Abstract
The inositol pyrophosphate, diphosphoinositol pentakisphosphate, regulates p53 and protein kinase Akt signaling, and its aberrant increase in cells has been implicated in apoptosis and insulin resistance. Inositol hexakisphosphate kinase-2 (IP6K2), one of the major inositol pyrophosphate synthesizing enzymes, mediates p53-linked apoptotic cell death. Casein kinase-2 (CK2) promotes cell survival and is upregulated in tumors. We show that CK2 mediated cell survival involves IP6K2 destabilization. CK2 physiologically phosphorylates IP6K2 at amino acid residues S347 and S356 contained within a PEST sequence, a consensus site for ubiquitination. HCT116 cells depleted of IP6K2 are resistant to cell death elicited by CK2 inhibitors. CK2 phosphorylation at the degradation motif of IP6K2 enhances its ubiquitination and subsequent degradation. IP6K2 mutants at the CK2 sites that are resistant to CK2 phosphorylation are metabolically stable.
Inositol phosphates are messenger molecules mediating a variety of cell functions, the best known being inositol 1,4,5-trisphosphate (IP3) which releases intracellular calcium (1). Inositol pyrophosphates contain a pyrophosphate bond with energetic qualities comparable to ATP (2). The most studied example, diphosphoinositol pentakisphosphate (IP7), displays a 5′-diphosphate (3, 4). Another isomer of IP7 has been reported with a pyrophosphate at position 1 or 3 (5, 6). The predominant mammalian form contains the 5′-diphosphate and will hereafter be designated IP7.
IP7 is generated in mammals by a family of inositol hexakisphosphate kinases (IP6Ks) with evidently disparate functions (7, 8). IP6K1 generates a pool of IP7 which physiologically inactivates the protein kinase Akt leading to marked upregulation of glycogen synthase kinase beta (GSK3β) and inhibition of the mTOR protein translation pathway (9). IP6K1 mediates insulin resistance and weight gain, as IP6K1 deleted mice manifest insulin sensitivity and diminished body weight (9). Functions for IP6K3 have not been elucidated. An enzyme called VIP1 generates the 1/3 isomer and is functionally most prominent in yeast where it regulates phosphate disposition (10).
Inositol hexakisphosphate kinase-2 (IP6K2), first identified by Lindner and coworkers (11) as a proapoptotic gene, sensitizes cells to apoptotic stimuli (12). Mice with targeted deletion of IP6K2 display relative resistance to ionizing radiation and an increased incidence of aerodigestive tract carcinoma (13). Cell survival associated with heat shock protein 90 (HSP90) involves IP6K2 (14). Thus, HSP90 physiologically binds IP6K2 to inhibit its catalytic activity. Drugs and mutations that block this binding activate IP6K2 leading to cell death (14).
IP6K2 killing is selective for p53 mediated cell death (15). Thus, HCT116 colon cancer cells with deletion of the IP6K2 gene manifest decreased sensitivity to agents that kill via p53 but not to agents whose apoptotic actions involve different mechanisms. IP6K2 prevents activation of p53-associated cell arrest thereby leading to augmentation of the cell death genetic program apparently by default.
Heretofore posttranslational modifications of IP6K2 have not been characterized. We chose to examine phosphorylation of IP6K2 by casein kinase-2 (CK2) for the following reasons. IP6K2 possesses a proline (P), glutamic acid (E), serine (S), and threonine (T) (PEST) sequence whose phosphorylation typically facilitates protein degradation (16). The PEST sequence in IP6K2 contains a consensus for CK2 phosphorylation. CK2 is a serine/threonine protein kinase with prominent prosurvival functions which are mediated in part by phosphorylation of IκBα (17). CK2 is markedly augmented in a wide range of cancers (18) and appears to enhance malignant transformation by creating a more hospitable environment for tumor growth (19). CK2 also stimulates angiogenesis (20) as well as multidrug resistance (21). Pandolfi and coworkers demonstrated that the tumor suppressor promyelocytic leukemia protein (PML) is a physiologic substrate of CK2 (22). CK2 phosphorylation of PML facilitates its degradation through the ubiquitin pathway leading to enhanced cell survival and increased tumor growth.
