Abstract
Transgenic (Tg) mouse models of Alzheimer's disease have served as valuable tools for investigating pathogenic mechanisms related to Aβ accumulation. However, assessing disease status in these animals has required time-consuming behavioral assessments or postmortem neuropathological analysis. Here, we report a method for tracking the progression of Aβ accumulation in vivo using bioluminescence imaging (BLI) on two lines of Tg mice, which express luciferase (luc) under control of the Gfap promoter as well as mutant human amyloid precursor protein. Bigenic mice exhibited an age-dependent increase in BLI signals that correlated with the deposition of Aβ in the brain. Bioluminescence signals began to increase in 7-mo-old Tg(CRND8:Gfap-luc) mice and 14-mo-old Tg(APP23:Gfap-luc) mice. When Tg(APP23:Gfap-luc) mice were inoculated with brain homogenates from aged Tg(APP23) mice, BLI detected the accelerated disease onset and induced Aβ deposition at 11 mo of age. Because of its rapid, noninvasive, and quantitative format, BLI permits the objective repeated analysis of individual mice at multiple time points, which is likely to facilitate the testing of Aβ-directed therapeutics.
Keywords: optical imaging, prion-like transmission, seeding
Alzheimer's disease (AD) is a progressive and fatal neurodegenerative disorder, and it is the most common cause of dementia in adults. Neuropathologically, AD is characterized by the accumulation of extracellular amyloid deposits in the brain composed of aggregated Aβ peptide as well as intracellular neurofibrillary tangles, which are made up of hyperphosphorylated and aggregated tau protein. The Aβ peptide is generated by sequential endoproteolytic cleavage of the amyloid precursor protein (APP) by β- and γ-secretase enzymes. The amyloid cascade hypothesis of AD states that increased levels of Aβ peptide in the brain, caused by aging, mutation in APP, or mutation in presenilin, result in the gradual aggregation of Aβ into higher-order structures that are neurotoxic (1–4).
The deposition of Aβ in the brain in the form of extracellular plaques has been accurately recapitulated in transgenic (Tg) mice overexpressing human APP containing mutations linked to familial AD (5–7). In these models, the kinetics of Aβ deposition are determined by the transgene expression level and the combination of mutations used. Two widely used AD mouse models, Tg(APP23) and Tg(CRND8) mice, develop robust AD-like Aβ pathology in their brains characterized by high cerebral Aβ(1-40) and Aβ(1-42) levels (8, 9). Mice from both lines show spatial learning and memory deficits, mimicking the prominent loss of cognitive function observed in patients with AD (10, 11). Consequently, these and similar mouse models have been extensively used to study the biochemistry of amyloid deposition and the effects of genetic modifiers on amyloid formation as well as to assess the efficacy of potential AD therapeutics.
Limiting the utility of these current AD mouse models, the Tg mice do not typically show overt progressive signs of neurological illness and do not die in response to the prominent amyloidosis present in their brains. Thus, in vivo assessment of AD-like pathology in the brains of these mice has proven challenging. Although neuropathological analysis of brain provides a definitive diagnosis, it can only be accomplished postmortem. Although behavioral tests such as the Morris water maze (12) can be used to assess the cognitive status of the mouse, these tests are labor-intensive, low throughput, and fraught with procedural difficulties and genetic background artifacts (13). Other maze techniques, such as the radial arm maze, are comparatively more rapid but still require large numbers of mice (14). Although imaging techniques might be useful, they have their limitations as well. Cranial window imaging requires complicated neurosurgical procedures (15). Amyloid imaging using radiolabeled tracers such as PiB (16), which has been effective for visualizing amyloid in humans, has not shown success when applied in Tg mouse models of AD (17). Therefore, a rapid and quantitative method is needed to assess the neuropathological status of living mice.
Recently, we showed that prion disease in mice can be diagnosed at ∼55 d postinoculation, less than one-half the time to the onset of clinical symptoms, by using Tg mice, which express firefly luciferase under the control of the glial fibrillary acidic protein (GFAP) promoter, denoted Tg(Gfap-luc) mice (18, 19). In these mice, astrocytic gliosis increases as prion disease progresses; this results in the up-regulation of Gfap mRNA and hence, luciferase protein, which can be visualized in live mice by using bioluminescence imaging (BLI) after injection of the mice with the luciferase substrate d-luciferin. Because reactive astrocytic gliosis occurs in the brains of patients with AD (20) and GFAP-reactive astrocytes are known to be associated with Aβ plaques in various Tg mouse models of AD (8, 9, 21, 22), we reasoned that the Tg(Gfap-luc) mice may be used to visualize the progression of Aβ deposition and corresponding neuropathology in live mice.
