect on developed or arising NK, NKT, and/or T cells. Conversely, Lin− Flt3− cells gave rise to these lymphoid populations, but not DCs (Fig. 3 A and B). Together these data confirm that IL-2 affects flt3L-dependent DC development by acting on Lin−flt3+ DC precursors. We also assessed the levels of another subset of cDCs expressing CD24, described to be the functional and phenotypic equivalent of CD8+ splenic cDCs (21) (Fig. S3A). When starting with an enriched population of Lin−flt3+ precursors, the development of CD24hi cDCs was also inhibited by IL-2 (Fig. 4A).
Fig. 3.
IL-2 blocks the development of DCs from Lin−flt3+ DC precursors. Lin−flt3+ cells or Lin−flt3− BM cells were cultured for 7 d with flt3L ± IL-2 and analyzed by flow cytometry. Plots were gated on (A) pDCs or cDCs and (B) NK, NKT, or T cells. Absolute numbers are for 5 × 104 cells plated.
Fig. 4.
IL-2 inhibits flt3L-dependent development of CD24hi cDCs and does not affect DC survival. Lin−flt3+ BM cells were cultured with flt3L ± IL-2 and harvested for flow analysis of (A) the absolute number and (B) percentage of apoptotic cells [annexin V(anxV)+] among pDCs (Top), CD11bhi DCs (Middle), and CD24hi DCs (Bottom) after the indicated days of culture. (C) Representative histograms for anxV staining on day 7 of culture are shown. Absolute numbers are for 2 × 104 cells plated.
Inhibitory Effect of IL-2 on DC Development Is Not Associated with an Increase in the Apoptosis of DC Subsets.
We next asked whether the IL-2–induced inhibition of DC development was due to increased apoptosis of developing DCs. Lin−flt3+ BM cells were cultured with flt3L ± IL-2 as above, and cells were collected at different time points. Early stages of apoptosis were detected by annexin V (anxV) staining. We observed no significant difference in the proportion of pDCs, CD11bhi cDCs, or CD24hi DCs that expressed anxV when IL-2 was added (Fig. 4 B and C). Therefore, the effect of IL-2 on DC development is not due to increased DC apoptosis in FL-BM cultures.
IL-2 Receptor Is Expressed on MDPs upon flt3L Stimulation and More MDPs Are Found in FL-BM Cultures When IL-2 Is Added.
Although Lin−flt3+ BM cells are enriched for DC precursors, it is a heterogenous population containing progenitors of different stages of DC development, including MPs, MDPs, and CDPs that can be distinguished phenotypically by their expression of c-kit, CX3CR1, and CD115 (8) (Fig. S3 C and D). To identify which of these DC progenitors can respond optimally to IL-2, we measured IL-2Rα expression on Lin−CD11c− cells stimulated with flt3L ± IL-2. To differentiate between CX3CR1− MPs and CX3CR1+ MDPs, we used BM from CX3CR1gfp/+ mice (Fig. S3D). Both the level of IL-2Rα expression on MDPs and the absolute number of MDPs expressing IL-2Rα increase after flt3L stimulation (Fig. 5A). These data suggest that flt3L can induce the expression of IL-2Rα on MDPs, predisposing them to the effect of IL-2.
Fig. 5.
IL-2 receptor is expressed specifically at the MDP stage of DC development and more MDPs are found in the presence of IL-2. (A) Lin−CD11c− cells from CX3CR1gfp/+ mice were cultured in media only, flt3L, or flt3L + 100 units/mL IL-2 for 18–20 h before flow analysis. IL2Rα expression on MPs, MDPs, and CDPs is shown. Absolute numbers are for 105 cells plated. (B) Lin−flt3+ cells were cultured with flt3L ± IL-2 and harvested at day 7 for flow analysis. CD11c− cells (gated at Top) were analyzed for CD115 and c-kit expression (Middle). (Bottom) CDPs and MDPs were gated as shown and quantified. Absolute numbers are for 5 × 104 cells plated.