In the present study we demonstrate that CK2 physiologically phosphorylates IP6K2 at two identified sites. Cell death elicited by CK2 inhibitors is prevented by deletion of IP6K2. Inhibition of CK2 enhances IP6K2 stability, whereas CK2 phosphorylation of IP6K2 destabilizes the enzyme. Thus, regulation of IP6K2 appears to mediate the prosurvival actions of CK2.
Results
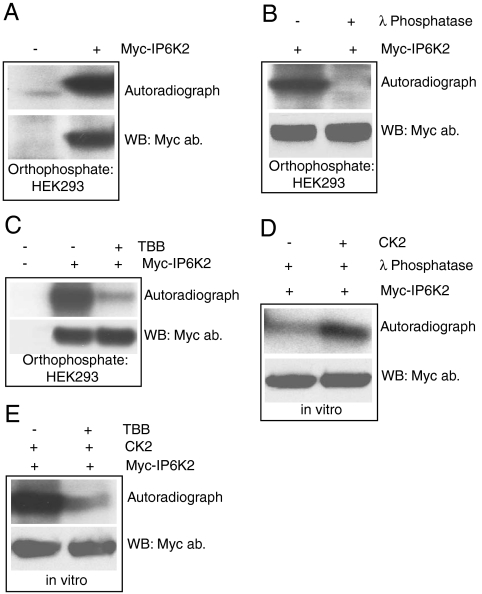
In HEK293 cells labeled with 32P-orthophosphate IP6K2 is prominently radiolabeled (Fig. 1A) with the label abolished by treatment with λ phosphatase (Fig. 1B). This phosphorylation predominantly involves CK2, as treatment with the selective CK2 inhibitor TBB (4, 5, 6, 7-tetrabromobenzotriazole) virtually abolishes the labeling of IP6K2 (Fig. 1C). Definitive evidence that CK2 phosphorylates IP6K2 is apparent utilizing λ phosphatase treated immunoprecipitated Myc-IP6K2 in which added CK2 directly phosphorylates IP6K2 (Fig. 1D). Specificity for this action is evident in its inhibition by treatment with TBB (Fig. 1E).
Fig. 1.
CK2 phosphorylates IP6K2 A. Myc-IP6K2 phosphorylation in HEK293 cells detected by orthophosphate labeling. B. Specificity of IP6K2 phosphorylation is confirmed by λ phosphatase treatment of the radiolabeled IP6K2 protein after orthophosphate labeling. C. TBB treatment (50 μM, 3 h) abolishes Myc-IP6K2 phosphorylation in HEK293 cells. D. CK2 phosphorylation of immunoprecipitated and λ phosphatase treated Myc-IP6K2 in vitro. E. TBB (5 μM) inhibits CK2 phosphorylation of Myc-IP6K2 in vitro.
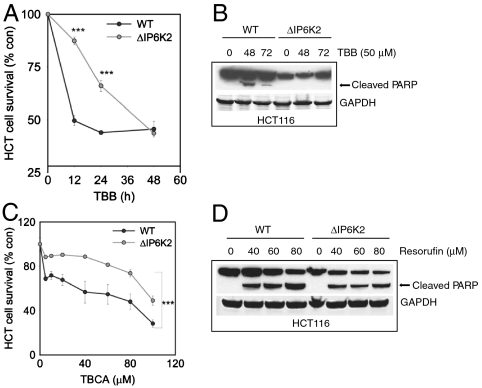
Previously, we developed preparations of the HCT116 colon cancer cell line with targeted deletion of IP6K2 and showed that the cells are resistant to the apoptotic actions of agents acting through p53 (15). The IP6K2 deleted cells are markedly resistant to the apoptotic effects of the CK2 inhibitor TBB in terms of cell survival monitored by MTT (Fig. 2A). Cleavage of PARP, another index of cell death, is greatly diminished in IP6K2 mutants (Fig. 2B). We also employed TBCA, a much more potent and selective derivative of TBB (23) (Fig. 2C). TBCA substantially reduces HCT116 cell survival at concentrations as little as 5 μM. We evaluated resorufin, a structurally distinct CK2 inhibitor (24) (Fig. 2D). In wild-type cells resorufin stimulates PARP cleavage in a concentration dependent fashion. In IP6K2 deleted cells PARP cleavage is reduced and does not manifest a concentration-response dependence upon resorufin.
