Abstract
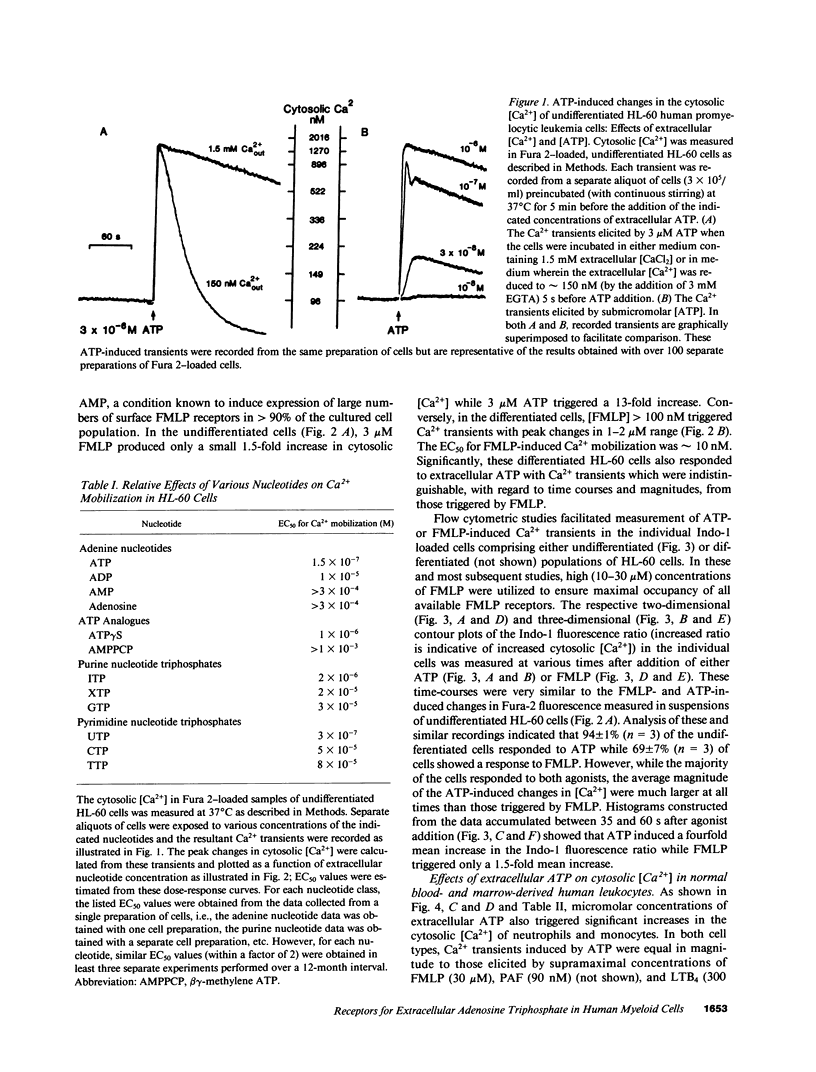
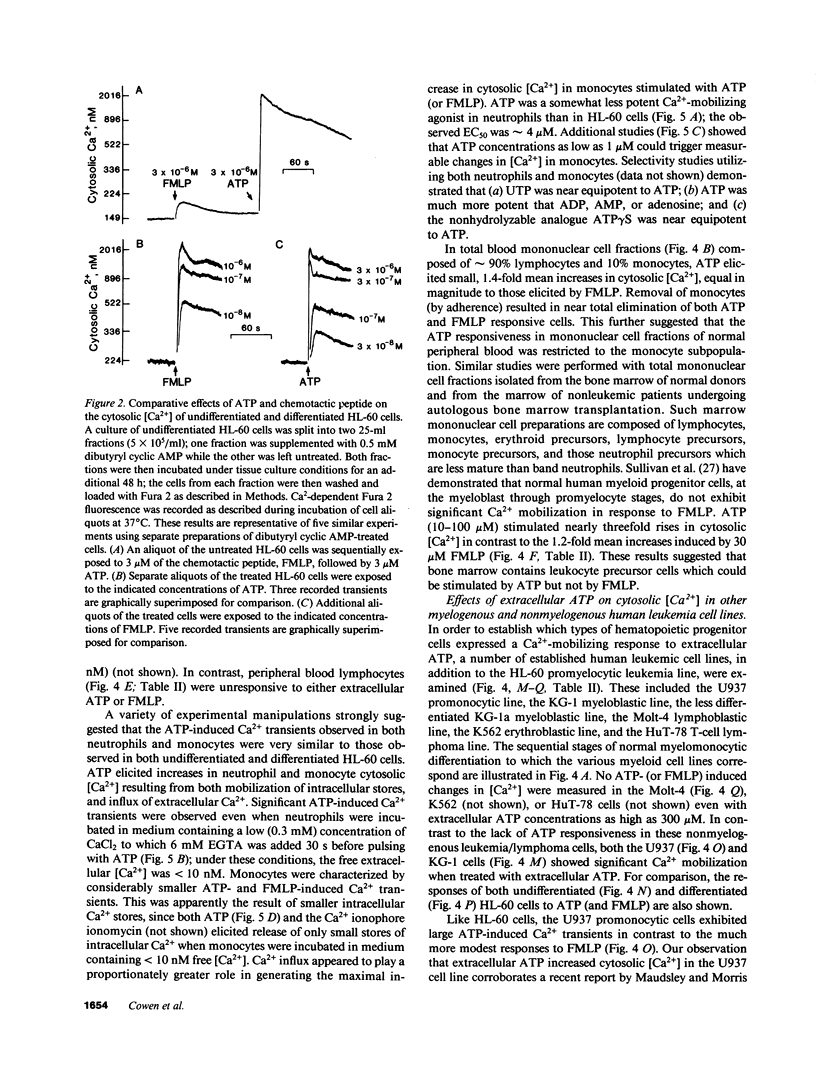
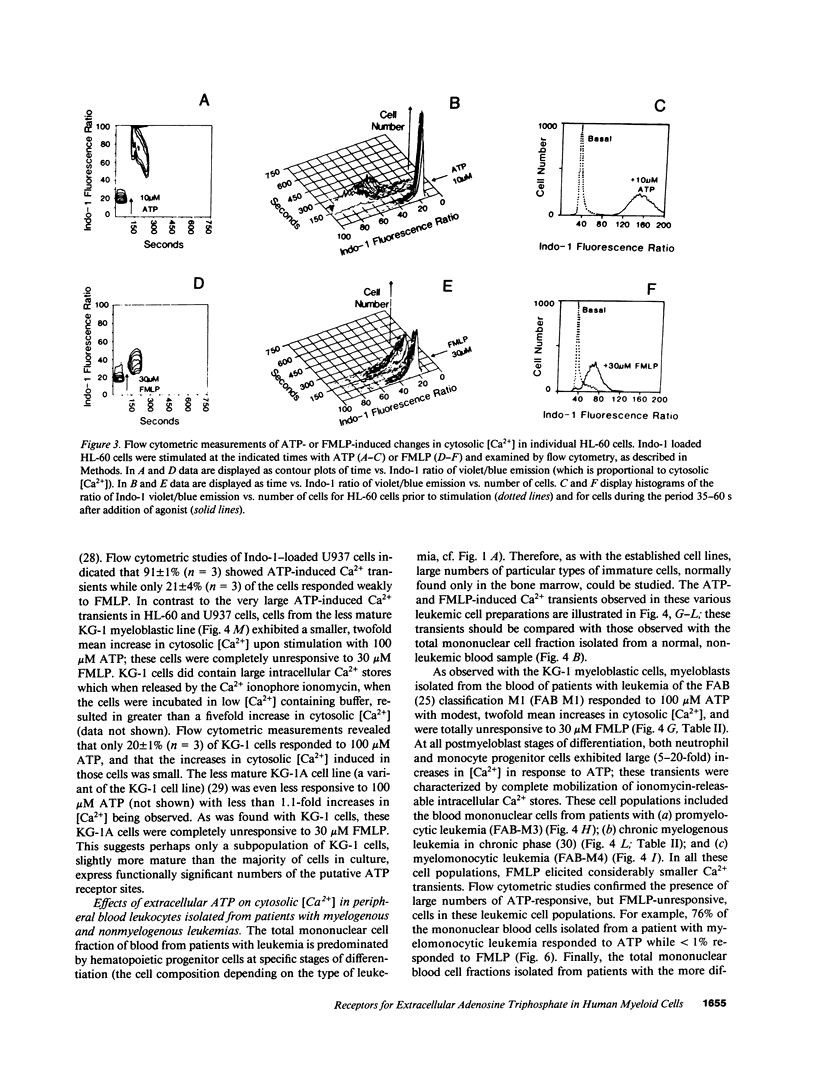
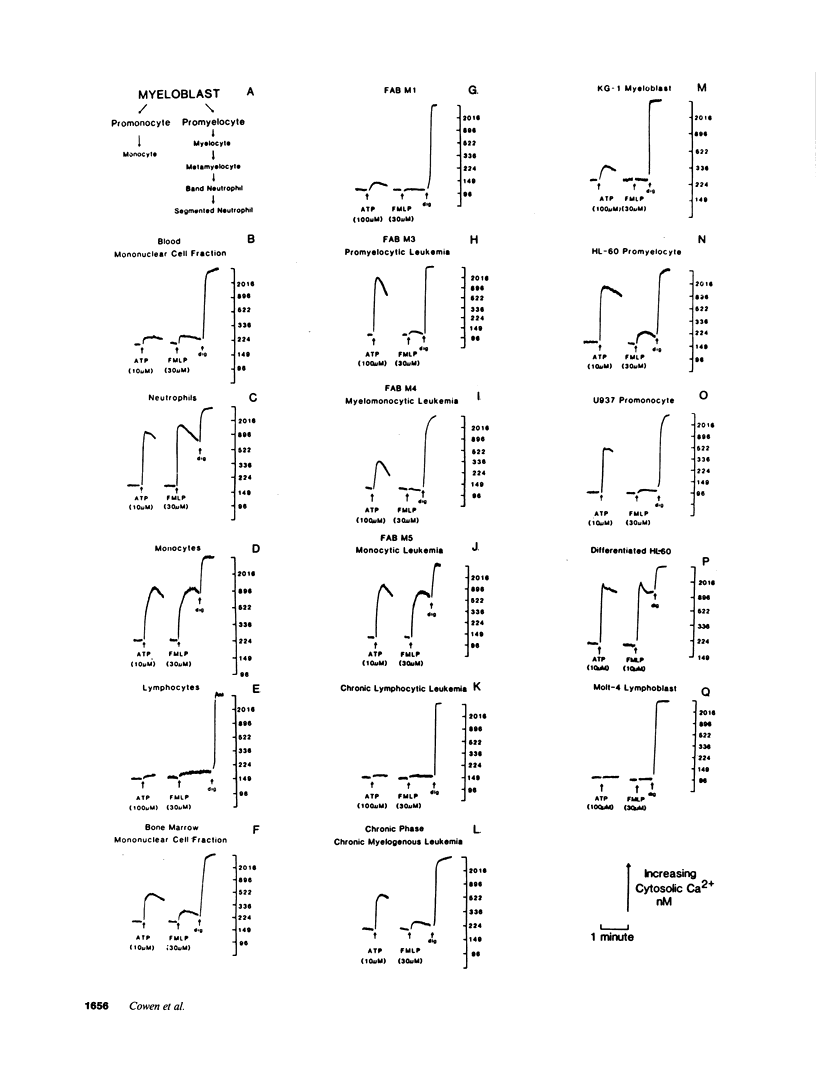
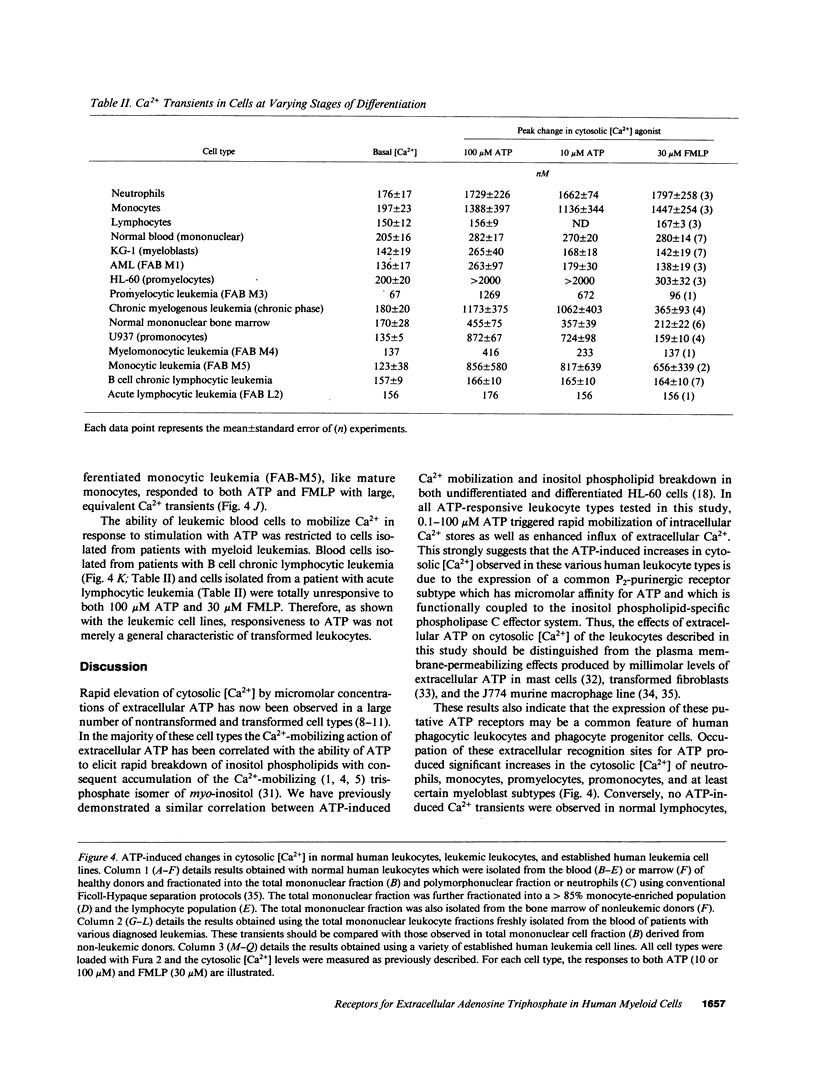
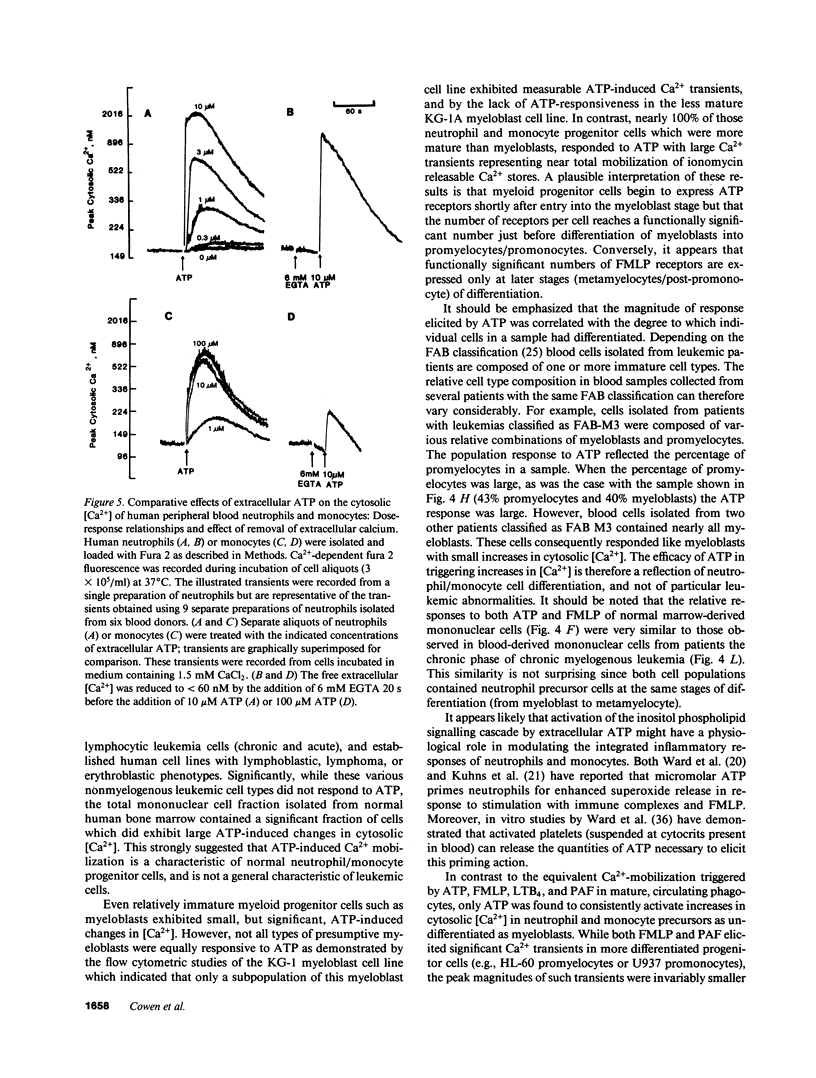
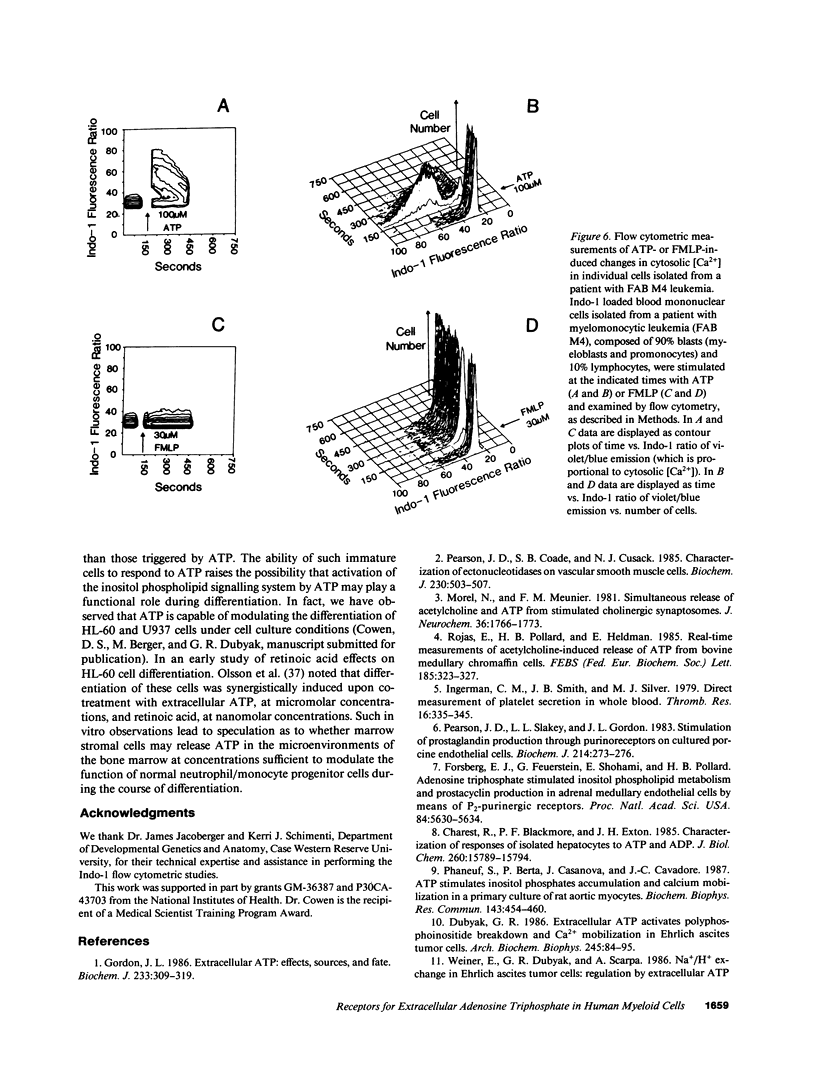
We have examined the ability of extracellular ATP to elicit intracellular Ca2+ mobilization in a broad range of human leukocytes at particular stages of hematopoietic differentiation. The average cytosolic [Ca2+] in various leukocyte populations was measured in Fura 2-loaded cell suspensions while the cytosolic [Ca2+] in individual, Indo 1-loaded leukocytes was assayed by flow cytometric methods. Utilizing normal blood- and marrow-derived cells, human leukemic cell lines, and mononuclear cell fractions derived from the blood of patients with various leukemias, we have found that ATP-induced Ca2+ mobilization appears restricted to leukocytes of neutrophil/monocyte ontogeny. Significant ATP-induced increases in cytosolic [Ca2+] were observed