To determine if BLI might be useful in studying AD mouse models, we crossed Tg(APP23) and Tg(CRND8) mice with Tg(Gfap-luc) mice to generate bigenic Tg(APP23:Gfap-luc) and Tg(CRND8:Gfap-luc) mice. In both lines of mice, age- and transgene-dependent increases in the brain BLI signal were observed, which correlated with the onset of robust Aβ deposition in the brain. Thus, these bigenic mice permit the diagnosis of AD-like neuropathology in living mice and will facilitate longitudinal studies in which individual mice can be followed over their lifetimes. Using BLI of AD mouse models may simplify and even accelerate studies designed to test the efficacy of Aβ-directed therapeutics in mice.
Results
Correlation of GFAP and Aβ Levels in the Brains of Tg Mice.
Motivated by our success in using BLI to diagnose prion disease in Tg mice expressing firefly luciferase under the control of the murine Gfap promoter (19), we asked if BLI could also be used for tracking the progression of Aβ accumulation in Tg mouse models of AD. We chose two well-characterized Tg lines as AD mouse models. One line, denoted Tg(APP23) mice (8), expresses Swedish mutant human APP and first develops disease-associated neuropathological changes by 6–10 mo of age. The onset of amyloid deposition is sex-dependent, with female mice exhibiting earlier Aβ deposition compared with males (23). The other line, Tg(CRND8) mice (9), expresses human APP with Swedish and Indiana mutations and exhibits Aβ deposition beginning at 3–4 mo of age. Despite the large quantities of Aβ present in the brains of aged animals, mice from both lines can survive in excess of 2 y without showing overt signs of neurological dysfunction.
We began by analyzing the relationship between brain GFAP and Aβ levels in these lines. Western immunoblots of brain homogenates from Tg(CRND8) mice of different ages showed increases in GFAP and Aβ levels beginning at 5 mo of age (Fig. 1A). Levels of GFAP and Aβ progressively increased until 10 mo of age. Quantification of GFAP levels showed a significant fourfold increase (P < 0.05) in Tg(CRND8) mice at age 8–10 mo compared with 3- to 4-mo-old Tg(CRND8) mice and aged-matched non-Tg littermates (Fig. 1C). Similarly, GFAP and Aβ levels also increased with age in Tg(APP23) mice, although with more protracted kinetics compared with the Tg(CRND8) mice: the first demonstrable increases in GFAP and Aβ by Western blotting were observed at 15 mo of age (Fig. 1B). GFAP and Aβ levels progressively increased in Tg(APP23) mice up to 24 mo of age. Quantification of GFAP levels also showed a significant increase (P < 0.001) in aged (>18 mo) Tg(APP23) mice compared with young (<12 mo) Tg(APP23) mice (Fig. 1D). A direct correlation (R2 = 0.72) was found between total Aβ levels and GFAP levels for both lines of mice (Fig. 1E). In a graph depicting GFAP levels against total Aβ levels, similar slopes were obtained for both Tg lines after linear regression, arguing that GFAP levels are directly indicative of Aβ levels. Prominent staining of GFAP-reactive astrocytes was observed in the vicinity of Aβ plaques in both mouse models (Fig. 1 F and G). Cumulatively, these results suggest that GFAP levels are a suitable proxy for assessing Aβ deposition in the brains of these two Tg mouse models of AD.
Fig. 1.
Correlation between Aβ and GFAP levels in the brains of Tg(CRND8) and Tg(APP23) mice. (A and B) Western immunoblots show that GFAP and Aβ increased with age (in months, shown at the top of each lane) in Tg(CRND8) mice (A) and Tg(APP23) mice (B). All molecular weight markers are in kilodaltons. In both lines of mice, expression of human APP and actin are shown for comparison. (C and D) Quantification by ELISA shows that GFAP levels in the brain were significantly increased in older Tg(CRND8) (C) and Tg(APP23) (D) mice. *P < 0.05; ***P < 0.001. Data are mean ± SEM. Tg(CRND8) mice at 8–10 mo (n = 6) are compared with age-matched non-Tg controls (n = 4) and Tg(CRND8) mice at 3–4 mo (n = 4). Tg(APP23) mice at 18–24 mo (n = 8) are compared with Tg(APP23) mice at 6–12 mo (n = 11). (E) GFAP and total Aβ [Aβ(1–40) + Aβ(1–42)] protein levels determined by ELISA are significantly correlated (P < 0.0001) in the brains of Tg(CRND8) mice (black; n = 22) and Tg(APP23) mice (red; n = 27). (F and G) Immunohistochemistry shows coincident GFAP and Aβ staining in the brains of an aged Tg(CRND8) mouse (12 mo; F) and in an aged Tg(APP23) mouse (21 mo; G). GFAP staining (red) surrounds the Aβ plaques (green) in both Tg mouse lines. (Scale bar: F and G, 50 μm.)
