Abstract
The larva of the invertebrate chordate Ciona intestinalis possesses only 36 striated muscle cells and lacks body segmentation. It can swim, however, like a vertebrate tadpole, and how its simple body achieves such sophisticated motor control remains puzzling. We found that muscle contractions in Ciona larvae are variable and can be changed by sensory stimuli, so that neuromuscular transmission can convert the variable neural inputs into graded muscle activity. We characterized the molecular nature of the nicotinic acetylcholine receptor (nAChR) at neuromuscular synapses. When heterologously expressed in Xenopus oocytes, this nAChR channel exhibited two biophysical features resembling vertebrate neuronal nAChRs rather than the muscle type: inward rectification and high Ca2+ permeability. Both of these properties were abolished by a simple mutation at the channel pore in one of the non-α subunits, called BGDE3, so as to adopt the sequence of related subunits in vertebrates, γ and ε. In vivo exchange of native BGDE3 with this mutant severely disrupted graded motor control, producing instead sporadic all-or-none–like flexions. The graded nature of excitation–contraction (E-C) coupling in this organism is based on the traits of the nAChR channel pore, which confer fine controllability on such a coarse motor architecture.
Keywords: ascidian larva, muscle physiology, locomotion, neuromuscular junction
In tailed aquatic vertebrates such as fish and amphibian tadpoles, swimming undulations are generated by sequential activation of the myotomal segments. These segments contain multiple classes of muscle fibers, including slow, fast, and intermediate types, with distinct mechanical and metabolic properties that constitute a “gearing” system adjustable to a wide range of speeds. The activity of a given segment is mostly independent of the others, and when, which, and how many fibers will be activated are determined by a neural network in the spinal cord (1–3). This neural mechanism by which contraction magnitude is varied is based on the logic for recruiting motor neurons into the active population, called the size principle (1–4). This control system for compound muscle fibers is likely a key innovation in vertebrates, but how far this basis can be traced back in invertebrate systems is poorly understood.
Ascidians are marine invertebrates that constitute a sister clade of the vertebrates (5, 6). The larva of the ascidian Ciona intestinalis (L) is tadpole shaped and possesses bilateral muscle bands, a dorsal neural tube supported by an axial notochord, and sensory organs including a photoreceptive ocellus (Fig. 1A and Fig. S1A). Despite this suite of organs, the body of the Ciona larva is quite simple. The muscle band on each side lacks a segmental plan and is composed of only 18 cells arranged in a single layer (Fig. 1A and Fig. S1). The 4–5 pairs of cholinergic motor neurons located in the motor ganglion (or the visceral ganglion) have accounted for the swimming behavior (Fig. S1) (7–10). These cholinergic neurons project axons down the tail and innervate a part of the (mainly dorsal) muscle cells, whereas others receive excitation via gap junctions (8–11). Despite such knowledge, how the Ciona larva swims like fish using such simplified motor units remains unknown.
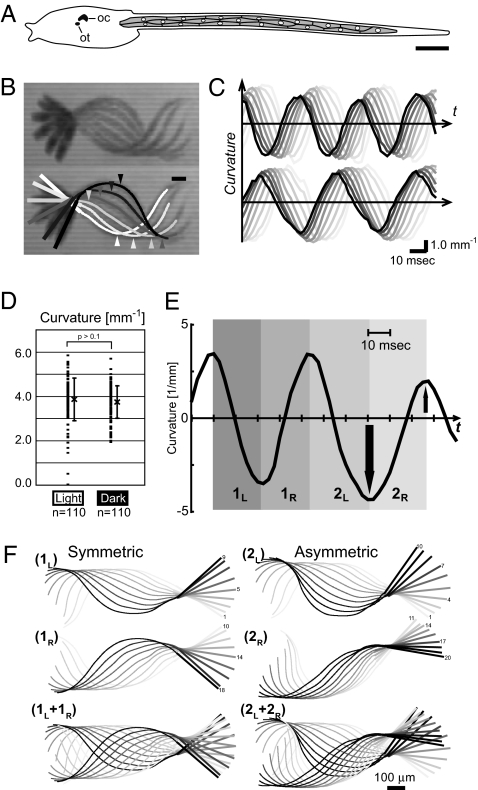
Fig. 1.
The Ciona larva swims by undulatory tail beats of varied magnitude. (A) Schematic drawing of the Ciona tadpole larva (from Fig. S1A). The tail harboring the muscle band (shaded) elongates posteriorly (Right). This arrangement of 18 mononucleated (circles) muscle cells is constant among individuals. On the dorsal side of the trunk resides a cerebral ganglion containing the ocellus photoreceptor (oc) and gravity sensor otolith (ot). (Scale bar, 100 μm.) (B) Merged image tracks showing half of a swimming cycle at 5-ms time intervals (Upper). The medial axis of trunk and the traced tail midline are depicted using motion analysis software (Lower; from white to black, 5-ms intervals). Thick and thin lines correspond to the trunk and tail regions, respectively. (Scale bar, 100 μm.) (C) Cyclic changes in the curvature along the tail. Black to light gray traces show the curvature changes at positions along the tail at 100-μm intervals (from black to light gray, 100–800 μm distant from the trunk–tail junction), clearly illustrating the rostrocaudally traveling wave. Frequency of cyclic bending is greater in the “dark” (Upper) than that in the “light” (Lower). (D) The magnitudes of bending curvatures are highly variable, although the average values are similar under dark and light conditions. (E and F) The left and right bending pairs can be symmetric (1L and 1R) or asymmetric (2L and 2R). (E) Curvature changes at 300 μm from the trunk–tail junction. The asymmetric bending cycles are indicated with arrows. (F) The motion tracks during the 1L, 1R, 2L, and 2R periods shown in (E). Colors show temporal order (light gray to black; intervals = 2.5 ms).