Fig. 2.
IP6K2 mediates cell death associated with CK2 inhibition A. IP6K2Δ HCT116 cells display resistance to inhibitory effect of TBB on cell survival compared to WT HCT cells. (***p < 0.001). B. TBB mediated PARP cleavage is absent in IP6K2Δ HCT116 cells. C. TBCA’s inhibitory effect on cell survival is greatly reduced in IP6K2Δ HCT116 cells. (***p < 0.001). D. Resorufin induced apoptosis, as indicated by PARP cleavage, is reduced in IP6K2Δ HCT116 cells.
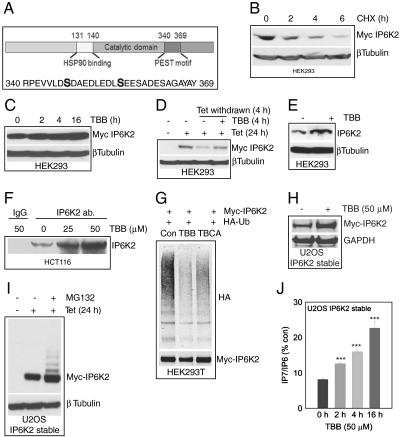
We wondered how IP6K2 mediates cell death associated with CK2 inhibition. The tumor suppressor protein promyelocytic leukemia (PML) is degraded through the ubiquitin pathway following CK2 phosphorylation at its PEST motif, a consensus sequence that signals degradation (22). IP6K2 possesses a PEST motif at amino acid residues 340–369 with potential CK2 phosphorylation sites (Fig. 3A). Treatment of HEK293 cells with the protein synthesis inhibitor cycloheximide (CHX) leads to a rapid loss of IP6K2 protein with a half-life of about 2 h (Fig. 3B). We explored potential influences of CK2 phosphorylation upon the stability of IP6K2 protein. If CK2 facilitates IP6K2 degradation, then one might expect increased IP6K2 levels when CK2 is inhibited. Treatment with TBB does increase IP6K2 protein concentration (Fig. 3C). In another approach to evaluating IP6K2 stability, we utilized HEK293TRex cells in which IP6K2 is induced by treatment with tetracycline (Fig. 3D). Withdrawal of tetracycline for 4 h depletes IP6K2 protein, while TBB treatment prevents this depletion. Endogenous levels of IP6K2 in HEK293 cells are markedly augmented by TBB treatment (Fig. 3E). In HCT116 cells TBB treatment also substantially enhances concentrations of IP6K2 protein (Fig. 3F).
Fig. 3.
IP6K2 stability is enhanced by CK2 inhibition A. CK2 phosphorylation sites at the degradation specific PEST motif of IP6K2. B. Myc-IP6K2 protein displays a half-life of 2 h as measured by cycloheximide treatment of HEK293 cells. C. TBB (50 μM) stabilizes Myc-IP6K2 protein levels in a time-dependent fashion. D. TBB (50 μM) protects tetracycline removal induced IP6K2 protein degradation. E. TBB (50 μM, 6 h) stabilizes endogenous IP6K2 levels in HEK293 cells. F. Immunoprecipitation of endogenous IP6K2 from HCT116 cells reveals increased protein levels after TBB treatment. G. IP6K2 ubiquitination is significantly reduced after TBB (50 μM) or TBCA (10 μM) treatment, H. TBB stabilizes Myc-IP6K2 in U2OS cells. I. The MG132 treatment reveals multiple ubiquitinated Myc-IP6K2. J. TBB increases IP7 levels in U2OS cells stably expressing Myc-IP6K2. (***p < 0.001).
To examine whether IP6K2 degradation involves the ubiquitin pathway, we monitored its ubiquitination (Fig. 3G). Treatment with both TBB and the more potent derivative TBCA diminishes IP6K2 ubiquitination consistent with the notion that CK2 destabilizes IP6K2 by increasing its ubiquitin conjugation.