BLI Monitoring of Disease Progression in Tg(CRND8:Gfap-luc) Mice.
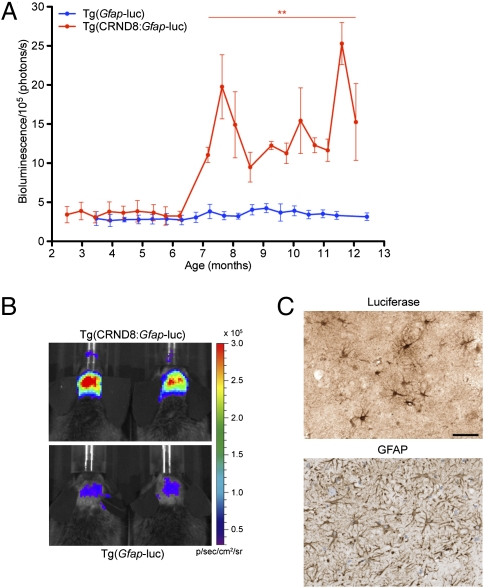
To test whether Aβ deposition in Tg(CRND8) mice could be monitored analogously to PrPSc accumulation as previously described for prion disease, we crossed the Tg(CRND8) mice with the Tg(Gfap-luc) reporter mice (18) to generate bigenic Tg(CRND8:Gfap-luc) mice. We performed BLI on the brains of Tg(CRND8:Gfap-luc) mice once every 14 d from 2.5 to 12 mo of age. Tg(CRND8:Gfap-luc) mice exhibited marked increases in the mean BLI signals compared with Tg(Gfap-luc) controls beginning at ∼7 mo of age and continuing for the remainder of the experiment (Fig. 2 A and B). Quantification of the mean BLI signals from all imaged mice revealed significant elevations (P < 0.01) in bigenic Tg(CRND8:Gfap-luc) mice from 7 to 13 mo of age compared with age-matched Tg(Gfap-luc) controls (Fig. 2A).
Fig. 2.
Bioluminescence imaging of disease progression in Tg(CRND8:Gfap-luc) mice. (A) Mean bioluminescence (±SEM) signals obtained from Tg(CRND8:Gfap-luc) mice (red; n = 5) were compared with Tg(Gfap-luc) mice (blue; n = 8) at 2.5–12.5 mo of age. Whereas Tg(Gfap-luc) mice did not exhibit an increase in the BLI signal with age, Tg(CRND8:Gfap-luc) mice showed a statistically significant increase in bioluminescence beginning at 7 mo of age, which continued for the duration of the experiment (**P < 0.01 at each time point). (B) BLI signals from the brains of 12-mo-old Tg(CRND8:Gfap-luc) mice (Upper) are prominent compared with age-matched Tg(Gfap-luc) controls (Lower). (C) Immunohistochemistry on the brain of a 12-mo-old Tg(CRND8:Gfap-luc) mouse shows prominent luciferase (Upper) and GFAP (Lower) staining in astrocytes within the hippocampus. (Scale bar: 50 μm.)
It is important to note that 50–60% of bigenic Tg(CRND8:Gfap-luc) mice, which derive from mice on a mixed B6/C3 background crossed with mice on an FVB/N background, died by 4 mo of age (Fig. S1). These mice exhibited no neurological signs of disease before death and did not exhibit rapid Aβ accumulation. This is a common problem that occurs when Tg(CRND8) are transferred to alternate genetic backgrounds (9). To alleviate this problem, we are attempting to cross Tg(CRND8) mice with Tg(Gfap-luc) mice, which have been transferred to the C57BL/6 background and then subsequently crossed with C3H mice. Whether this crossing will prevent early mortality of bigenic Tg(CRND8:Gfap-luc) mice remains to be determined.
Neuropathologic analysis of the brains of Tg(CRND8:Gfap-luc) mice was identical to that observed in Tg(CRND8) mice at 12 mo of age. Large numbers of Aβ-containing amyloid plaques were found in the cortical regions as well as the hippocampus. GFAP-reactive astrocytes as well as astrocytes that stained positively for firefly luciferase were observed in Tg(CRND8:Gfap-luc) mice (Fig. 2C). Levels of Aβ in the brains of Tg(CRND8:Gfap-luc) mice at 12 mo of age, as determined by ELISA, were also similar to those observed in Tg(CRND8) mice. Thus, Tg(CRND8:Gfap-luc) mice develop AD-like pathology in their brains, which can be monitored by increased BLI signals resulting from increased expression of the Gfap-luc reporter in astrocytes. Despite a clear increase in the BLI signal, no clinical signs of neurological illness were present in aged Tg(CRND8:Gfap-luc) mice. Thus, BLI permitted the in vivo diagnosis of neurological disease in these mice in the absence of any overt signs of neurological dysfunction.