in neutrophils, monocytes, and myeloid progenitor cells as immature as myeloblasts, but not in lymphocytes. Extensive characterization of the ATP-induced changes in [Ca2+] observed in the HL-60 promyelocytic cell line have indicated these Ca2+-mobilizing effects of ATP can be correlated with an activation of inositol phospholipid breakdown via the occupation of P2-purinergic receptors Significantly, of the various agonists (FMLP, platelet-activating factor, LTB4, and ATP) which elicit equivalent and maximal Ca2+ mobilization in mature neutrophils and monocytes, ATP was the most efficacious stimulant of Ca2+ mobilization in immature neutrophil/monocyte precursors. Thus, expression of putative P2-purinergic receptors for ATP appears to precede expression of other receptor types known to activate the inositol phospholipid signaling cascades in terminally differentiated phagocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Gerard C., Olbrantz P., Wilson J., McCall C. E., McPhail L. C. Priming of the respiratory burst of neutrophils by diacylglycerol. Independence from activation or translocation of protein kinase C. J Biol Chem. 1987 May 15;262(14):6643–6649. [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Chaplinski T. J., Niedel J. E. Cyclic nucleotide-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1982 Nov;70(5):953–964. doi: 10.1172/JCI110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman C. M., Smith J. B., Silver M. J. Direct measurement of platelet secretion in whole blood. Thromb Res. 1979;16(3-4):335–344. doi: 10.1016/0049-3848(79)90081-1. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Billing R., Lusis A. J., Sparkes R., Golde D. W. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980 Aug;56(2):265–273. [PubMed] [Google Scholar]
- Kuhns D. B., Wright D. G., Nath J., Kaplan S. S., Basford R. E. ATP induces transient elevations of [Ca2+]i in human neutrophils and primes these cells for enhanced O2- generation. Lab Invest. 1988 Apr;58(4):448–453. [PubMed] [Google Scholar]