Recent research on the Ciona genome provides an opportunity to examine the molecular mechanisms underlying the physiology of this animal (12, 13). Given the correspondence between the body plans yet nevertheless the extreme contrast between their body complexities (minimal ascidian larva vs. much larger vertebrates), detailed comparison of their apparently similar swimming dynamics could uncover some novel control mechanisms. Here, we show that swimming flexions in Ciona larvae are of varied strength, or graded. Molecular characterization of Ciona muscle nicotinic ACh receptor channel (nAChR) illuminates an amino acid at the channel pore as an essential factor for the graded motor control, which the nAChR of ascidian larval muscle retains, but which vertebrates have lost.
Results
Tail Flexions Are Graded in Ciona Larvae.
The swimming movement of the Ciona tadpole larva was quantitatively analyzed through high-speed video imaging. The midline of the larval body was traced (Fig. 1B) (14), and the magnitude of local tail flexure (“curvature,” the reciprocal of the measured curvature radius) was calculated at successive points along the tail (100-μm intervals from the trunk–tail junction). Larvae beat their tail in an alternating left–right manner, and waves of contraction are propagated from anterior to posterior, as in vertebrate fish (Fig. 1C and Movie S1) (11). When a long-pass filter was used to cut off the light visible to the larvae (<590 nm), they swam more vigorously in what is called a shading response (Movie S1) (11, 15). In the “dark,” the tail-beating frequency (ϕ) and the velocity of the traveling wave (ϖ) increased significantly (15), whereas the wavelength of the swimming cycles (λ = ϖ/ϕ) and the mean of the flexure strength (curvature) were relatively constant (Fig. 1D and S2 A–C). Despite the constant flexure, the local force generated by the muscle cells should be much greater under dark conditions, because the beating frequency is significantly higher (Fig. S3). This suggests that the local force generated in the muscle bands must change in response to light stimuli.
The magnitudes of the muscle contractions were highly variable also under a constant light (Fig. 1D). Even in a neighboring cycle pair, the flex became spontaneously larger or smaller, and the undulation became bilaterally asymmetrical, in contrast to the typical symmetrical cycle (Fig. 1 E and F). When such asymmetries were seen, a corresponding change in swimming direction occurred (Fig. S2 D–F). Thus, the muscle bands are able to generate variable bending patterns stochastically or in response to sensory stimuli, which leads to observable changes in the force and/or direction of swimming.
Ci-nAChR-A1, -BGDE3, and -B2/4 Form the Larval Muscle nAChR.
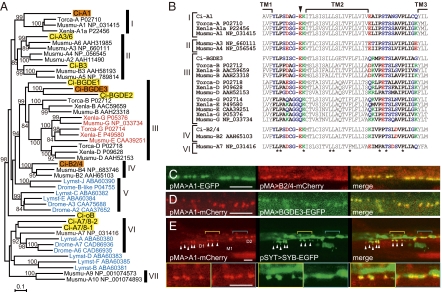
To determine the molecular basis for neuromuscular transmission, we characterized the nAChR subunits expressed in the larval muscle. In an earlier (13) and subsequent surveys of the Ciona genome, we identified 10 nAChR subunit genes and a rapsyn homolog gene (Fig. 2A, Fig. S4A, and Table S1). We cloned all of them and determined their expression patterns during larval development (Fig. S4B). Larval muscle cells express three subunit genes, Ci-nAChR-A1, -BGDE3, and -B2/4 (called here A1, BGDE3, and B2/4 for short), as well as Ci-rapsyn. A1 is orthologous to the vertebrate muscle α1 subunit, and BGDE3 is one of the three paralogs of the vertebrate muscle non-α nAChR subunits β1/γ/δ/ε (Fig. 2A). Although B2/4 is the cognate of the vertebrate β2/β4 subunits that function in the nervous system, the B2/4 gene was expressed in both the nervous system and muscle cells (Fig. 2A and Fig. S4).
Fig. 2.