Furthermore, upon addition of IL-2, we also observed an increase in two distinct cell populations: CD11c− and CD11c+SiglecH−CD24−CD11b− (Fig. S3A). To identify whether the CD11c− population are DC progenitors, we measured the expression of c-kit and CD115. We found that without IL-2, the few CD11c− cells present are mainly CD115+ c-kitlo/−, phenotypically similar to CDPs (Fig. 5B). In contrast, upon addition of IL-2, the CD11c− cells are mostly CD115− c-kithi, resembling the phenotype of MDPs (Fig. 5B). With the addition of IL-2, the proportion and absolute number of MDPs increase and the proportion and absolute number of CDPs decrease. This result correlates with our earlier data showing that IL-2Rα is predominantly expressed on MDPs upon flt3L stimulation (Fig. 5A). To define the second population of cells that arise upon addition of IL-2 (CD11c+SiglecH−CD24−CD11b−), we measured expression of MHCII and SIRPα. We find that this population of cells is MHCII− SIRPαlo/− (Fig. S3B), resembling the phenotype of pre-cDCs (8). Together our data suggest that the inhibitory effect of IL-2 on DC development is predominantly on the differentiation and expansion of a specific subset of DC progenitors: MDPs.
Decreased IL-2 Signaling in Vivo Correlates with Increased pDC Frequencies.
IL-2 signals mainly via phosphorylation of STAT5. Because a complete STAT5 knockout is neonatally lethal, conditional STAT5 knockout mice (tie2-cre, stat5fl/fl) were generated in which the STAT5 locus was deleted in hematopoietic stem cells and endothelial cells (22). We found a significantly increased proportion of pDCs in the spleens and BMs of these conditional STAT5 knockout mice compared with wild-type controls (Fig. 6A, Left); cDC numbers were not significantly increased in these mice although a similar increasing trend can be observed (Fig. 6A, Right). These data are consistent with our hypothesis that in the absence of IL-2/STAT5 signaling, there is less inhibition on DC development. However, the absence of signaling by other STAT5-dependent cytokines could also contribute to the observed phenotype.
Fig. 6.
Decreased IL-2 signaling in vivo correlates with increased frequency of pDCs. (A) Cells from the indicated tissues were isolated from either stat5fl/fl,tie2-cre+ mice (stat5 deleted) or control Cre− mice, and the proportion of pDCs and cDCs was determined by flow cytometry. (B) Spleen cells were isolated from NOD and NOD.Idd3/5 mice, and the number of pDCs was determined by flow cytometry. Each dot represents one mouse.
Nonobese diabetic (NOD) mice, a model for spontaneous autoimmune diabetes, are known to produce less IL-2 compared with B6 mice (14). NOD.Idd3/5 mice, a congenic strain that has the B10 or B6 alleles for Idd5 and for Idd3 that contains IL-2, are almost completely protected from the development of diabetes (diabetes incidence at 30 wk of age: 80% in NOD vs. <3% in NOD.Idd3/5) and express more IL-2 compared with NOD mice (23). These mice provide another model in which the effect of different levels of IL-2 on DCs can be assessed. We measured the number of pDCs in the spleens of these two strains and found that NOD mice had significantly more pDCs compared with NOD.Idd3/5 (Fig. 6B). Therefore, in two in vivo models, reduced levels of IL-2 signaling or IL-2 production correlate with an increase in pDCs.
Presence of IL-2 During flt3L-Dependent DC Development Alters DC Phenotype and Reduces DC Function.
We next examined the phenotype and function of DCs derived in the presence of flt3L and IL-2. We observed that FL-cDCs developed in the presence of IL-2 (FL+IL2-cDCs) express increased levels of major histocompatability complex II (MHCII) and costimulatory molecules including CD40, CD80, CD86, and programmed death ligand 1 (PD-L1) (Fig. 7A). pDCs express far lower levels of costimulatory markers and MHCII as previously reported (6), but nonetheless the presence of IL-2 during their development can enhance their surface expression of costimulatory proteins (Fig. 7B). For comparison, Toll-like receptor ligands (TLR-L) [Lipopolysaccharride (LPS) and CpG-2216] were added for the last 18–22 h of the FL-BM culture (Fig. 7 A and B). The levels of costimulatory molecules observed in FL+IL2-DCs were lower than those induced by TLR-L stimulation, suggesting that these FL+IL2-DCs display a semimature phenotype.
Fig. 7.
The presence of IL-2 during DC development reduces T-cell stimulatory ability of DCs and predisposes DCs to apoptosis. (A and B) Total BM cells were cultured with flt3L ± IL-2. For +TLRL, LPS (A) or CpG-2216 (B) was added to FL-BM cultures on day 6. Cells were harvested on day 7 for flow cytometry. (C and D) Sorted cDCs were cocultured with naive B6 or OTII CD4+CD25− splenic T cells under the indicated stimulation. (C) After 3 d of coculture, CFSE dilution and CD25 expression were analyzed. (D) Cells were harvested after 16–20 h of coculture to compare anxV expression and CD25 expression.