- Maudsley D. J., Morris A. G. Rapid intracellular calcium changes in U937 monocyte cell line: transient increases in response to platelet-activating factor and chemotactic peptide but not interferon-gamma or lipopolysaccharide. Immunology. 1987 Jun;61(2):189–194. [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Morel N., Meunier F. M. Simultaneous release of acetylcholine and ATP from stimulated cholinergic synaptosomes. J Neurochem. 1981 May;36(5):1766–1773. doi: 10.1111/j.1471-4159.1981.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R., Gallo R. C. Priming of human myeloid leukemic cell lines HL-60 and U-937 with retinoic acid for differentiation effects of cyclic adenosine 3':5'-monophosphate-inducing agents and a T-lymphocyte-derived differentiation factor. Cancer Res. 1982 Oct;42(10):3928–3933. [PubMed] [Google Scholar]
- Pearson J. D., Coade S. B., Cusack N. J. Characterization of ectonucleotidases on vascular smooth-muscle cells. Biochem J. 1985 Sep 1;230(2):503–507. doi: 10.1042/bj2300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. D., Slakey L. L., Gordon J. L. Stimulation of prostaglandin production through purinoceptors on cultured porcine endothelial cells. Biochem J. 1983 Jul 15;214(1):273–276. doi: 10.1042/bj2140273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Rider L. G., Niedel J. E. Diacylglycerol accumulation and superoxide anion production in stimulated human neutrophils. J Biol Chem. 1987 Apr 25;262(12):5603–5608. [PubMed] [Google Scholar]
- Rojas E., Pollard H. B., Heldman E. Real-time measurements of acetylcholine-induced release of ATP from bovine medullary chromaffin cells. FEBS Lett. 1985 Jun 17;185(2):323–327. doi: 10.1016/0014-5793(85)80931-5. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A., Friedberg I. Effect of exogenous ATP on the permeability properties of transformed cultures of mouse cell lines. J Biol Chem. 1977 Jul 10;252(13):4584–4590. [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Sokal J. E., Baccarani M., Russo D., Tura S. Staging and prognosis in chronic myelogenous leukemia. Semin Hematol. 