(A) A neighbor-joining tree constructed from all of the C. intestinalis nAChR subunits found in the genome and the related subunits from vertebrates (Musmu, Mus musculus; Torca, Torpedo californica), snail (Lymst, Lymnaea stignalis), and fruit fly (Drome, Drosophila melanogaster). Bootstrap values >80 are shown. I, chordate muscle α1; II, chordate neuronal α2–6/β3; III, chordate muscle non-α (β/γ/δ/ε); IV, chordate β2/4; V, α7/8; VI, α9/10. (B) Sequence alignment around the TM1–TM3 regions. Shown are sequences from the A1, B2/4, and BGDE3 subunits from C. intestinals; the vertebrate muscle α, β, γ, δ, and ε subunits (Torca, Torpedo californica; Xenla, Xenopus laevis; Musmu, Mus musculus); and neuronal subunits of M. musculus. Asterisks mark the amino acids conserved among all of the sequences. The position of the “intermediate ring” is indicated by an arrowhead. Acidic (red), basic (green), and polar (blue) amino acids are highlighted. (C and D) Focal colocalization of fluorescent protein-tagged A1 and B2/4 (C), and A1 and BGDE3 (D), expressed under the control of the muscle actin gene promoter (pMA). (Scale bars, 10 μm.) (E) Close association between the A1-mCherry patches (red) and nerve fibers or termini (green). Anterior muscle cells are shown, and the approximate positions of the D1, D2, and M1 muscle cell nuclei are labeled (anterior is left). The anterior dorsal muscle cell (D1) receives branched nerve termini, which are visualized by Ci-Synaptobrevin-EGFP (SYB-EGFP, green, arrowheads). A1-mCherry makes patches at sites corresponding to those EGFP signals. Such branches are not found at the D2 level, and A1-mCherry foci are associated with the nerve fiber extending down the tail (see also Fig. S5E). The D1 and D2 regions shown in yellow and blue, respectively, are magnified in the Lower panels. (Scale bars, 10 μm.)
When we expressed EGFP or mCherry fusions with A1, BGDE3, and B2/4 under the control of an ascidian muscle actin gene promoter (pMA) (16), a chain of fluorescent patches was seen along the dorsal edge of the muscle band (Fig. S5A). The foci of A1-EGFP or -mCherry matched up with those of B2/4-mCherry, BGDE3-EGFP, and also of rapsyn-EGFP (Fig. 2 C and D and Fig. S5 B–D). The diameter of these patches was 0.9 ± 0.2 μm (mean ± SD; n = 215), and their average density was 3.7 ± 0.6 (mean ± SD; n = 26)/10 μm along the anterior–posterior axis. Neither the size nor density of the patches appeared related to the mosaic expression levels of the exogenous fluorescent nAChR subunits. We next coinjected pMA > A1-mCherry together with a pSYT > EGFP or pSYT > Syb-EGFP construct containing an ascidian neuron-specific synaptotagmin promoter to drive expression of EGFP or a Ci-synaptobrevin-EGFP fusion protein (Fig. S1D) (9, 17). The A1-mCherry patches merged precisely with the motor terminals on D1 muscle cells and were also in contact with motor axons posteriorly (Fig. 2E and Fig. S5E) (9). These obervations suggest that A1, BGDE3, and B2/4 are targeted to neuromuscular junctions to form postsynaptic receptor aggregates.
To test whether the three subunits form a functional nAChR channel, we expressed their cRNAs in Xenopus oocytes. Under a two-electrode voltage clamp at a holding potential of –50 mV, robust inward currents were elicited in response to ACh application in a dose-dependent manner [Fig. 3 A and D and Fig. S6A; EC50 = 180 ± 102 μM (mean ± SD)]. Much reduced currents were observed in the absence of the B2/4 subunit, and no currents were detected when A1 with only B2/4 or A1 alone was expressed (Fig. 3 B–D and Fig. S6A). These results, together with our pharmacological tests (Fig. S6), suggest that A1, BGDE3, and B2/4 constitute functional nAChRs.
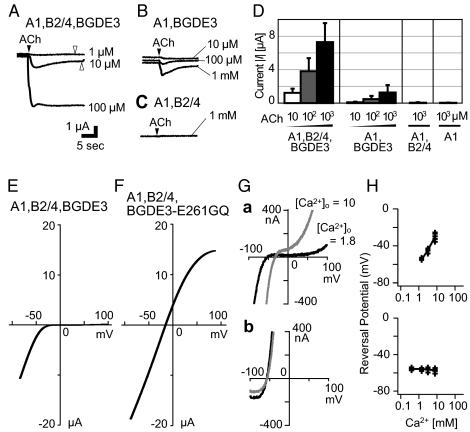
Fig. 3.
