Abstract
The RNA virus, hepatitis E virus (HEV) is the most or second-most important cause of acute clinical hepatitis in adults throughout much of Asia, the Middle East, and Africa. In these regions it is an important cause of acute liver failure, especially in pregnant women who have a mortality rate of 20–30%. Until recently, hepatitis E was rarely identified in industrialized countries, but Hepatitis E now is reported increasingly throughout Western Europe, some Eastern European countries, and Japan. Most of these cases are caused by genotype 3, which is endemic in swine, and these cases are thought to be zoonotically acquired. However, transmission routes are not well understood. HEV that infect humans are divided into nonzoonotic (types 1, 2) and zoonotic (types 3, 4) genotypes. HEV cell culture is inefficient and limited, and thus far HEV has been cultured only in human cell lines. The HEV strain Kernow-C1 (genotype 3) isolated from a chronically infected patient was used to identify human, pig, and deer cell lines permissive for infection. Cross-species infections by genotypes 1 and 3 were studied with this set of cultures. Adaptation of the Kernow-C1 strain to growth in human hepatoma cells selected for a rare virus recombinant that contained an insertion of 174 ribonucleotides (58 amino acids) of a human ribosomal protein gene.
Keywords: emerging virus, zoonosis
Hepatitis E virus (HEV) gained notoriety as the cause of epidemics and sporadic cases of acute hepatitis in developing countries; examples include the 29,300 cases that occurred during the New Delhi outbreak in 1956 and the 2,621 cases reported over 6 mo in an Internally Displaced Persons Camp in Darfur in which pregnant women, as has been reported previously (1), had the highest mortality rate (26–31%) (2). HEV is the most or second-most important cause of acute hepatitis in adults in developing countries. Contrary to recent dogma, however, the virus is not restricted to developing countries, and sporadic cases are recognized increasingly in industrialized countries as awareness of the potential for infection spreads and tests for the virus are performed.
Historically, hepatitis E was described as an enterically transmitted, self-limiting hepatitis that never progressed to chronicity (3). However, 4 y ago a case of chronic hepatitis E was identified in Europe, and since then chronicity has been documented in immunocompromised solid-organ transplant recipients and HIV-infected individuals (4–7). Although hepatitis E infection generally causes mild to moderate disease, it occasionally has caused fulminant liver failure in acute cases, in chronically infected patients, and especially in those with underlying chronic liver disease or pregnancy (1, 2, 4–8). Additionally, hepatitis E has been misdiagnosed as drug-induced liver injury, thus complicating drug trials or treatment regimens (9). From its discovery in 1983, documented HEV transmission was linked almost exclusively to contaminated water; that association changed abruptly with the discovery of HEV infection following ingestion of uncooked deer meat (10, 11). Hepatitis E now is recognized as being not just a waterborne-disease of developing countries but also an emerging food-borne disease of industrialized countries (11, 12).
HEV is a nonenveloped, positive-stranded RNA virus with a genome size of 7.2 kb (3). All three ORFs are used. ORF1 occupies the 5′ two-thirds of the genome and encodes enzymes required for RNA replication. ORF2 encodes the structural protein comprising the virion capsid, and ORF3, which mostly overlaps ORF2, encodes a short protein of 114 amino acids that is required for virus egress from cells and that is proposed to perturb numerous cellular pathways (13–15). ORF2 and ORF3 proteins are translated from a single, bicistronic, subgenomic mRNA in which the ORF3 methionine initiation codon is located 11 nucleotides upstream of that of ORF2 (16).
Thus far, four HEV genotypes that infect humans are recognized (17). Genotype 1 and 2 infections have been identified exclusively in humans, whereas genotype 3 and 4 viruses have been isolated from swine, deer, mongoose, cattle, and rabbits in addition to humans (18). Genotypes 3 and 4 are ubiquitous in swine, and undercooked pork may be a major source of zoonotic infections of humans (12, 18). However, cross-species transmission has not been studied extensively, and additional zoonotic reservoirs probably exist.
HEV usually replicates to low titers in vivo; growing it in cultured cells is exceedingly difficult, and much of the virus life cycle is unknown. In a major breakthrough, Okamoto and coworkers recently adapted a genotype 3 and a genotype 4 strain to replicate to high titers in two human cell lines, A549 lung cells and PLC/PRF/5 hepatoma cells (19, 20).
The epidemiology of HEV is far from understood, and the zoonotic aspects, in particular, require further study. In the present report, genotype 3 virus isolated from a chronically infected patient (5) was characterized, adapted to grow in human hepatoma cells, and used to identify a set of human, swine, and deer cell cultures permissive for HEV infection. These cultures were used to examine cross-species infections by HEV genotypes 1 and 3.