BLI Monitoring of Disease Progression in Tg(APP23:Gfap-luc) Mice.
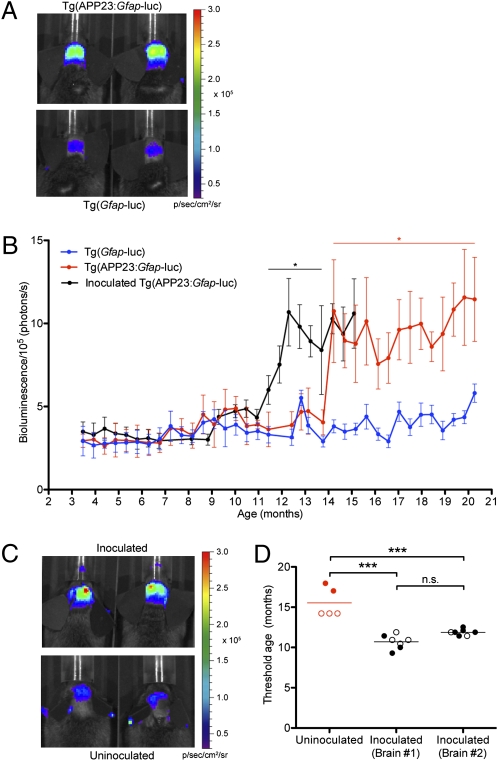
We next crossed the Tg(Gfap-luc) mice with Tg(APP23) mice in which pathological changes develop much more slowly than in the Tg(CRND8) mice. Mice were again scanned semimonthly using BLI beginning at 3 mo of age and continuing to 21 mo. Tg(APP23:Gfap-luc) mice began to show an increase in mean BLI signals at ∼14.5 mo of age (Fig. 3 A and B, red line), and the BLI signal remained elevated for the duration of the experiment. This observation is consistent with the slower kinetics of GFAP accumulation and Aβ deposition in Tg(APP23) mice, as detected by Western immunoblotting (15 mo of age) (Fig. 1B). Quantification of the mean BLI signals from all imaged mice revealed significant elevations (P < 0.05) in bigenic Tg(APP23:Gfap-luc) mice from 14.5 to 21 mo of age compared with age-matched Tg(Gfap-luc) controls (Fig. 3B). Thus, disease progression could also be followed in live Tg(APP23) mice using BLI.
Fig. 3.
Bioluminescence imaging of disease progression in uninoculated and inoculated Tg(APP23:Gfap-luc) mice. (A) BLI signals from the brains of 20-mo-old Tg(APP23:Gfap-luc) mice (Upper) are greater compared with age-matched Tg(Gfap-luc) controls (Lower). (B) Mean brain bioluminescence (±SEM) signals obtained from Tg(APP23:Gfap-luc) mice (red; n = 5) were compared with Tg(Gfap-luc) mice (blue; n = 8) at 3.5–21 mo of age. Tg(APP23:Gfap-luc) mice showed a statistically significant increase in bioluminescence beginning at 14.5 mo of age, which continued for the duration of the experiment (*P < 0.05). In Tg(APP23:Gfap-luc) mice inoculated with brain homogenates prepared from 31-mo-old Tg(APP23) mice (black; n = 14), a significant increase in the bioluminescence signal is apparent between 11.5 and 14 mo of age compared with uninoculated Tg(APP23:Gfap-luc) mice (*P < 0.05). After 14.5 mo of age, the BLI signals from inoculated and uninoculated Tg(APP23:Gfap-luc) mice were both elevated, indicating Aβ deposition in both lines. (C) The BLI signals from the brains of inoculated Tg(APP23:Gfap-luc) mice (Upper) are greater than those of age-matched uninoculated Tg(APP23:Gfap-luc) mice (Lower), both shown at 12 mo of age. (D) The mean BLI threshold ages from two groups of Tg(APP23:Gfap-luc) mice inoculated with homogenate prepared from the brains of two different 31-mo-old Tg(APP23) mice (black) were significantly earlier (***P < 0.001) compared with uninoculated Tg(APP23:Gfap-luc) controls (red). On average, the threshold age was decreased by ∼4 mo in the inoculated mice. In uninoculated mice, females (open circles) exhibited lower threshold ages than males (closed circles).
BLI of Accelerated Disease in Tg(APP23:Gfap-luc) Mice Inoculated with Brain Homogenates Containing Aβ.
Recently, it has been shown that inoculation of brain homogenates from aged Tg(APP23) mice into the brains of young Tg(APP23) mice resulted in the accelerated induction of Aβ deposition in proximity to the inoculation site (24, 25). We asked whether this accelerated Aβ deposition also coincided with an increase in GFAP levels that could be monitored using our bigenic Tg(APP23:Gfap-luc) mice.