The A1,B2/4,BGDE3 channel is an inward rectifier and permeable to Ca2+. (A–D) Upon combinatorial expression of A1, B2/4, and BGDE3 subunits, large ACh-dependent inward currents are elicited in a dose-dependent manner (A and D), and the responses are markedly reduced in the absence of B2/4 (B and D). In the absence of BGDE3, or both BGDE3 and B2/4, ACh-evoked currents are not seen (C and D). The holding potential was set at −50 mV. The unit bar is common for A–C, and the filled and open arrowheads indicate the time points of the puff and wash of ACh, respectively. For more details, see also Fig. S6A. (E and F) Current–voltage (I-V) relationship for ACh-evoked currents through the A1,B2/4,BGDE3 receptor (E) or A1,B2/4,BGDE3-E261GQ, an intermediate ring mutant (F); see also Fig. S7A. (G) I-V curves for A1,B2/4,BGDE3 (a) and A1,B2/4,BGDE3-E261GQ (b) in the external Ca2+ concentration at 1.8 mM (black) or 10 mM (gray). In both external solutions, [Na+]o = 10 mM and [K+]o = 2 mM (Table S2). The reversal potential is shifted substantially in a but not in b. (H) Changes in the reversal potential at different [Ca2+]o with A1,B2/4,BGDE3 (Upper) and A1,B2/4,BGDE3-E261GQ (Lower) channels. Used external solutions are shown in Table S2. Reversal potentials read from multiple I-V traces (>3) in each solution are plotted against the external Ca2+ activities (Table S2). The fitted lines were drawn using the Goldman–Hodgkin–Katz equation (SI Materials and Methods). For all of the records in this figure, the oocytes were injected in advance with the Ca2+ chelator BAPTA, which inhibited activation of the intrinsic Ca2+-activated channels (Fig. S7A).
The Ciona Muscle nAChR is a Ca2+-Permeable Inward Rectifier.
The current–voltage (I-V) relationship of the ACh-induced currents through the A1,BGDE3,B2/4 channel exhibited striking inward rectification (Fig. 3E), when oocytes were injected in advance with a Ca2+ chelater BAPTA to inhibit the cell-intrinsic Ca2+-activated ion (mainly Cl−) channels (Fig. S7A) (18). A similar I-V pattern was seen in the absence of B2/4 (Fig. S7B). Such inward rectification is characteristic of neuronal nAChR subtypes in vertebrates, such as the α7, α3β4, and α4β2, but vertebrate muscle nAChRs show linear I-V patterns (19–22). It is also known that when these vertebrate neuronal nAChRs are mutated at a Glu residue in the “intermediate ring” (Fig. 2B), which faces the channel pore to form a part of the gate, the mutant channels show a linear I-V pattern (21–23). In fact, A1, BGDE3, and B2/4 all possess the Glu (E) at the intermediate ring, whereas in vertebrate muscle nAChRs with a symmetric I-V curve, the γ/ε subunits invariably possess a Gly-Gln (GQ) in place of the Glu (Fig. 2B). When this Glu of BGDE3 was mutated to Ala, Gln, or Asp, the inward rectification was more or less relieved (Fig. S7 C and D). With BGDE3-E261GQ mutant, which mimics the intermediate ring of the vertebrate γ/ε subunits, the I-V curve became linear (Fig. 3F), although the current amplitudes were less affected.
This Glu at the intermediate ring is also responsible for high Ca2+ permeability (21, 22). Vertebrate neuronal nAChRs in general show higher permeability to Ca2+ relative to Na+, PCa/PNa, than muscle nAChRs (19–22). We next assessed the reversal potential changes in the different extracellular concentrations of Na+, K+, or Ca2+ ([Na+]o, [K+]o, or [Ca2+]o). In the wild-type A1,B2/4,BGDE3 channel, the reversal potential was shifted by changes of [Na+]o, [K+]o, or [Ca2+]o (Fig. 3 G and H and Fig. S7 E and F). However, the reversal potential of the mutant A1,B2/4,BGDE3-E261GQ channel was unaffected by changes in [Ca2+]o (Fig. 3 G and H and Fig. S7 E and F). The Goldman–Hodgkin–Katz equation gave an estimate for PCa/PNa of 1.8 ± 0.4 (mean ± SD) with the wild-type channel, whereas <0.01 for the A1,B2/4,BGDE3-E261GQ channel. The value for the wild type is comparable to those for the α3β4 and α4β2 subtypes of vertebrate neuronal nAChRs (20, 21). Thus, Glu261 of BGDE3, which the vertebrate γ/ε subunits have lost, is essential for the inward rectification and high Ca2+ permeability of the Ciona muscle nAChR.
Functional Alteration of Muscle nAChRs Disrupts the Graded Motor Control.
The above results show that BGDE3 is expressed only in the muscle and is indispensable for the formation of functional channels. When a morpholino antisense-oligo (MO) against BGDE3 was injected into fertilized eggs, the larvae showed no sign of tail beating, although they hatched at the appropriate time and had a normal looking albeit slight curling of the tail (Fig. 4 Ba and Ca and Movie S2). A control MO containing five-base mismatches did not affect swimming behavior (Fig. 4 Ab, Bb, and Cb and Movie S3). When the antisense MO was injected into one of the blastomeres of two-cell stage embryos, the derived larvae beat their tail unilaterally (Fig. 4 Ae, Be, and Ce). This unilateral activity was enhanced by the dark stimulus (Movie S4), implying that BGDE3-MO did not affect the neural signals from the ocellus to the muscle.
Fig. 4.