Results
Genotype 3 Infection of Cells from 10 Different Species.
Although certain genotype 3 and 4 strains are known to infect swine and/or deer as well as humans, there are no virus–cell culture systems suitable for exploring host-range parameters. In an effort to develop such a system, genotype 3 Kernow-C1 strain of HEV was semipurified from the feces of an HIV-1 patient infected with HEV (5). The patient had been chronically infected with HEV for 2 y when his feces were collected and found to contain ≈1010 viral genomes per gram. The virus was inoculated onto five human and one rhesus cell lines, and 7 d later cells were stained for immunofluorescence microscopy with antibodies to ORF2 capsid protein and to ORF3 protein. Because these viral proteins are translated from a subgenomic mRNA, their presence indicates viral RNA synthesis has occurred. Infected foci were found in all six cultures, but the number of foci was more than 7.5-fold higher in HepG2/C3A human hepatoma cells than in human Huh7.5 or PLC/PRF/5 hepatoma cells, A549 lung carcinoma cells, Caco-2 intestinal cells, or rhesus kidney cells, suggesting that HepG2/C3A cells were the most permissive.
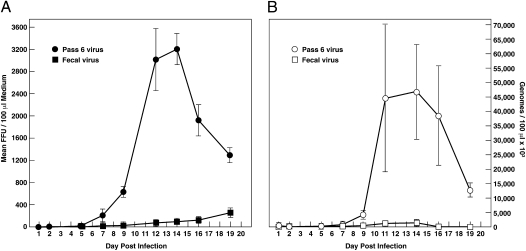
Semipurified virus was serially passed six times in HepG2/C3A cells for 7 mo total. The virus in feces formed 80 and 90 times more foci on HepG2/C3A cells than on A549 or PLC/PRF/5 cells, respectively, and by passage 4 the virus produced 400 and 500 times more foci in the HepG2/C3A cells than in these other two cell lines. Growth curves on HepG2/C3A cells comparing production of infectious virus and virion RNA by fecal and passage 6 viruses confirmed that serial passage of the fecal virus had produced a virus able to grow more efficiently in HepG2/C3A cells [P = 0.008 for fluorescent focus units (FFU) and 0.013 for RNA] (Fig. 1). At day 14 the fecal virus had released 89 FFU and 1.3 × 106 genome equivalents (GE) of RNA/100 μL medium to give a specific infectivity of 1 FFU/15,083 GE; on day 14 the passage 6 virus released 3,203 FFU and 46.1 × 106 GE RNA/100 μL to give a specific infectivity of 1 FFU/14,399 GE. Similar attempts to adapt the fecal virus to grow on A549 cells or PLC/PRF/5 cells were unsuccessful.
Fig. 1.
Adaptation of Kernow-C1 virus to grow in human hepatoma cells. Approximately equal amounts of virus present in the feces (square) or serially passed six times in HepG2/C3A cells (circle) were inoculated at a low multiplicity of infection onto HepG2/C3A cells and infectious viruses (A) and total viruses (B) released into the medium were quantified by focus-forming assay on HepG2/C3A cells and by RT-PCR, respectively. Focus assays of all harvested samples and of the reserved inocula were performed at the same time, in triplicate and under code. Direct comparison indicated that the fecal inoculum, which produced many fewer viruses, actually had contained five times more infectious virus than the passaged inoculum. Note the difference in scales for the FFU and RNA. Error bars show SD.
The fecal virus also was tested for the ability to infect a variety of nonprimate cells available from the American Type Culture Collection (ATCC). Genotype 3 viruses have been isolated from pigs and deer, and each of three pig kidney cell lines contained numerous ORF2- and ORF3-stained foci. The deer cell line had a moderate number (Fig. 2). Remarkably, the cow, mouse, chicken, cat, dog, and rabbit cell cultures each also contained a few cells stained for both ORF2 and ORF3 proteins (Fig. S1).
Fig. 2.
Kernow-C1 infection of swine and deer cells. Fecal virus was inoculated onto LLC-PK1 pig cells (Upper) and OHH1.Li deer cells (Lower), and cells were immunostained 3 d later for ORF2 protein (green) and ORF3 protein (red).
Titration of Genotypes 1 and 3 on Human, Pig, and Deer Cells.
