Abstract
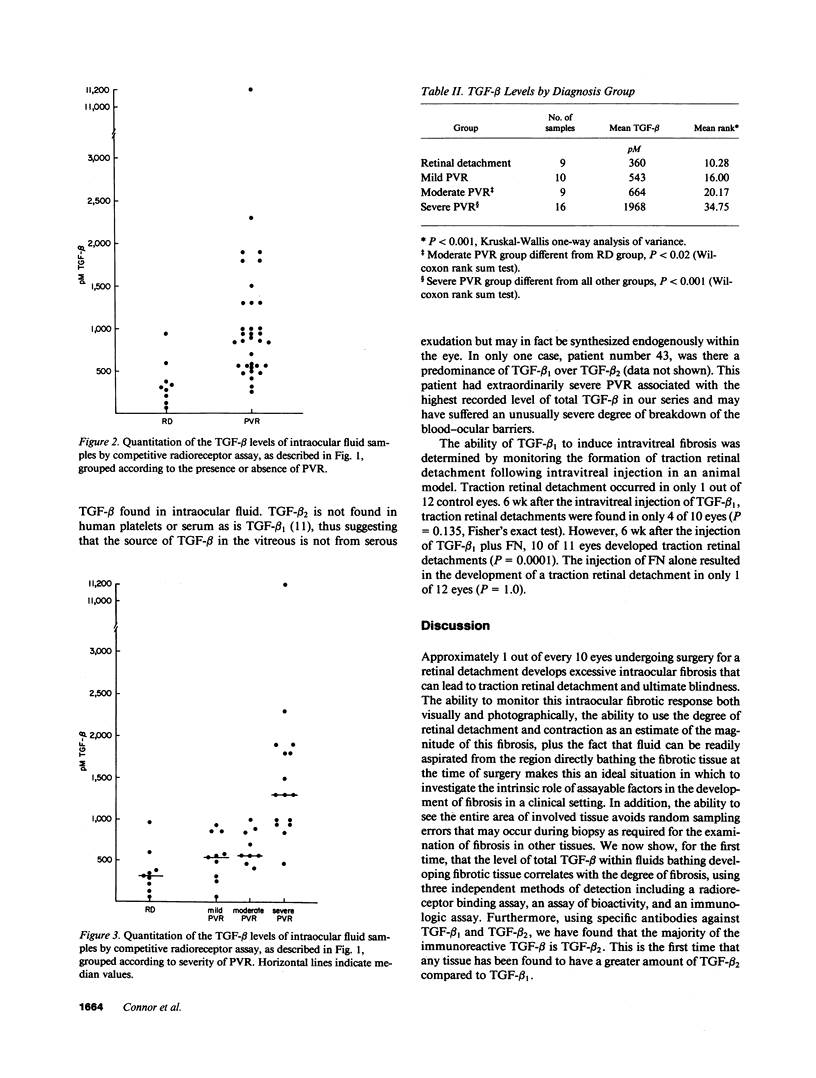
Approximately 1 out of every 10 eyes undergoing surgery for retinal detachment develops excessive intraocular fibrosis that can lead to traction retinal detachment and ultimate blindness. This disease process has been termed proliferative vitreoretinopathy (PVR). The ability to monitor and grade this fibrotic response accurately within the eye as well as the ability to aspirate vitreous cavity fluid bathing the fibrotic tissue makes this an ideal setting in which to investigate the development of fibrosis. Although laboratory studies have recently shown that transforming growth factor-beta (TGF-beta) can enhance fibrosis, little clinical evidence is yet available correlating the level of this or other growth factors with the degree of fibrosis in a clinical setting. We have found that vitreous aspirates from eyes with intraocular fibrosis associated with PVR have more than three times the amount of TGF-beta (1,200 +/- 300 pM [SEM]) found in eyes with uncomplicated retinal detachments without intraocular fibrosis (360 +/- 91 pM [SEM]). Using an in vitro assay, 84-100% of the TGF-beta activity could be blocked with specific antibodies against TGF-beta 2, whereas only 10-21% could be blocked by specific antibodies against TGF-beta 1. TGF-beta 1 was used in an animal model of traction retinal detachment. Since beta 1 and beta 2 have essentially identical biologic effects and only human beta 1 was available in quantities required, beta 1 was chosen for these in vivo studies. The injection of TGF-beta1 plus fibronectin (FN) but not TGF-beta1 alone into the vitreous cavity of rabbits resulted in the increased formation of intraocular fibrosis and traction retinal detachments as compared to control eyes. In previous studies, intravitreal FN levels were also found to be elevated in eyes with intraocular fibrosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Campochiaro P. A., Jerdan J. A., Glaser B. M., Cardin A., Michels R. G. Vitreous aspirates from patients with proliferative vitreoretinopathy stimulate retinal pigment epithelial cell migration. Arch Ophthalmol. 1985 Sep;103(9):1403–1405. doi: 10.1001/archopht.1985.01050090155053. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Clarkson J. G., Green W. R., Massof D. A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol. 1977 Jul;84(1):1–17. [PubMed] [Google Scholar]
- Cromack D. T., Sporn M. B., Roberts A. B., Merino M. J., Dart L. L., Norton J. A. Transforming growth factor beta levels in rat wound chambers. J Surg Res. 1987 Jun;42(6):622–628. doi: 10.1016/0022-4804(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Kimura A., Maehama S., Tong B. D. Platelet-derived growth factor: roles in normal and v-sis transformed cells. Cancer Surv. 1985;4(4):633–653. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Ikeda T., Lioubin M. N., Marquardt H. Human transforming growth factor type beta 2: production by a prostatic adenocarcinoma cell line, purification, and initial characterization. Biochemistry. 1987 May 5;26(9):2406–2410. doi: 10.1021/bi00383a002. [DOI] [PubMed] [Google Scholar]