To examine whether the presence of IL-2 during flt3L-dependent DC development also alters DC function, we cultured FL-BM for 7 d with or without low-dose (10 units/mL) IL-2 and then sorted for pDCs and cDCs (Fig. S2C). To examine cytokine secretion by DCs, the sorted DCs were plated out with or without TLR stimulation (CpG-2216 for pDCs; LPS for cDCs). We observed that without TLR stimulation, the DCs did not secrete proinflammatory cytokines; and upon TLR stimulation, there was no significant difference in secretion of either IL-6 or IFN-α between DCs developed with and without IL-2, whereas FL+IL2-cDC secreted increased tumor necrosis factor-α (TNF-α) (Fig. S4 A and B). Although no IL-12 was detected after stimulation with LPS, we also stimulated FL-cDCs with CpG-1826, a stronger IL-12 stimulator for these cells (21). With CpG-1826 stimulation, FL+IL2-cDCs produced less IL-12 than FL-cDCs (Fig. S4A).
To test the T-cell stimulatory function of FL+IL2-DCs, the sorted cDCs were cocultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled naive CD4+CD25− splenic T cells from either B6 or T-cell receptor transgenic ovalbumin (OVA)-specific OTII mice, using either soluble anti-CD3 or OVA peptide 323–339, respectively, for stimulation. We observed reduced proliferation and activation of naive T cells induced by FL+IL2-cDCs compared with FL-cDCs (Fig. 7C). Similar DC–T-cell cocultures were also set up using DCs derived from purified Lin-flt3+ BM precursors rather than total BM, and a decrease in the ability of FL+IL2-DCs to stimulate T cells, compared with FL-DCs, was also observed (Fig. S5A). This result suggests that the IL-2-dependent change in DC function is not due to indirect effects from other cell types, such as NK, T, and NKT cells, that arise in the total BM cultures when IL-2 is present.
To determine why FL+IL2-DCs displayed decreased stimulatory capacity, we assayed several known regulatory pathways. A similar low level of forkhead box P3 (Foxp3)+ cells (indicative of regulatory T cells) was observed in all cultures (Fig. S6 A and B). No IL-10 or IL-12 was detected in the DC–T-cell coculture supernatants (Fig. S6C). However, because FL+IL2-cDCs secrete less IL-12 upon CpG-1826 stimulation (Fig. S4A), we tested whether blocking IL-12 activity affected T-cell proliferation. Addition of anti-IL-12p40 did not change the level of CFSE dilution induced by either FL-cDCs or FL+IL2-cDCs (Fig. S6D). We also measured cytokine levels directly from day 7 of FL-BM cultures ± IL-2 (Fig. S4C). We detected no IL-12 secretion from any of the supernatants. We observed a low level of IL-10 secretion from Lin−flt3+ BM-derived FL+IL2-cultures, but not from total BM-derived FL+IL2 cultures. Because IL-2 inhibits T-cell stimulatory capacity of DCs derived from both total BM and Lin−flt3+ BM (Fig. 7 and Fig. S5), this IL-10 production is not likely to account for the differences observed. Together these data show that differences in Foxp3+ regulatory T cells or IL-10 or IL-12 production do not account for the reduced ability of FL+IL2 DCs to stimulate T-cell proliferation.
We next assessed the level of DC apoptosis in the cocultures. Although most DCs die by the end of the 3-d DC–T cocultures (when T-cell proliferation is assessed), increased DC apoptosis upon T-cell interaction at early time points can decrease T-cell stimulation. Interestingly, at 16–20 h of DC–T-cell coculture, we observed more DC apoptosis, measured by an increased proportion of anxVhi cells among CD11c+ cells with FL+IL2-DCs, which inversely correlated with CD25 expression on T cells at this early time point (Fig. 7D), suggesting that increased apoptosis of FL+IL2-DCs results in reduced T-cell activation. Similar results were also obtained using DCs derived from purified Lin−flt3+ BM precursors (Fig. S5B).