To revisit the question of host-range restrictions on genotype 1, serial dilutions of the highest-titered stocks available of genotypes 1 (Sar-55 and Akluj), and 3 (US-2, Kernow-C1 fecal, and Kernow-C1 passage 6) were inoculated onto HepG2/C3A, LLC-PK1 pig cells and deer cells, and cultures were immunostained for ORF2 and ORF3 proteins 3 d later. The number of ORF2+ foci at the last one or two positive dilutions was used to calculate the infectious titer (Fig. 3). As expected, both genotype 1 strains infected HepG2/C3A cells; surprisingly, they also infected LLC-PK1 cells, albeit less efficiently (P = 0.016 for Sar-55 and 0.009 for Akluj). In contrast, both genotype 3 strains infected LLC-PK1 cells more efficiently than they did HepG2/C3A cells (P = 0.006 for fecal, 0.010 for passage 6, and 0.008 for US-2). Even though the passage 6 virus was adapted to grow in the HepG2/C3A cells, it still infected more pig cells than human cells. Similar results (with one exception, for US-2) were obtained in multiple experiments, although the virus titers and therefore the ratios varied from experiment to experiment (Table S1). Because of this variation, it is necessary to include at least one genotype 1 and one genotype 3 strain in each assay for comparison (Table S1).
Fig. 3.
Comparative titration of HEV on human and swine cells. Serial dilutions of each virus were inoculated in triplicate onto human HepG2/C3A cells (solid bars) or LLC-PK1 pig cells (open bars) in eight-well chamber slides. Three days later, slides were coded and immunostained for ORF2 protein, and foci at the end point were counted manually. The code was not broken until all samples were counted. Student's t test P values ranged from 0.006–0.016.
The lower titer of the passage 6 virus compared with that of the fecal Kernow-C1 virus reflects a lower specific infectivity of the cell-cultured virus. The cultured viruses in Fig. 1 had a specific infectivity of about 1 FFU/15,000 GE on HepG2/C3A cells; the Kernow-C1 virus in the feces had a specific infectivity of 1 FFU/450 GE on these same cells.
The infection of deer cells was more complicated. US-2 did not infect the deer cells in this experiment, but each of the other strains did, with a titer 8–11 times lower than that on LLC-PK1 cells. Interestingly, dual staining for ORF2 and ORF3 proteins suggested that genotype 1, but not genotype 3, strains were deficient in ORF2 capsid protein production. All stained deer cells in each well were counted: two-thirds of the cells containing genotype 1 ORF3 protein had no detectable ORF2 protein, whereas every cell containing genotype 3 ORF3 protein contained ORF2 protein (Fig. 4A). This imbalance was not seen in human cells infected with genotype 1, Sar-55: Of 73 randomly selected cells that scored positive for ORF3 protein, only one cell lacked detectable ORF2 protein. Because translation of ORF2 and ORF3 initiates from closely spaced methionine codons on the same bicistronic mRNA (16), this result suggested a translation bias toward initiation of ORF3 protein synthesis at the expense of ORF2 protein synthesis in deer cells infected with genotype 1 strain but not in those infected with genotype 3 strains.
Fig. 4.
Differential translation of ORF2 in deer cells. (A) Deer cells were infected with indicated strains and were immunostained 3 d later. All cells containing ORF2 protein (solid bars), ORF3 protein (open bars), or both proteins (hatched bars) were counted. (B) Deer cells and S10-3 human cells were transfected with a CMV plasmid expressing a bicistronic mRNA containing the sequence of Sar-55 (CMV-Sar), Kernow-C1 (CMV-Kernow), or the Sar-55 sequence in which the first 29 nucleotides were replaced by those of Kernow-C1 (CMV-MT29). Two days later cells immunostained for ORF2 or ORF3 protein were quantified by FACS, and the ratio of ORF3 to ORF2 was calculated according to the percent of cells stained.
Viral protein production in deer cells was not robust enough to permit FACS analysis. Therefore, FACS analysis of cells transfected with CMV promoter-driven mRNAs was performed to confirm the initiation bias. Bicistronic mRNAs of wild-type Sar-55, wild-type Kernow-C1, and Sar-55 with the first 29 nucleotides mutated to those of Kernow-C1 were transiently expressed in S10-3 human hepatoma cells and in deer cells. FACS analysis of cultures stained separately for ORF2 and ORF3 proteins demonstrated that significantly more ORF2 protein than ORF3 protein was produced by the mutant CMV-MT29 (P = 0.024) and CMV-Kernow (P = 0.052) than by CMV-Sar (P = 0.003) (Fig. 4B). Because the CMV-Sar and CMV-MT29 differed only by these 29 nucleotides, the increased relative production of ORF2 by the mutant suggested that translation of Sar-55 ORF2 capsid protein was diminished in deer cells. Indeed, when the same 29-nucleotide mutation was introduced into the infectious full-length Sar-55 clone (pSK-E2-MT29), and it and the wild-type Sar-55 transcripts were transfected into deer cells and scored by immune microscopy 5 d later for ORF2 and ORF3 protein production, the average ratio of ORF3-/ORF2-containing cells decreased from 3.68 for wild-type to 0.4 for the mutant (P = 0.004). These results confirm that the 29-nucleotide genotype 3 sequence at the translation initiation site was sufficient to increase ORF2 production of Sar-55 in deer cells (Table 1). In comparison, a similar ratio of ORF3-/ORF2-containing cells was obtained for human S10-3 cells transfected with either the wild-type or mutant clone (Table 2).