- Jaccoma E. H., Conway B. P., Campochiaro P. A. Cryotherapy causes extensive breakdown of the blood-retinal barrier. A comparison with argon laser photocoagulation. Arch Ophthalmol. 1985 Nov;103(11):1728–1730. doi: 10.1001/archopht.1985.01050110124039. [DOI] [PubMed] [Google Scholar]
- Johnson N. F., Foulds W. S. Observations on the retinal pigment epithelium and retinal macrophages in experimental retinal detachment. Br J Ophthalmol. 1977 Sep;61(9):564–572. doi: 10.1136/bjo.61.9.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampik A., Green W. R., Michels R. G., Nase P. K. Ultrastructural features of progressive idiopathic epiretinal membrane removed by vitreous surgery. Am J Ophthalmol. 1980 Dec;90(6):797–809. doi: 10.1016/s0002-9394(14)75195-5. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laqua H., Machemer R. Clinical-pathological correlation in massive periretinal proliferation. Am J Ophthalmol. 1975 Nov;80(5):913–929. doi: 10.1016/0002-9394(75)90289-5. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Machemer R., van Horn D., Aaberg T. M. Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol. 1978 Feb;85(2):181–191. doi: 10.1016/s0002-9394(14)75946-x. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Michels R. G. A clinical and histopathologic study of epiretinal membranes affecting the macula and removed by vitreous surgery. Trans Am Ophthalmol Soc. 1982;80:580–656. [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Jensen K., Machemer R., Azarnia R. Autotransplantation of retinal pigment epithelium in intravitreal diffusion chamber. Am J Ophthalmol. 1975 Sep;80(3 Pt 2):530–537. doi: 10.1016/0002-9394(75)90222-6. [DOI] [PubMed] [Google Scholar]
- Pircher R., Jullien P., Lawrence D. A. Beta-transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem Biophys Res Commun. 1986 Apr 14;136(1):30–37. doi: 10.1016/0006-291x(86)90872-7. [DOI] [PubMed] [Google Scholar]
- Rentsch F. J. The ultrastructure of preretinal macular fibrosis. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977 Sep 28;203(3-4):321–337. doi: 10.1007/BF00409837. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Flanders K. C., Kondaiah P., Thompson N. L., Van Obberghen-Schilling E., Wakefield L., Rossi P., de Crombrugghe B., Heine U., Sporn M. B. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res. 1988;44:157–197. doi: 10.1016/b978-0-12-571144-9.50010-7. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprugel K. H., McPherson J. M., Clowes A. W., Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987 Dec;129(3):601–613. [PMC free article] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Van Horn D. L., Aaberg T. M., Machemer R., Fenzl R. Glial cell proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol. 1977 Sep;84(3):383–393. doi: 10.1016/0002-9394(77)90684-5. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]