BGDE3-E261GQ mutation alters the swimming pattern in vivo. (A) Histograms depicting the incidences of particular flexion magnitudes (maximum curvature during a half cycle at 300 μm from the trunk–tail junction) during swimming-like repetitive movements triggered by “dark” stimuli. The pattern during normal swimming (a) is as depicted in Fig. 1 and shown here as a reference. The combination of injections (MO, cRNA, and/or plasmid DNA) and the number of examined contractions are shown above each histogram. (B) Representative trace of the bending cycles under each experimental condition. (a) BGDE3-MO injected larva. Traces in (b–g) correspond to those in A. Changes in curvature at three points along the tail are represented in red (100 μm from the trunk–tail junction), blue (300 μm), and green (500 μm). The delays before the posterior points reach the peak reflect the rostrocaudal propagation of bending. A time scale bar is included in each trace. In d and g, the top traces are shown at five-times-longer duration. The curvature changes during the periods indicated by i and ii (framed yellow and blue, respectively) were expanded below for comparison with other traces. (C) Merged midline traces during a bending cycle. Intervals are 2.5 ms in a–f and 5 ms in g. (Scale bar, 100 μm for a–g). The first and last tracks are colored purple and orange, respectively, and the track with maximum curvature in contralateral bending is colored red.
Coinjection of BGDE3 cRNA with BGDE3-MO largely restored the swimming behavior (Fig. 4 Ac, Bc, and Cc and Movie S5), verifying the target specificity of the MO. Next, to address the functional importance of the molecular properties of the Ciona muscle nAChR, we used cRNA encoding the BGDE3-E261GQ mutant to rescue the MO phenotype. When BGDE3-E261GQ cRNA was introduced together with BGDE3-MO, most flexions became feeble, but heavy bending also occurred on occasions (Fig. 4 Ad, Bd, and Cd and Movie S6). These sporadic heavy contractions did not appear to propagate down the tail (Fig. 4 Bd and Cd). In this experiment, however, the ubiquitous expression from BGDE3-E261GQ cRNA might have altered some neuronal nAChRs within the locomotor network. To localize the expression to the muscle, we expressed BGDE3 under the control of the muscle-specific promoter (pMA). Coinjection of BGDE3-MO and the plasmid DNA encoding pMA > BGDE3 (wild type) again restored the swimming behavior (Fig. 4 Af, Bf, and Cf), although the larvae frequently showed weak flexions or failures during a swimming bout, as predicted by the mosaic expression from the plasmid DNA in the muscle cells. When we coinjected BGDE3-MO and pMA > BGDE3-E261GQ, most flexions again became feeble with sporadic larger contractions interjected (Fig. 4 Ag, Bg, and Cg and Movie S7). These results suggest that without Ca2+ permeability and/or inward rectification of the muscle nAChR, the tail muscle is unable to respond in a graded manner to variable neural inputs but shows discrete patterns of twitching that are either too weak or too large.
Discussion
It has been documented that muscle and neuronal nAChRs of vertebrates can be distinguished on the basis of their ion selectivity and rectification of current flow. Whereas neuronal receptors show considerable Ca2+ permeability and inward rectification, muscle receptors are less permeable to Ca2+ and are not rectifying (19–22). Although recent findings have to some extent revised this view (24, 25), the distinction between neuronal and muscle nAChRs of vertebrates still appears to be significant: PCa/PNa (or PCa/PCs) in vertebrate muscle nAChRs mostly ranges from 0.1 to 0.8, whereas in neuronal nAChRs, PCa/PNa is generally >1.0 and often >5.0 (20, 21, 24). These properties can be made to change by point mutations at the intermediate ring (21, 22). In fact, the vertebrate muscle-specific γ and ε subunits have consistently lost the Glu at this position, whereas all others depicted in Fig. 2A retain this Glu residue (except for the anion-permeable Lymnaea B and F subunits). In our survey of the genome of amphioxus, a chordate group different from olfactores (vertebrates + urochordates), we found no substitution of this Glu, except for a change to Gln in an α3/6-like subunit. Thus, the substitution seen in the γ/ε subunits is specific to vertebrates.
Amphioxus has body segments, each of which contains myocytes with different metabolic properties. The muscle cells extend a thin process to form a synapse on the central neural tube (26) and express both voltage-gated Na+ (NaV) and L-type Ca2+ (l-CaV) channels (27, 28). Expression of these two channels enables transmission of the electrical signal through the thin process by a NaV-based action potential (AP) to instantaneously trigger the excitation–contraction (E-C) coupling mechanism via l-CaV (27–29). On the other hand, muscle cells of the Ciona larva are electrically coupled and innervated polyneuronally and multisynaptically (refs. 9, 11, and this study). With this configuration, graded flexions that travel down the tail should never be derived from an all-or-none mode of E-C coupling, based on an AP of the sort documented in vertebrate fast skeletal myotubes. Ascidian larval muscle cells lack NaV channels, but possess l-CaV channels and ryanodine receptors (RyRs) (30–33). The acute increase of [Ca2+]i for contraction is mediated by Ca2+-induced Ca2+ release (CICR) through the RyRs, not by the physical coupling of l-CaV channels and RyRs as in vertebrate skeletal muscle (32–34). We propose here that the Ca2+ flux through nAChRs, together with that through l-CaV, stimulates RyRs to direct CICR for E-C coupling in the Ciona muscle (Fig. 5). This sets the muscle response range in proportion to variable neural inputs. The I-V relationship of the ascidian l-CaV has the typical V shape, and depolarization to approximately −10 to +10 mV is required for full-activation (33). By contrast, nAChR currents ensure large influxes of Ca2+ at lower membrane potentials due to the large driving force. Endplate potentials (EPPs) induced by nAChR can stimulate l-CaV channels in the vicinity and even those in neighboring cells, given the electrical coupling within the muscle band (35). In fact, the APs recorded to date from ascidian larval muscle cells appear too slow to represent each swimming contraction (refs. 7, 11; Fig. S7G). Instead, EPP-like membrane potential changes of variable amplitude have been observed at the swimming frequency (refs. 7, 11; Fig. S7H), supporting that EPP-like transients, involving Ca2+ through nAChRs, activate muscle in proportion to the inputs.