Young (∼2-mo-old) Tg(APP23:Gfap-luc) mice were stereotactically inoculated with brain homogenate prepared from aged (∼31-mo-old) Tg(APP23) mice, and GFAP levels in their brains were monitored one time every 14 d using BLI. An increase in the mean BLI signal was observed in inoculated mice at 11.5 mo of age (9.5 mo postinoculation), 3 mo earlier than uninoculated mice (Fig. 3 B and C, compare black and red curves). To further assess this acceleration, we calculated the threshold age, defined as the age at which two successive BLI scans exceeded a threshold value of 7 × 105 photons/s, for uninoculated and inoculated Tg(APP23:Gfap-luc) mice. The mean threshold age for the inoculated mice was 11.3 ± 0.2 mo, ∼4 mo earlier compared with that of uninoculated mice at 15.5 ± 0.8 mo (Fig. 3D). In agreement with previous studies showing earlier onset of amyloid deposition in female Tg(APP23 mice) (23), uninoculated female Tg(APP23:Gfap-luc) mice exhibited lower threshold ages compared with males. Examples of interanimal variability in the BLI curves and threshold ages for inoculated and uninoculated Tg(APP23:Gfap-luc) mice are shown in Fig. S2. There was no significant difference in the threshold age between groups of mice inoculated with brain homogenate prepared from either of two aged Tg(APP23) mice. These data indicate that perturbations in Aβ deposition kinetics can be monitored using BLI of Tg(APP23:Gfap-luc) mice.
Neuropathological and Biochemical Analyses of Aβ-Inoculated Bigenic Mice.
The brains of inoculated Tg(APP23:Gfap-luc) mice were analyzed for induced Aβ deposition at 12 mo of age (10 mo postinoculation). Whereas uninoculated mice of this age exhibited a few compact Aβ deposits in the hippocampus and overlying cortical regions, large numbers of small Aβ plaques as well as more diffuse Aβ deposits were evident in inoculated Tg(APP23:Gfap-luc) mice (Fig. 4A Upper). The most striking pathology was observed in the subcallosal region, where a confluent layer of Aβ deposition was present. This localized pathology was unique to the inoculated mice and thus, facilitated the discrimination between spontaneous and induced Aβ deposits. These induced Aβ deposits were accompanied by increased GFAP staining in the subcallosal region (Fig. 4A Lower). Double labeling of Aβ and GFAP confirmed that GFAP-positive astrocytes were coincident with the Aβ deposition (Fig. 4B). The induced diffuse Aβ deposition in the hippocampus was similar to that previously observed in inoculated Tg(APP23) mice (24). Notably, induced Aβ deposition was also present along the walls of cerebral blood vessels (cerebral amyloid angiopathy) in the thalamus (Fig. 4C).
Fig. 4.
Accelerated Aβ deposition in Tg(APP23:Gfap-luc) mice inoculated with brain homogenates prepared from aged Tg(APP23) mice. (A) Immunohistochemistry for Aβ and GFAP in the CA1 region of the hippocampus and overlying cortex from the brains of inoculated Tg(APP23:Gfap-luc) mice at 12 mo of age (10 mo postinoculation). Induced plaque-like Aβ deposition was prominent in the subcallosal region (black arrows), and diffuse Aβ deposition was observed in the lacunosum moleculare layer of the hippocampus (black arrowheads). Increased GFAP staining (Lower; red arrows) was coincident with the induced Aβ deposition. In comparison, the brains of age-matched uninoculated Tg(APP23:Gfap-luc) mice showed occasional Aβ plaques (Upper Left) and minimal GFAP staining (Lower Left). (Scale bar: 200 μm.) (B) Strong GFAP labeling (red) was observed surrounding the induced Aβ deposition (green) in the subcallosal region of inoculated mice. (Scale bar: 50 μm.) (C) Cerebral amyloid angiopathy in the brains of inoculated Tg(APP23:Gfap-luc) mice. Prominent Aβ deposition in cerebral blood vessels was observed in an inoculated mouse (Right) at 12 mo of age but not in an uninoculated age-matched control (Left). (Scale bar: 100 μm.) (D) By Western blotting, increased GFAP (Top) and Aβ (Middle) protein levels were apparent in brain homogenates prepared from inoculated Tg(APP23:Gfap-luc) mice at 13.5 mo of age (two right lanes) compared with age-matched uninoculated controls (two left lanes). Actin is shown as a control. Molecular weight markers are indicated in kilodaltons. (E) As determined by ELISA, Aβ(1-40) levels were increased approximately sevenfold (*P < 0.05) and Aβ(1-42) levels were increased approximately eightfold (**P < 0.01) in inoculated Tg(APP23:Gfap-luc) mice at 13.5 mo of age compared with age-matched uninoculated controls (n = 4 each). Data are mean ± SEM. (F) As determined by ELISA, GFAP levels were approximately twofold greater (*P < 0.05) in the brains of inoculated Tg(APP23:Gfap-luc) mice at 13.5 mo of age compared with uninoculated controls (n = 4 each). Data are mean ± SEM.