Table 1.
Production of ORF2 and ORF3 proteins in deer cells transfected with infectious transcripts of wild-type and mutant cDNA clones
| pSK-E2* |
pSK-E2-MT29† |
||||||
| Experiment no.‡ | ORF2§ | ORF3§ | ORF3/ORF2 | Experiment number‡ | ORF2§ | ORF3§ | ORF3/ORF2 |
| 1 | 149 | 510 | 3.42 | 4 | 43 | 19 | 0.44 |
| 2 | 146 | 420 | 2.88 | 5 | 56 | 24 | 0.43 |
| 3 | 74 | 351 | 4.74 | 6 | 28 | 9 | 0.32 |
*The infectious cDNA clone of HEV strain Sar-55.
†The infectious cDNA clone of HEV in which the first 29 nucleotides of the Sar-55 bicistronic region in pSK-E2 were replaced with that of Kernow-C1.
‡Three cultures were transfected with each viral genome.
§Transfected cells were transferred under code to eight-well chamber slides and were immunostained on day 5. All ORF2- and ORF3- positive cells in each well were counted before the code was broken.
Table 2.
FACS analysis of ORF2 and ORF3 proteins in S10-3 cells after transfection with infectious transcripts of wild-type and mutant cDNA clones
| pSK-E2* |
pSK-E2-MT29† |
||||||
| Experiment no.‡ | ORF2§(%)¶ | ORF3§(%)¶ | ORF3/ORF2 | Experiment number‡ | ORF2§(%)¶ | ORF3§(%)¶ | ORF3/ORF2 |
| 1 | 22.45 | 37.73 | 1.68 | 4 | 21.97 | 29.07 | 1.32 |
| 2 | 23.16 | 38.84 | 1.67 | 5 | 22.21 | 26.96 | 1.21 |
| 3 | 19.78 | 39.70 | 2.00 | 6 | 21.67 | 31.71 | 1.46 |
*The infectious cDNA clone of HEV strain Sar-55.
†The infectious cDNA clone of HEV in which the first 29 nucleotides of the Sar-55 bicistronic region in pSK-E2 were replaced with that of Kernow-C1.
‡Three cultures were transfected with each viral genome.
§Cells were immunostained for ORF2 and ORF3 proteins on day 5 posttransfection.
¶Percentage of cells stained for indicated viral proteins.
HepG2/C3A-Adapted Virus and Host-Cell Recombination.
The RT-PCR consensus sequence of the virus in the feces and at passage 6 was determined. Sixteen amino acid differences (10 in ORF1, five in ORF2, and one in ORF3) along with an in-frame insert of 58 amino acids in the hypervariable region (HVR) of ORF1 (21) differentiated passage 6 from the fecal virus (Table S2). A Blast search identified the inserted sequence as belonging to the ribosomal S17e superfamily, which is highly conserved across species. One hundred sixty-seven of 170 nucleotides and 53 of 57 amino acids were identical to those in the human ribosomal protein S17 (GenBank accession no. DQ896701.2) (Fig. 5), compared with only 155 of 171 nucleotides in the swine S17 protein (GenBank accession no. AY5500731.1). RT-PCR with paired HEV and insertion sequence primers detected viral genomes with the insertion in the original fecal suspension, indicating that a double-recombination event had occurred either in the patient or in a previous host. It is noteworthy that the recombinant genomes in the feces were a minor species, because they were not detected by direct sequencing of the RT-PCR products from the feces or from first-passage virus in the culture medium 70 d postinfection. The entire HVR was amplified from the feces with HEV-specific primers, cloned, and sequenced. Of 120 clones sequenced, none contained the insert.
Fig. 5.
Insertion of a human sequence into the HVR of Kernow-C1. Alignment of human ribosomal gene S17 sequence and that obtained by direct sequencing of RT-PCR product amplified from virus passed six times in HepG2/C3A cells. HEV sequences flanking the insert are underlined. (A) Nucleotide. (B) Amino acid.
In an attempt to determine whether the inserted sequence or its size was relevant, the insert sequence was cloned in frame into the HVR of the Sar-55 infectious clone in the sense, reverse, or reverse-complementary orientation, and in vitro transcribed genomes were transfected into S10-3 cell. The wild-type genomes and those with the sense orientation of the insert were indistinguishable and produced many more virus-positive cells than could be counted; in contrast, wells containing cells transfected with the genomes containing the reversed or reversed-complementary insert contained only 16 and 12 virus-positive cells, respectively (Fig. S2). Determination of whether the inserted sequence and/or one or more point mutations played a role in adaptation awaits the construction of an infectious cDNA clone of the Kernow-C1 strain.