Discussion
IL-2 is recognized for its wide spectrum of effects on lymphocytes, including T-cell homeostasis and NK cell development (19). In this study we reveal a role for IL-2 in DC development and function. We have shown that the addition of IL-2 can inhibit flt3L-dependent development of pDCs and both subsets of cDCs from BM cells in vitro, an effect that is not mediated by increased DC apoptosis during their development. By using Lin−flt3+ cells, a population enriched for DC precursors and that does not develop into other lymphoid cells, we separate the effect of IL-2 on DCs from any effect on fully developed or developing NK, T, or NKT cells. Our data also suggest that IL-2 inhibits DC development at the MDP progenitor stage, before the progenitors commit to becoming either a monocyte/macrophage or a common DC precursor. We noted that IL-2 also increases a population that resembles pre-cDCs, suggesting that precursor cells that are not stopped at the MDP stage may be blocked later at the pre-cDC stage.
In addition to the effect of IL-2 on flt3L-dependent DC development, IL-2 can also abrogate the ability of these DCs to stimulate T cells via an increase in DC apoptosis upon interaction with T cells. It is currently unclear why increased DC apoptosis is observed in these DC–T cocultures. Productive T-cell interaction with DCs usually provides a survival signal to DCs (24), so perhaps FL+IL2-cDCs either do not interact as well with T cells or are less responsive to T-cell–mediated survival signals.
IL-2 is also important for the maintenance of immune homeostasis: IL-2 promotes the growth of T cells during an immune response, but can also limit T-cell responses by predisposing activated T cells to apoptosis and by enhancing the survival and expansion of regulatory T cells (15, 25). Our findings suggest an additional role of IL-2 during an immune response: By reducing the number and function of DCs, IL-2 may indirectly dampen T-cell responses and restore immune homeostasis. Because MDPs are found in the BM, this is likely to be where IL-2 could exert this function in vivo (26); activated and/or memory T cells found in the BM may be a cellular source of IL-2 (27).
In the context of autoimmunity, the IL2 and IL2Ra genes are associated with human type 1 diabetes and other autoimmune diseases (13). In mice, the IL-2 gene is located at the insulin-dependent diabetes (Idd) locus Idd3 that confers diabetes susceptibility in NOD mice and is also linked to other autoimmune diseases. In NOD mice, reduced IL-2 results in decreased survival of regulatory T cells and progressive breakdown of self-tolerance (14). Our data suggest that IL-2 may also modulate autoimmune susceptibility via effects on DC homeostasis: Lower levels of IL-2 could result in more DCs and their increased ability to stimulate T cells. Accordingly, we have observed enhanced numbers of pDCs in NOD mice compared with the non-diabetes-prone NOD.Idd3/5 mice, which is consistent with an effect of IL-2 on DCs in vivo, but could also be due to other genes at these two loci. A more direct characterization of the effects of IL-2 on DCs in vivo is needed. Our observation also suggests that pDC may be pathogenic in the context of autoimmune diabetes as increased pDC number correlates with diabetes. Although pDCs play a tolerogenic role in experimental autoimmune encephalomyelitis (EAE) (28, 29), pDCs are implicated to be pathogenic in many other autoimmune conditions including lupus, arthritis, and psoriasis (30, 31): pDCs may contribute to type 1 diabetes pathogenesis via their production of type 1 IFN that can initiate diabetes in NOD mice (32–34). Interestingly, IFN-α therapy used to treat patients infected with hepatitis C virus can precipitate autoimmune diabetes (35).
Our findings may also have implications for the therapeutic use of IL-2 in cancer. In the context of tumor responses, IL-2 treatment can reduce tumor growth via the activation of NK cells (36), but inhibition of DC development and function induced by IL-2 could potentially dampen the antitumor responses (37, 38). Interestingly, when IL-2 was used in patients with advanced melanoma and renal cell cancer, a decrease in the frequencies of both circulating myeloid and plasmacytoid DCs was observed, consistent with our results (39).
Overall, our study has revealed a significant role of IL-2 for controlling flt3L-dependent development and function of DCs. These findings have important implications for our current view of events that can lead to the loss of immune tolerance in autoimmune diseases as well as for the therapeutic use of IL-2.
Materials and Methods
Animals.
Six- to 10-wk-old C57BL/6 mice, CX3CR1gfp/+ heterozygous mice (40), conditional STAT5KO (tie2-cre, STAT5fl/fl) mice (for details, see SI Materials and Methods), or 6- to 8-wk-old NOD or NOD.Idd3/5 mice were used. All animals were housed in specific pathogen-free conditions and handled according to protocols approved by the National Institutes of Health animal care and use committee.
Isolation and Culture of FL-BMDCs.