Brain homogenates prepared from Tg(APP23:Gfap-luc) mice at 13.5 mo of age (11.5 mo postinoculation) contained elevated levels of both GFAP and total Aβ compared with age-matched uninoculated mice as detected by Western blotting (Fig. 4D). Quantification of Aβ(1-40) and Aβ(1-42) peptides in the brains of inoculated Tg(APP23:Gfap-luc) mice revealed statistically significant increases of seven- and eightfold, respectively, compared with age-matched uninoculated controls (Fig. 4E). GFAP protein levels were also significantly up-regulated (P < 0.05) in the brains of inoculated Tg(APP23:Gfap-luc) mice compared with uninoculated controls (Fig. 4F). Collectively, these results show that the earlier BLI signal increases observed in inoculated Tg(APP23:Gfap-luc) mice were accompanied by induced Aβ deposition and increased Aβ levels in the brain.
Discussion
We describe here the successful deployment of BLI for assessing the status of Aβ deposition in living bigenic mice. Because Tg mouse models of AD do not typically exhibit overt signs of neurological dysfunction, our BLI paradigm may represent a major advance in the study of AD. Assessing disease endpoints in vivo will not only facilitate kinetic studies of AD pathogenesis but may also revolutionize the development of AD therapeutics.
Both the Tg(CRND8) and Tg(APP23) mouse models of AD exhibited age-dependent increases in brain bioluminescence signals (Figs. 2 and 3), which accurately reflected the development of neuropathological changes in these animals. These pathogenic changes are reminiscent of Aβ aggregation observed in the brains of AD patients and likely represent the initiating event in AD. Several observations argue that Aβ aggregation and accumulation are sufficient to cause AD. First, early-onset AD occurs in patients with mutations in the APP gene. Additionally, an increased incidence of AD has been found in Down's syndrome patients, who have three copies of APP, and early-onset AD patients with a duplication of the APP locus (26). In contrast, tau aggregation likely represents a downstream event, because mutations in APP result in both Aβ and tau pathology; however, mutations in tau that result in the formation of neurofibrillary tangles cause frontotemporal dementia but not AD (27–29). Thus, BLI of Aβ deposition in bigenic mice may facilitate the study of important early events in AD.
Comparison Between BLI and Other Techniques Used for Assessing Disease in Tg AD Mouse Models.
The BLI paradigm described here for the Tg(APP23) and Tg(CRND8) mouse models of AD can presumably be adapted to any mouse model of AD in which there is a notable GFAP response. Thus, this paradigm should also work with other popular models of AD (5, 6, 21). Furthermore, the effects of genetic modifiers on the kinetics of Aβ deposition can be readily monitored using this strategy.
Assessing disease status in Tg mouse models of AD by bioluminescence has numerous advantages over traditional techniques that have been used (Table 1). First, AD-like pathology can be diagnosed in mice without relying on clinical signs, which do not manifest in many Tg mouse models of AD. Second, because BLI is noninvasive, repeated measurements can be performed on a single mouse over the lifespan of the animal, permitting longitudinal studies and reducing the total number of mice required for an experiment. This is particularly useful when conducting drug discovery studies in which the effectiveness of a candidate therapeutic can be monitored over time. Attempting to perform such studies using traditional paradigms, such as the Morris water maze or postmortem neuropathological analysis, would require a separate cohort of mice for each time point tested. Third, BLI is less affected by mouse genetic background artifacts compared with behavioral tests. For instance, FVB/N mice have substantial visual impairment that makes them a poor choice for spatial behavioral tests (30). In comparison, bioluminescence scans can be conducted in FVB/N mice without problems (18, 19). Fourth, BLI generates objective quantitative results compared with subjective assessments of clinical symptoms or behavioral testing. Fifth, variation in BLI is limited and can be monitored, because it is likely caused by interanimal differences in the kinetics of Aβ peptide deposition. Finally, the throughput of BLI is considerably higher than either water-maze tasks or neuropathological analysis. A single BLI reading on a group of animals can be completed in minutes, whereas neuropathological analysis requires days and a complete behavioral assessment takes more than 1 wk. Given these advantages, BLI should be deployed for routine assessment of mice and the selection of appropriate time points for more exhaustive studies using behavioral tests. Thus, BLI is not expected to replace behavioral testing but should facilitate early and rapid evaluation of AD-like neuropathology.