Discussion
The Kernow-C1 strain, from a chronically infected patient, was grown in cell culture and among other characteristics, it exhibited a broad host range. Not only did it infect cells from nonprimate species, the range of cross-species infections spanning animals as diverse as chickens and mice was totally unexpected and would not have been predicted based on current knowledge. Note that none of the viruses used have been plaque purified, so each inoculum probably represents a mixed population. Therefore, the virus infecting primate cells may differ substantially from that infecting cells of other species. It should be possible to study the effects of biological diversity and cell culture-acquired mutations once an infectious cDNA clone with robust replication capacity is constructed.
Although the passage 6 virus produces sufficient extracellular virus to permit experiments previously impossible, the low specific infectivity of cell-cultured HEV imposes some difficulties. Both genotype 1 (14) and genotype 3 (13) viruses produced in cell culture differ significantly from those excreted in the feces, in that they contain ORF3 protein and their virions are not precipitated by anti-ORF2 antibody that readily precipitates fecal virions. If these differences in virus structure or composition are responsible for the reduced specific infectivity of the cultured viruses, it may be impossible to develop a truly robust cell culture system for HEV.
The demonstration that genotype 3 viruses infect swine cells more efficiently than human cells is consistent with the documented ubiquitous infection of swine worldwide compared with the sporadic infection of humans by this genotype (18). The extent and consistency of the opposite tropism of genotype 1 and 3 strains evidenced for human cells versus swine or deer cells in this study (Fig. 3) suggests that the cell-culture systems described here will be quite useful for further studying factors that affect cross-species HEV infections.
The question of how production of ORF2 versus ORF3 protein is regulated is unanswered, but the observed bias against Sar-55 ORF2 production in deer cells and its amelioration following introduction of a short 5′ RNA sequence from the Kernow-C1 strain (Fig. 4B) suggests that modulation of translation from closely spaced codons can differ significantly according to host species, and this difference may provide one mechanism for restricting host range. Clearly, inhibition of ORF2 capsid protein synthesis would compromise the ability to assemble the virions that could infect additional cells.
Selection of an AUG codon for initiation of translation is directed by position and by the nucleotides adjacent to the codon, according to rules defined by Kozak (22). Although genotype 1 and 3 bicistronic mRNAs have the same canonical Kozak sequences, the relevant AUG codons for ORF3 and ORF2 of genotype 3 are three nucleotides closer together than those of genotype 1, and distance between codons is known to affect initiation preferences. Therefore, this difference in AUG spacing (which is conserved within genotypes) probably explains the different translation patterns of genotypes 1 and 3 in deer cells.
In pig cells, differential translation of ORF2 was not observed, and Kernow-C1 (genotype 3) and Sar-55 (genotype 1) appeared to have a similar ratio of the two proteins in human and pig cells. However, because titer determinations were based on detectable ORF2 production, inefficient genotype-specific translation of ORF2 in one species relative to the other could explain why the titer of Sar-55 was consistently lower on pig cells than on human cells and why the opposite held for Kernow-C1 (Fig. 3).
Receptor differences, either quantitative or qualitative, offer an alternative explanation for host-range differences. Specific receptors for HEV have not been identified. In favor of receptor-determined host range, the passage 6 virus maintained a higher titer for pig cells than human cells even though adapted to grow in human cells. There are 54 amino acid differences (8.2%) between Sar-55 and Kernow-C1 capsid proteins and only five between the fecal and passage 6 capsid proteins, suggesting that the adapted virus may have retained the receptor-interacting specificity of the fecal virus.
ORF3 also is a serious candidate for restricting host range. ORF3 protein is required for virus egress, perhaps through interactions with one or more cellular proteins (13, 14). Because the Sar-55 and Kernow-C1 ORF3 proteins differ by 17.5% (20 of 114 amino acids), Kernow-C1, but not Sar-55 ORF3 may be able to interact efficiently with pig cellular proteins potentially involved in virus exit; thus, the replication cycle of Sar-55 would be aborted.
Inter- and intragenomic recombination for HEV has been reported only rarely (23). Therefore it is quite remarkable that a human RNA sequence was acquired in the passage 6 virus. Because genomes with this insertion were detected in the feces, the insertion is not an artifact of cell culture. It would be interesting to know whether the recombination occurred in this patient or previously. Because the insertion must reflect a rare event, it suggests that the lengthy period of chronic infection permitted the virus to mutate to an unusual extent. That this sequence was inserted into the HVR has multiple implications.