Fig. 5.
Proposed model of the E-C coupling mechanism in the Ciona larval muscle band. (A) Schematic representation of the I-V relationships of the Ciona larval muscle nAChR (red) and l-CaV (green). Note that the relative magnitudes of currents through the nAChR and l-CaV are not known. (B and C) Hypothetical mechanism by which Ca2+ is loaded into the cytosol (see Discussion). The muscle cells arranged in a single layer are coupled via gap junctions (dotted lines). ACh activates postsynaptic nAChRs (red) to elicit inward currents containing Ca2+ (straight arrows), which stimulates cytosolic CICR (circular arrows) (Upper). The endplate potential (EPP) induced by nAChRs stimulates l-CaV channels (green), which also activates CICR (Lower). The propagation of the depolarization is dependent on the size of the cation current, channel density, and other factors. At times when and in regions where the membrane potential is high, currents through nAChRs are diminished, irrespective of the presence of ACh, due to their rectification. The sizes of arrows reflect the magnitudes of the events. (B) At moderate levels of ACh, nAChRs locally elicit EPPs, which spatially decline and only weakly affect l-CaV (green). (C) When ACh levels are high, EPPs will effectively activate l-CaV channels and may evoke a Ca2+-based action potential (AP). The Ca2+ carried by both nAChRs and l-CaV channels contribute to stimulating CICR, more or less in proportion to the ACh levels. Without the Ca2+-permeability of nAChRs, the Ca2+-loading process shown in Upper section would be lost, but l-CaV-based APs would still occur. This dual source of Ca2+ with different properties can provide a mechanism for postsynaptic cells to respond in proportion to variable inputs.
In our model, when BGDE3-E261GQ replaces the endogenous BGDE3, the channel would largely lose the Ca2+ flux and thus induce CICR only via activation of l-CaV, so that muscle contractions would be feeble in most cases and be sporadically large. Thus, the situation becomes close to the all-or-none fashion, as in vertebrate twitch muscles. The mutant nAChR also lacks the property of rectification, so that it would conduct outward currents at membrane potentials appropriate for l-CaV activation. On occasions that ACh strongly stimulates the mutant receptors, l-CaV would be activated, but the outward current through the mutant receptor may counteract the depolarization and shunt the Ca2+ influx through l-CaV. This provides another possible explanation for why the tail beats weakened. Rectification of the receptor channel can therefore ensure successful relay of the depolarizing signal to l-CaV (21). It is intriguing that switching between GluR2-containing (Ca2+-impermeable/nonrectifying) and GluR2-free (Ca2+-permeable/rectifying) AMPA receptors in central synapses could be relevant to memory and disease, such as epilepsy, in the mammalian brain (36). Such a relay mechanism from a Ca2+-permeable ligand-gated inward rectifier to a voltage-dependent Ca2+ channel may, in general, represent a quasilinear means of loading Ca2+ in proportion to varied inputs.
Vertebrates have a variety of skeletal muscle systems. In fish, the slow (red, tonic) muscle fibers are responsible for slow sustained swimming, whereas the fast fibers are for heavy bending during fast swimming or escapes. Graded control of the motion is achieved through regulation of the number and types of the fibers activated (1–3), and in fish, moreover, the slow fibers can make graded contractions by themselves (35, 37). The slow muscle fibers in zebrafish larvae lack NaV-dependent APs and are tightly coupled across myotomes to distribute the synaptic signal to neighboring cells for averaging of the depolarization drive, as well as for priming the activation of the next segment (35, 37). Such a signaling process may also work in the Ciona muscle band, whereas it remains unclear how these tonic fibers of vertebrates load Ca2+ to make graded responses. The body of Ciona larva, by contrast, lacks such compound muscle systems of multiple fiber types, so that the mechanism we proposed here may be the only means to provide fine controllability upon the muscle band of a small number of single-typed fibers. The evolutionary contrast found in the chordate nAChRs may represent an adjustment of ion channel properties to their distinct lives and body forms, which can be attributed to a single amino acid substitution at the channel pore.
Materials and Methods
Detailed methods are provided in the SI Materials and Methods. Wild C. intestinalis adults were collected in Mikawa Bay, Aichi, Japan, and kept for weeks in laboratory tanks. The embryos were reared in artificial seawater (“Jamarin U,” Jamarin Laboratory, Osaka, Japan) at 18 °C. They hatched 17 h postfertilization (hpf), and the swimming patterns of 22- to 27-hpf larvae were recorded using a high-speed camera (FAST-CAM Rabbit mini 2; Photron) at 400 frames per second and an exposure time of 0.5 ms. The body shape in each frame was analyzed using “Bohboh ver. 3 (Bohboh Software, Tokyo)” a motion analyzer originally developed to study flagellar beating (14).