1988 Jan;25(1):49–61. [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Steinberg T. H., Silverstein S. C. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987 Mar 5;262(7):3118–3122. [PubMed] [Google Scholar]
- Sullivan R., Melnick D. A., Malech H. L., Meshulam T., Simons E. R., Lazzari K. G., Proto P. J., Gadenne A. S., Leavitt J. L., Griffin J. D. The effects of phorbol myristate acetate and chemotactic peptide on transmembrane potentials and cytosolic free calcium in mature granulocytes evolve sequentially as the cells differentiate. J Biol Chem. 1987 Jan 25;262(3):1274–1281. [PubMed] [Google Scholar]
- Verghese M. W., Charles L., Jakoi L., Dillon S. B., Snyderman R. Role of a guanine nucleotide regulatory protein in the activation of phospholipase C by different chemoattractants. J Immunol. 1987 Jun 15;138(12):4374–4380. [PubMed] [Google Scholar]
- Verghese M. W., Fox K., McPhail L. C., Snyderman R. Chemoattractant-elicited alterations of cAMP levels in human polymorphonuclear leukocytes require a Ca2+-dependent mechanism which is independent of transmembrane activation of adenylate cyclase. J Biol Chem. 1985 Jun 10;260(11):6769–6775. [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Role of guanine nucleotide regulatory protein in polyphosphoinositide degradation and activation of phagocytic leukocytes by chemoattractants. J Cell Biochem. 1986;32(1):59–69. doi: 10.1002/jcb.240320107. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., McCulloch K. K., Johnson K. J. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Invest. 1988 Apr;58(4):438–447. [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., McCulloch K. K., Phan S. H., Powell J., Johnson K. J. Platelet enhancement of O2-. responses in stimulated human neutrophils. Identification of platelet factor as adenine nucleotide. Lab Invest. 1988 Jan;58(1):37–47. [PubMed] [Google Scholar]
- Wiener E., Dubyak G., Scarpa A. Na+/H+ exchange in Ehrlich ascites tumor cells. Regulation by extracellular ATP and 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1986 Apr 5;261(10):4529–4534. [PubMed] [Google Scholar]