The HVR of Sar-55 could be experimentally truncated but not eliminated, suggesting that the sequence per se was not critical (21). The HVRs compared by Pudupakam et al. (21) correspond to amino acids 706–792 of Kernow-C1 ORF1. The HVR and surrounding region approximately encompassing amino acids 215–957 of ORF1 in all strains have no defined functions, and they are designated simply as “Y and papain-like domains” upstream of HVR and as “proline hinge and X domain” downstream. Therefore, insertions within the HVR would not be expected to disrupt any function. The HVR has not been characterized extensively, but one comparison (21) suggests that, within each genotype, certain sequence patterns may be conserved; the HVR sequences of genotypes 1 and 3 differed substantially in this comparison. The Kernow-C1 fecal consensus sequence contains 86 amino acids, compared with 71 for Sar-55. However, both the Kernow-C1 and the constructed Sar-55 chimera (Fig. S2) were viable when the S17 insert was present, demonstrating that this region is able to tolerate substantial changes. On the other hand, the greatly decreased transfection efficiency observed when the insert was reversed indicates that there are limitations that need to be understood. It remains to be shown whether simply increasing the length of the HVR led to selection of this quasispecies or whether the inserted sequence performed a particular function. The inserted sequence was fully conserved during passage, suggesting that the sequence itself may be relevant for growth in cell culture. In any case, the plasticity of this region and its possible relationship to growth in cell culture opens avenues for exploration.
The discovery of the recombination event leads to speculation that the HVR regions of each genotype differ so much because they originally arose by random recombination events and subsequently acquired mutations that disguised their origins.
Takahashi et al. (24) recently showed that virtually any serum with a high HEV titer can infect cultured cells. RNA viruses exist as quasispecies, and, given the tremendous difficulties in developing a cell culture system for HEV, it appears that a sample with a high titer has an increased probability of containing a variant with the correct constellation of mutations needed to permit infection of a cultured cell. The extraordinary ability of the Kernow-C1 strain to infect cells from such a broad spectrum of species, ranging from rodent to primate, probably reflects a high titer and a complex quasispecies generated during a prolonged infection in an immunocompromised host. That possibility, along with the demonstration that HEV can acquire new information through recombination with host cell sequences, leads to the conclusion that chronic HEV infection of a patient has important implications for the evolution of this “emerging virus.” For instance, it is tempting to speculate that the wider range of cells infected by this recombinant quasispecies also might explain the virus's ability to infect the central nervous system and potentially cause the neurological illness seen in the source patient. It is unfortunate that no cerebral spinal fluid remained for molecular analyses, but this report should spur such analysis in future cases.
Therefore, it may be prudent to cure HEV infections before they become chronic, not only for the patient's well-being but also for future control of the virus. With recent discoveries, HEV has become an even more intriguing human pathogen and deserves more attention.
Materials and Methods
Source Patient.
HEV particles were purified from the feces of a 48-y-old man infected with HIV-1 who was chronically coinfected with HEV for at least 2 y (5). At presentation, the patient had established liver cirrhosis with an active inflammatory component. In addition, he had clinical features of peripheral neuropathy that was considered an HEV-related complication because HEV was detected in his CSF and symptoms resolved with viral clearance (25). The virus strain obtained from this patient was designated Kernow-C1 HEV.
Cell Culture.
Huh-7 human hepatoma cells were originally isolated in Japan (26). Both S10-3 cells (a subclone of Huh-7 cells), and C25j cells (a subclone of Caco-2 cells, HTB-37), were isolated in-house. All other cell lines were purchased from the ATCC and are described in SI Materials and Methods. Most cell lines were propagated in DMEM (Cellgro; Mediatech) supplemented with 2 mM L-glutamine, penicillin/streptomycin (Sigma), and 10% FBS (20% for C25j) (Bio-Whittaker). Deer liver cells and chicken liver cells were cultured in Opti-MEM (Gibco) supplemented with 20% FBS (Bio-Whittaker). The HepG2/C3A, C25j, deer, and chicken cells were grown on rat tail collagen type 1 (Millipore). All cell stocks were grown at 37 °C in the presence of 5% CO2.
Virus Stocks.
All virus stocks except passage 6 virus consisted of 10% fecal suspension in PBS (pH 7.4); RT-PCR titers ranged from 106 to 108 GE/100 μL and were not predictive of infectivity titers. Genotype 1 strains Sar-55 (GenBank accession no. M80581.1) and Akluj (GenBank accession no. AF107909) were isolated from patients in Pakistan and India, respectively. Genotype 3 US-2 strain (GenBank accession no.AF060669) was obtained from a patient in the United States and was amplified in a rhesus macaque. Genotype 3 Kernow-C1 (ancient Cornish for “Cornwall”) strain was obtained from a chronically infected hepatitis E patient coinfected with HIV as described above. The passage 6 virus is the Kernow-C1 fecal virus that was adapted to grow in HepG2/C3A cells by serial passage.