Total bone marrow cells were collected and cultured as described previously (12) and in SI Materials and Methods, in media supplemented with 100 ng/mL mouse flt3L (mflt3L) (Peprotech) for 7 d. Human IL-2 (hIL-2) (R&D Systems) was added as indicated.
Flow Cytometry.
Cells were treated with Fc block (anti-CD16/32) and stained with fluorochrome-conjugated antibodies. Details of antibodies used can be found in SI Materials and Methods.
Isolation of Lin− flt3+ Cells.
Total BM cells were depleted of Lineage+ cells as listed in SI Materials and Methods. To sort flt3+ cells, Lin− cells were stained with anti-mouse PE-flt3 (A2F10) (eBioscience) and isolated using FACS Aria (BD Biosciences), as shown in Fig. S2A.
Testing T-Cell Stimulatory Capacity of Dendritic Cells.
CD4+CD25− T cells were labeled with CFSE and cocultured with sorted DCs and either anti-mouse CD3 (145-2C11) (Biolegend) or OVA peptide 323–339 (Anaspec). Cells were harvested after either 16–20 h or 3 d for analyses of cell viability, CFSE dilution, and T-cell activation (details in SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Alice Franks, the flow cytometry core facilities at the National Heart, Lung and Blood Institute and the National Institute of Allergic and Infectious Disease, and Helen Su and Qian Zhang for technical support, and Dr. Yasmine Belkaid for critical reading of the manuscript. This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Cancer Institute, and National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009738108/-/DCSupplemental.
References
- 1.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darrasse-Jèze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 4.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenna HJ, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 6.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vremec D, et al. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur J Immunol. 1997;27:40–44. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- 10.Naik SH, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 11.Esashi E, et al. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity. 2008;28:509–520. doi: 10.1016/j.immuni.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilliet M, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wicker LS, Santamaria P. IL-2 and its high-affinity receptor: Genetic control of immunoregulation and autoimmunity. Semin Immunol. 2009;21:363–371. doi: 10.1016/j.smim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Yamanouchi J, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segura E, Wong J, Villadangos JA. Cutting edge: B220+CCR9- dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol. 2009;183:1514–1517. doi: 10.4049/jimmunol.0901524. [DOI] [PubMed] [Google Scholar]
- 17.Blasius AL, et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 18.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Nelson BH, Lord JD, Greenberg PD. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature. 1994;369:333–336. doi: 10.1038/369333a0. [DOI] [PubMed] [Google Scholar]
- 21.Naik SH, et al. Cutting edge: Generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 22.Zhu BM, et al. Hematopoietic-specific Stat5-null mice display microcytic hypochromic anemia associated with reduced transferrin receptor gene expression. Blood. 2008;112:2071–2080. doi: 10.1182/blood-2007-12-127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton-Williams EE, et al. Expression of diabetes-associated genes by dendritic cells and CD4 T cells drives the loss of tolerance in nonobese diabetic mice. J Immunol. 2009;183:1533–1541. doi: 10.4049/jimmunol.0900428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkel KD, Kronin V, Krummel MF, Shortman K. The nature of the signals regulating CD8 T cell proliferative responses to CD8alpha+ or CD8alpha- dendritic cells. Eur J Immunol. 1997;27:3350–3359. doi: 10.1002/eji.1830271234. [DOI] [PubMed] [Google Scholar]
- 25.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 27.Di Rosa F. T-lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol Cell Biol. 2009;87:20–29. doi: 10.1038/icb.2008.84. [DOI] [PubMed] [Google Scholar]
- 28.Bailey-Bucktrout SL, et al. Cutting edge: Central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irla M, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G. Type I interferon: Friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, et al. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart TA, et al. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- 34.Downes K, et al. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS ONE. 2010;5:e12646. doi: 10.1371/journal.pone.0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devendra D, Eisenbarth GS. Interferon alpha—a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin Immunol. 2004;111:225–233. doi: 10.1016/j.clim.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Jin GH, Hirano T, Murakami M. Combination treatment with IL-2 and anti-IL-2 mAbs reduces tumor metastasis via NK cell activation. Int Immunol. 2008;20:783–789. doi: 10.1093/intimm/dxn036. [DOI] [PubMed] [Google Scholar]
- 37.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 38.Salio M, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 39.van der Vliet HJ, et al. Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res. 2007;13:2100–2108. doi: 10.1158/1078-0432.CCR-06-1662. [DOI] [PubMed] [Google Scholar]
- 40.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.