Table 1.
Comparison of various modalities for assessing disease status in mouse models of Alzheimer's disease
| BLI | Morris water maze | Neuropathology | |
| Performed in living mice | Yes | Yes | No* |
| Longitudinal studies on individual animals | Yes | No† | No |
| Number of animals required per experiment | 1 | 12–16 per time point | 2–3 per time point |
| Performed on all genetic backgrounds | Yes | No | Yes |
| Time required for single experiment per measurement | ∼15 min | 10–12 d | 3–4 d |
| Interanimal variability | None‡ | High | Low/medium |
| Throughput | High | Low | Medium |
*Some in vivo neuropathology can be performed by using two-photon imaging on mice with surgically implanted cranial windows.
†Although limited longitudinal Morris water maze studies can be performed, previous training reduces discrimination in later tests.
‡Individual mouse is its own control.
How does the sensitivity of BLI compare with the sensitivity of water-maze tests? Elevated BLI signals in the Tg(CRND8:Gfap-luc) mice were apparent at 7 mo of age. Using the Morris water maze, Tg(CRND8) mice showed delayed escape latency at ∼5 mo, but impaired performance in the probe trail was not evident until 9–10 mo of age (31). Delayed escape latency in the Morris water maze was reported in Tg(APP23) mice at 17–25 mo of age (23), whereas elevated BLI signals occurred in Tg(APP23:Gfap-luc) mice at ∼14 mo of age. Although some behavioral deficits may precede elevated BLI signals in certain Tg mice, BLI seems to be as sensitive as water-maze tests in monitoring the pathologic effects of Aβ deposition. Moreover, because of the multiple experimental design variables inherent with behavioral testing that often lead to poor reproducibility, BLI is likely to represent a better, more quantitative system for direct data comparisons between different laboratories.
BLI for Other Neurodegenerative Diseases.
Accumulating evidence argues that prion-like seeding of protein aggregates is involved in several neurodegenerative diseases (32). For instance, injection of brain homogenate prepared from old Tg(APP23) mice into young Tg(APP23) animals caused the induction of Aβ deposition in the vicinity of the inoculation site (24, 25). In those studies, the induction of protein aggregates was determined only by neuropathological analysis after euthanasia of the animals. Using BLI, we visualized the accelerated induction of Aβ deposition in live Tg(APP23:Gfap-luc) mice at 9 mo after inoculation with brain homogenates from aged Tg(APP23) mice (Fig. 3). Whether induced Aβ deposition and the corresponding BLI signal increases in inoculated Tg(APP23:Gfap-luc) mice can be accelerated even more is currently under investigation.
Could the Tg(Gfap-luc) mice be useful in studying other neurodegenerative diseases? Previously, Tg(Gfap-luc) mice have been used to show astrocytosis in the spinal cords of Tg mice expressing mutant superoxide dismutase 1 (SOD1), which is linked to familial amyotrophic lateral sclerosis (ALS) (33). BLI might be applied with Tg mouse models of Parkinson's disease, because some lines expressing mutant human α-synuclein also exhibit increased levels of GFAP (34). Furthermore, Tg models of frontotemporal dementia expressing mutant human tau clearly develop astrocytosis in response to tau deposits (35, 36). Whether induced tau deposition (37) is discernible by BLI after inoculation of Tg mice expressing WT human tau with brain homogenates from patients with frontotemporal dementia or from aged Tg mice expressing mutant human tau remains to be determined. Although studies using BLI to monitor neuronal dysfunction in mice are still in their infancy, noninvasive imaging paradigms, such as BLI or near-infrared fluorescence (38), promise to revolutionize the way neurodegenerative research is conducted and will likely prove to be essential tools for developing therapeutics that slow or halt disease-specific neurodegeneration.
Materials and Methods
Detailed methods are provided in SI Materials and Methods.
Mice.
Tg(APP23) mice expressing Swedish mutant human APP (751-aa isoform) under the control of the Thy-1.2 promoter (8) were maintained on a C57BL/6 background. Tg(CRND8) mice expressing Swedish and Indiana mutant human APP (695-aa isoform) under the control of the hamster Prnp promoter (9) were maintained on a mixed B6/C3 background. Tg(Gfap-luc) mice expressing firefly luciferase under the control of the murine Gfap promoter (18) were a gift from Caliper Life Sciences and were maintained on an FVB/N background. To create bigenic mice, Tg(APP23) and Tg(CRND8) mice were crossed with Tg(Gfap-luc) animals and screened for the presence of both transgenes. In all experiments, both male and female mice were used. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee at the University of California San Francisco.
BLI.