Plasmid Constructs.
The infectious cDNA clone of HEV strain Sar-55, pSK-E2 (GenBank accession no. AF444002) and plasmid CMV-Sar were described previously (16, 27). Plasmid CMV-MT29 was generated by replacing the first 29 nucleotides of Sar-55 subgenomic RNA with those of Kernow-C1 HEV in the plasmid CMV-Sar. Plasmid CMV-Kernow was constructed by amplifying the entire bicistronic mRNA of the Kernow-C1 virus in the feces and cloning it into pCMV5122 as had been done for Sar-55 (16). The Sar-55 cDNA clones containing human S17 gene sequences in sense (Sar55-S17), reverse (Sar55-S17R), and reverse-complement (Sar55-S17RC) orientation were constructed by amplifying the human S17 gene from passage 6 Kernow-C1 virus and inserting it in frame by fusion PCR between nucleotides 2251 and 2252 within the HVR region of pSK-E2. The infectious plasmid pSK-E2-MT29 was generated by replacing the first 29 nucleotides of the Sar-55 bicistronic region in pSK-E2 with those of Kernow-C1.
In Vitro Transcription and Transfection of Cultured Cells.
Full-length viral cDNA was transcribed with T7 polymerase, and capped transcripts were transfected into S10-3 or deer cells with DMRIE-C (Invitrogen) as described previously (27) and detailed in SI Materials and Methods. LLC-PK1 cells were killed by all transfection methods tried. CMV plasmids were transfected into S10-3 and deer cells using Lipofectamine 2000 (Invitrogen) as described in SI Materials and Methods.
Infection of Cultured Cells.
We seeded 100,000 cells per well onto eight-well Lab-Tek II CC2 slides (Nunc) 1 d before infection. Virus stocks were diluted in Opti-MEM (Gibco), and 100 μL of the diluted virus was added to each chamber and incubated for 5 h at 34.5 °C in a CO2 incubator. The virus mixture was removed, cells were washed with PBS, and medium was added, followed by incubation at 34.5 °C for 3 d.
Immunofluorescence Analysis and Focus-Forming Assay.
Cells on eight-well chamber slides were fixed with acetone and doubly stained with chimpanzee anti-ORF2 and rabbit anti-ORF3. Stained cells or foci were visualized with a fluorescence microscope and manually counted as described previously (28) and in SI Materials and Methods.
Flow Cytometric Analysis for the Quantification of ORF2 and ORF3 Proteins.
Transfected cells cultured in 100-mm dishes (Corning) were trypsinized and fixed with 1 mL methanol for 15 min at 4 °C. Immunostaining was the same as for adherent cells, except separate aliquots of cells were stained for ORF2 and ORF3 proteins. After washing with PBS, cells were resuspended in 1 mL PBS and analyzed using a FACScan flow cytometer (Becton Dickinson). A total of 20,000 events was acquired for each sample, and the data were analyzed using BD CellQuest software.
RT-PCR.
RNA was extracted with TRIzol LS (Invitrogen), reverse transcribed, and amplified with a Qiagen kit. PCR products eluted from agarose gels were directly sequenced to provide the fecal and passage 6 consensus sequences or were cloned and then sequenced to provide representative HVR sequences. Details are given in SI Materials and Methods.
Growth Curve.
A T25 flask seeded with 106 HepG2/C3A cells was inoculated with 1 mL of previously titrated fecal or passage 6 virus stock diluted to contain approximately equal FFU for HepG2/C3A cells. An aliquot of each diluted inoculum was frozen at −80 °C for retitration at the end of the experiment. After 5 h incubation at 37 °C, medium was removed, cells were washed three times with Opti-MEM, 2.5 mL of DMEM with 10% FBS and antibiotics were added, and the flasks were incubated at 37 °C. The medium was collected and replaced with fresh medium on the days indicated. The collected medium was passed through a 0.45-μm filter and frozen at −80 °C as 100-μL aliquots. Triplicates of all frozen samples, including the inocula, were processed in parallel to determine FFU and RNA concentration under identical conditions. Direct comparison in the same test indicated the fecal inoculum contained 22,000 FFU, compared with 4,200 FFU for the passage 6 virus. The Wilcoxon test was performed on the values from day 7 onward.
Statistics.
Statistics were performed by mathematical statisticians in the Biostatistics Research Branch of the National Institute of Allergy and Infectious Diseases. Student's t test was used except for the growth-curve analysis.