Our extensive surveys of the genome databases (Joint Genome Institute, C. intestinalis ver. 1, http://genome.jgi-psf.org/ciona4/ciona4.home.html, and ver. 2, http://genome.jgi-psf.org/Cioin2/Cioin2.home.html) revealed 10 nAChR subunit genes and a rapsyn homolog gene (Table S1). We obtained full-length cDNAs for these through 5′ and 3′ rapid amplification of cDNA ends (DDBJ/GenBank accession nos. AB539786–AB539796).
Experiments using Xenopus laevis oocytes were performed in accordance with the guidelines of the Animal Care Committee of the National Institute for Physiological Sciences. The A1, B2/4, and BGDE3 cRNAs were synthesized from cDNAs subcloned into pSD64TF (from T. Snutch, University of British Columbia, Vancouver, BC, Canada). Site-specific mutagenesis was performed using a QuikChange kit (Stratagene). Injection of cRNA into defolliculated oocytes and two-electrode voltage clamp were performed using standard protocols. Unless otherwise noted, 50–100 nL of 25 mM BAPTA was injected into oocytes at least 15 min before recording. Oocytes were clamped at −50 mV in a normal external solution [ND96 (in mM): 96 NaCl, 2 KCl, 2 CaCl2, 1.8 MgCl2, 5 Hepes (pH 7.5)] containing 1–2 μM of atropine, and 2.5 μL of agonist solution was puffed onto their surface. The I-V relationship was examined by applying a ramp pulse. After subtracting leak currents (before puffing), reversal potential values were read by eye. The chemical compositions and estimated ionic activities of the external solutions used for examining ion permeability are listed in Table S2.
The sequence of BGDE3-MO was AAGACAGTTAATCATTCTCTATCTC; that of the control MO had five mismatches and was AAcACAcTTAATgATTCTgTATgTC (GeneTools). Aliquots of MO (0.5 mM MO in 200 mM KCl) were manually injected into fertilized eggs having an intact chorion. The BGDE3 cRNA was synthesized as described above. The pMA > BGDE3 plasmid was prepared from a pEGFP-N1 backbone (Clontech), an ascidian muscle actin gene promoter (16), and BGDE3 cDNA. The cRNA and plasmid DNA for morphant rescuing were injected at 0.4 μg/μL and 6–8 μg/mL, respectively.
Supplementary Material
Acknowledgments
Dr. Hiroaki Misonoh helped us greatly in the initial steps of this work. We thank Drs. R. Tsien for mCherry; N. Satoh for Ciona synaptobrevin cDNA; T. Snutch for pSD64TF; Drs. Hiroki Takahashi and Shigehiro Yamada for cooperation with the animal care and supply and for plasmids; and Drs. Edward Cooper, Ian Meinertzhagen, and Quentin Bone for critical reading of the manuscript. We also thank Dr. Shin-ichi Higashijima for partial support and discussion and Dr. Hiroki Nishida for encouragement. This study was supported by Japan Society for the Promotion of Science Postdoctoral Fellowship 02291 (to A.N.) and by the Research Institute of Marine Invertebrates Foundation (A.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences reported in this paper have been deposited in the DDBJ/GenBank database (accession nos. AB539786–AB539796).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013547108/-/DCSupplemental.
References
- 1.Jayne BC, Lauder GV. How swimming fish use slow and fast muscle fibers: Implications for models of vertebrate muscle recruitment. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1994;175:123–131. doi: 10.1007/BF00217443. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DH, McLean DL, Hale ME, Fetcho JR. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron. 2007;53:91–102. doi: 10.1016/j.neuron.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 3.McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- 4.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 5.Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 6.Putnam NH, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 7.Ohmori H, Sasaki S. Development of neuromuscular transmission in a larval tunicate. J Physiol. 1977;269:221–254. doi: 10.1113/jphysiol.1977.sp011900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meinertzhagen IA, Lemaire P, Okamura Y. The neurobiology of the ascidian tadpole larva: Recent developments in an ancient chordate. Annu Rev Neurosci. 2004;27:453–485. doi: 10.1146/annurev.neuro.27.070203.144255. [DOI] [PubMed] [Google Scholar]
- 9.Imai JH, Meinertzhagen IA. Neurons of the ascidian larval nervous system in Ciona intestinalis: I. Central nervous system. J Comp Neurol. 2007;501:316–334. doi: 10.1002/cne.21246. [DOI] [PubMed] [Google Scholar]
- 10.Horie T, Nakagawa M, Sasakura Y, Kusakabe TG, Tsuda M. Simple motor system of the ascidian larva: Neuronal complex comprising putative cholinergic and GABAergic/glycinergic neurons. Zoolog Sci. 2010;27:181–190. doi: 10.2108/zsj.27.181. [DOI] [PubMed] [Google Scholar]
- 11.Bone Q. On the locomotion of ascidian tadpole larvae. J Mar Biol Assoc U K. 1992;72:161–186. [Google Scholar]