Mice were imaged one time every 14 d using an IVIS 200 imaging system (Caliper Life Sciences). BLI was initiated at 3–4 mo of age and continued until ∼12 mo of age for the Tg(CRND8:Gfap-luc) mice and ∼21 mo of age for the Tg(APP23:Gfap-luc) mice. Before each imaging scan, the heads of the mice were shaved bald. Mice were given an i.p. injection of d-luciferin potassium salt solution (Gold Biotechnology) prepared in PBS, pH 7.4 (Invitrogen). Each mouse (n = 5–8 for each time point) received 30 μL of 30 mg/mL luciferin solution (a dose of ∼30 mg/kg). The mice were then anesthetized using an isoflurane/oxygen gas mix and imaged 10 min later for 60 s under constant anesthesia. Black construction paper cutouts were placed over the ears to minimize extraneous signals. Bioluminescence values were quantified from images displaying surface radiance using circular regions of interest and then converted to total flux of photons (photons per second) using Living Image 3.0 software (Caliper Life Sciences). The threshold age for BLI curves was defined as the age at which two successive scans revealed bioluminescence values of greater than 7 × 105 photons/s.
Stereotactic Inoculations.
Brain homogenates from two 31-mo-old Tg(APP23) mice [10% (wt/vol) in calcium- and magnesium-free PBS] were prepared using an Omni Tip (Omni International) with a Fisher Scientific PowerGen homogenizer (Fisher Scientific); 25 μL of 1% brain homogenate diluted in PBS containing 5% BSA were injected into 2-mo-old Tg(APP23:Gfap-luc) mice (n = 7 for each inoculum) using a Hamilton syringe into the hippocampus and overlying cortex (anterior/posterior = −2.5 mm, dorsal/ventral = −1.0/−1.8 mm) on the right side of the brain. Injection speed was 5 μL/min, and the needle was kept in place for an additional 2 min before it was slowly withdrawn.
Supplementary Material
Acknowledgments
We thank Jeff Wilkins for many insightful and stimulating discussions of the work. We are grateful to Paul Fraser for providing the Tg(CRND8) mice and Matthias Staufenbiel for providing the Tg(APP23) mice and aged brains. We thank Caliper Life Sciences for the Tg(Gfap-luc) mice and Kevin Francis for his continued support and advice on bioluminescence imaging. This work was supported by grants from the National Institutes of Health (R37 AG031220) and the Hillblom Foundation as well as gifts from the Sherman Fairchild Foundation, the Lincy Foundation, and Robert Galvin. J.C.W. was supported by a postdoctoral fellowship from the Canadian Institutes of Heath Research (CIHR).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019034108/-/DCSupplemental.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 3.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 4.O'Nuallain B, et al. Amyloid β-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci. 2010;30:14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 7.Mucke L, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturchler-Pierrat C, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chishti MA, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 10.Kelly PH, et al. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol Aging. 2003;24:365–378. doi: 10.1016/s0197-4580(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 11.Janus C, et al. A β peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 12.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 14.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Luehmann M, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 17.Klunk WE, et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer's disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, et al. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci Lett. 2004;367:210–212. doi: 10.1016/j.neulet.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Tamgüney G, et al. Measuring prions by bioluminescence imaging. Proc Natl Acad Sci USA. 2009;106:15002–15006. doi: 10.1073/pnas.0907339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin WS, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 22.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A β deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Syková E, et al. Changes in extracellular space size and geometry in APP23 transgenic mice: A model of Alzheimer's disease. Proc Natl Acad Sci USA. 2005;102:479–484. doi: 10.1073/pnas.0408235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 25.Eisele YS, et al. Induction of cerebral β-amyloidosis: Intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci USA. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rovelet-Lecrux A, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 27.Hutton M, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 28.Spillantini MG, et al. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poorkaj P, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 30.Võikar V, Kõks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- 31.Hyde LA, et al. Age-progressing cognitive impairments and neuropathology in transgenic CRND8 mice. Behav Brain Res. 2005;160:344–355. doi: 10.1016/j.bbr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Miller G. Neurodegeneration. Could they all be prion diseases? Science. 2009;326:1337–1339. doi: 10.1126/science.326.5958.1337. [DOI] [PubMed] [Google Scholar]
- 33.Keller AF, Gravel M, Kriz J. Live imaging of amyotrophic lateral sclerosis pathogenesis: Disease onset is characterized by marked induction of GFAP in Schwann cells. Glia. 2009;57:1130–1142. doi: 10.1002/glia.20836. [DOI] [PubMed] [Google Scholar]
- 34.Giasson BI, et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 35.Allen B, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshiyama Y, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamura N, et al. In vivo detection of amyloid plaques in the mouse brain using the near-infrared fluorescence probe THK-265. J Alzheimers Dis. 2010 doi: 10.3233/JAD-2010-100270. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.