Supplementary Material
Acknowledgments
We thank Rebecca DerSimonian and Xiao Liu Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, for performing the statistical analyses. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [HQ389543 (Kernow-C1 fecal virus) and HQ709170 (Kernow-C1 passage 6 virus)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018878108/-/DCSupplemental.
References
- 1.Kumar A, Beniwal M, Kar P, Sharma JB, Murthy NS. Hepatitis E in pregnancy. Int J Gynaecol Obstet. 2004;85:240–244. doi: 10.1016/j.ijgo.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Boccia D, et al. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis. 2006;42:1679–1684. doi: 10.1086/504322. [DOI] [PubMed] [Google Scholar]
- 3.Meng XJ. Recent advances in hepatitis E virus. J Viral Hepat. 2010;17:153–161. doi: 10.1111/j.1365-2893.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- 4.Péron JM, et al. Prolonged hepatitis E in an immunocompromised patient. J Gastroenterol Hepatol. 2006;21:1223–1224. doi: 10.1111/j.1440-1746.2006.04209.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 6.Legrand-Abravanel F, et al. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202:835–844. doi: 10.1086/655899. [DOI] [PubMed] [Google Scholar]
- 7.Pischke S, Wedemeyer H. Chronic hepatitis E in liver transplant recipients: A significant clinical problem? Minerva Gastroenterol Dietol. 2010;56:121–128. [PubMed] [Google Scholar]
- 8.Kc S, Sharma D, Basnet BK, Mishra AK. Effect of acute hepatitis E infection in patients with liver cirrhosis. JNMA J Nepal Med Assoc. 2009;48:226–229. [PubMed] [Google Scholar]
- 9.Dalton HR, et al. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429–1435. doi: 10.1111/j.1365-2036.2007.03504.x. [DOI] [PubMed] [Google Scholar]
- 10.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 11.Purcell RH, Emerson SU. Hepatitis E: An emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Purcell RH, Emerson SU. Hidden danger: The raw facts about hepatitis E virus. J Infect Dis. 2010;202:819–821. doi: 10.1086/655900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K, et al. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. J Gen Virol. 2009;90:1880–1891. doi: 10.1099/vir.0.010561-0. [DOI] [PubMed] [Google Scholar]
- 14.Emerson SU, et al. Release of genotype 1 hepatitis E virus from cultured hepatoma and polarized intestinal cells depends on open reading frame 3 protein and requires an intact PXXP motif. J Virol. 2010;84:9059–9069. doi: 10.1128/JVI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra V, Taneja S, Kalia M, Jameel S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci. 2008;33:451–464. doi: 10.1007/s12038-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 16.Graff J, Torian U, Nguyen H, Emerson SU. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J Virol. 2006;80:5919–5926. doi: 10.1128/JVI.00046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerson SU, et al. Hepevirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy, VIIIth Report of the ICTV. London: Elsevier/Academic; 2004. pp. 851–855. [Google Scholar]
- 18.Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet Res. 2010;41:46–65. doi: 10.1051/vetres/2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada K, et al. Construction of an infectious cDNA clone of hepatitis E virus strain JE03-1760F that can propagate efficiently in cultured cells. J Gen Virol. 2009;90:457–462. doi: 10.1099/vir.0.007559-0. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, et al. Development and characterization of a genotype 4 hepatitis E virus cell culture system using a HE-JF5/15F strain recovered from a fulminant hepatitis patient. J Clin Microbiol. 2009;47:1906–1910. doi: 10.1128/JCM.00629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pudupakam RS, et al. Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: Evidence for attenuation of HVR deletion mutants in vivo. J Virol. 2009;83:384–395. doi: 10.1128/JVI.01854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak M. Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proc Natl Acad Sci USA. 1995;92:2662–2666. doi: 10.1073/pnas.92.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, et al. Recombination analysis reveals a double recombination event in hepatitis E virus. Virol J. 2010;7:129–134. doi: 10.1186/1743-422X-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, et al. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: Characterization of HEV virions in blood circulation. J Clin Microbiol. 2010;48:1112–1125. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalton HR, et al. Neurological sequelae of acute and chronic HEV genotype 3 infection. Gut. 2010;59(Suppl II):A36. [Google Scholar]
- 26.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 27.Emerson SU, et al. Recombinant hepatitis E virus genomes infectious for primates: Importance of capping and discovery of a cis-reactive element. Proc Natl Acad Sci USA. 2001;98:15270–15275. doi: 10.1073/pnas.251555098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emerson SU, et al. In vitro replication of hepatitis E virus (HEV) genomes and of an HEV replicon expressing green fluorescent protein. J Virol. 2004;78:4838–4846. doi: 10.1128/JVI.78.9.4838-4846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.