- 12.Dehal P, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- 13.Okamura Y, et al. Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes. Physiol Genomics. 2005;22:269–282. doi: 10.1152/physiolgenomics.00229.2004. [DOI] [PubMed] [Google Scholar]
- 14.Baba SA, Mogami Y. An approach to digital image analysis of bending shapes of eukaryotic flagella and cilia. Cell Motil Cytoskeleton. 1985;5:475–489. [Google Scholar]
- 15.Zega G, Thorndyke MC, Brown ER. Development of swimming behaviour in the larva of the ascidian Ciona intestinalis. J Exp Biol. 2006;209:3405–3412. doi: 10.1242/jeb.02421. [DOI] [PubMed] [Google Scholar]
- 16.Satou Y, Satoh N. Two cis-regulatory elements are essential for the muscle-specific expression of an actin gene in the ascidian embryo. Dev Growth Differ. 1996;38:565–573. doi: 10.1046/j.1440-169X.1996.t01-1-00013.x. [DOI] [PubMed] [Google Scholar]
- 17.Katsuyama Y, et al. Regulation of synaptotagmin gene expression during ascidian embryogenesis. Dev Biol. 2002;244:293–304. doi: 10.1006/dbio.2002.0584. [DOI] [PubMed] [Google Scholar]
- 18.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 19.Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 20.Costa ACS, Patrick JW, Dani JA. Improved technique for studying ion channels expressed in Xenopus oocytes, including fast superfusion. Biophys J. 1994;67:395–401. doi: 10.1016/S0006-3495(94)80494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haghighi AP, Cooper E. A molecular link between inward rectification and calcium permeability of neuronal nicotinic acetylcholine α3β4 and α4β2 receptors. J Neurosci. 2000;20:529–541. doi: 10.1523/JNEUROSCI.20-02-00529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson GG, Karlin A. The location of the gate in the acetylcholine receptor channel. Neuron. 1998;20:1269–1281. doi: 10.1016/s0896-6273(00)80506-1. [DOI] [PubMed] [Google Scholar]
- 24.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Fucile S, Sucapane A, Grassi F, Eusebi F, Engel AG. The human adult subtype ACh receptor channel has high Ca2+ permeability and predisposes to endplate Ca2+ overloading. J Physiol. 2006;573:35–43. doi: 10.1113/jphysiol.2006.108092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flood PR. Structure of the segmental trunk muscle in amphioxus. With notes on the course and “endings” of the so-called ventral root fibres. Z Zellforsch Mikrosk Anat. 1968;84:389–416. [PubMed] [Google Scholar]
- 27.Hagiwara S, Kidokoro Y. Na and Ca components of action potential in amphioxus muscle cells. J Physiol. 1971;219:217–232. doi: 10.1113/jphysiol.1971.sp009658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagiwara S, Henkart MP, Kidokoro Y. Excitation-contraction coupling in amphioxus muscle cells. J Physiol. 1971;219:233–251. doi: 10.1113/jphysiol.1971.sp009659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue I, Tsutui I, Bone Q, Brown ER. Evolution of skeletal muscle excitation-contraction coupling and the appearance of dihydropyridine-sensitive intramembrane charge movement. Proc Biol Sci. 1994;255:181–187. [Google Scholar]
- 30.Miyazaki S, Takahashi K, Tsuda K. Calcium and sodium contributions to regenerative responses in the embryonic excitable cell membrane. Science. 1972;176:1441–1443. doi: 10.1126/science.176.4042.1441. [DOI] [PubMed] [Google Scholar]
- 31.Davis AK, Greaves AA, Dallman JE, Moody WJ. Comparison of ionic currents expressed in immature and mature muscle cells of an ascidian larva. J Neurosci. 1995;15:4875–4884. doi: 10.1523/JNEUROSCI.15-07-04875.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajo K, Okamura Y. Development of transient outward currents coupled with Ca2+-induced Ca2+ release mediates oscillatory membrane potential in ascidian muscle cells. J Neurophysiol. 2004;92:1056–1066. doi: 10.1152/jn.00043.2004. [DOI] [PubMed] [Google Scholar]
- 33.Nakajo K, Chen L, Okamura Y. Cross-coupling between voltage-dependent Ca2+ channels and ryanodine receptors in developing ascidian muscle blastomeres. J Physiol. 1999;515:695–710. doi: 10.1111/j.1469-7793.1999.695ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Biase V, Franzini-Armstrong C. Evolution of skeletal type e-c coupling: A novel means of controlling calcium delivery. J Cell Biol. 2005;171:695–704. doi: 10.1083/jcb.200503077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna VM, Brehm P. An electrically coupled network of skeletal muscle in zebrafish distributes synaptic current. J Gen Physiol. 2006;128:89–102. doi: 10.1085/jgp.200609501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: Synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Buckingham SD, Ali DW. Sodium and potassium currents of larval zebrafish muscle fibres. J Exp Biol. 2004;207:841–852. doi: 10.1242/jeb.00839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.