To further substantiate the stabilization of IP6K2 protein by CK2 inhibition, we employed U2OS cells in which IP6K2 is stably expressed. In these cells treatment with TBB greatly increases levels of IP6K2 (Fig. 3H). To demonstrate ubiquitination directly, we treated cells with the proteasome inhibitor MG132 which causes a pronounced laddering of overexpressed IP6K2 (Fig. 3I). To assess whether IP6K2 protein stabilized by TBB represents the functional enzyme, we monitored its catalytic activity (Fig. 3J). TBB increases IP7 generation presumably by IP6K2 in a time-dependent fashion with a tripling of catalytic activity at 16 h following TBB addition.
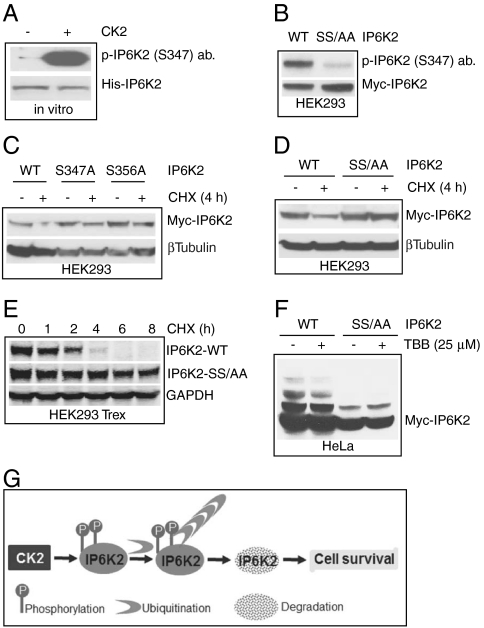
To elucidate the molecular mechanism whereby CK2 phosphorylates and destabilizes IP6K2, we conducted mass spectrometric analysis of GST-IP6K2 overexpressed in HEK293 cells. The enzyme is phosphorylated at serines 347 and 356 within the PEST motif (Fig. 3A).
To characterize the sites of phosphorylation, we developed an antibody that is selective for IP6K2 phosphorylated at S347 and clearly recognizes CK2-phosphorylated IP6K2 purified from bacteria (Fig. 4A). This phosphorylation is abolished in immunoprecipitated Myc-IP6K2 with mutations of both S347 and S356 (IP6K2 SS/AA) (Fig. 4B).
Fig. 4.
IP6K2 destabilization is associated with CK2 phosphorylation at serines 347 and 356 A. CK2 phosphorylation of bacterially purified His-tagged IP6K2 is detected by α-pIP6K2 (S347) antibody. Total protein was detected by coommassie blue staining. B. Myc-IP6K2 S347/356AA double mutant is not recognized by the α-pIP6K2 (S347) antibody. WT and mutant IP6K2 were overexpressed in HEK293 cells and were immunoprecipitated using α-Myc antibody. C. IP6K2 S347A and S356A single mutants display partial but not total stability after cycloheximide treatment. D. IP6K2 S347/356AA double mutant does not degrade after 4 h cycloheximide treatment. E. Substantial increase in half-life of IP6K2 S347/356AA double mutant. The half-life of the mutant is 8 h as compared to 2 h for WT IP6K2. F.TBB decreases ubiquitination of WT-IP6K2, whereas the mutant is resistant to ubiquitination and hence unaffected by TBB treatment. G. Model showing CK2 phosphorylation mediated ubiquitination and degradation of IP6K2.
If phosphorylation of serines 347/356 destabilizes IP6K2, then their mutations should slow turnover of the protein. Mutations of S347 and S356 singly partially alter IP6K2 stability, monitored in terms of degradation following cycloheximide treatment (Fig. 4C); whereas the double mutant is stable (Fig. 4D). Wild-type IP6K2 displays a half-life of about 2 h, whereas very little degradation of IP6K2-SS347/356AA is evident over an 8 h time course (Fig. 4E).
Resistance to degradation of IP6K2 is associated with diminished ubiquitination. Thus, TBB treatment decreases the ubiquitin ladder of IP6K2 in wild-type HeLa cells (Fig. 4F). The mutant IP6K2-SS347/356AA displays a further reduction of ubiquitination in the presence or absence of TBB (Fig. 4F).
Discussion
In the present study we obtained evidence that IP6K2 mediates the antiapoptotic actions of CK2. Thus, the proapoptotic effects of a number of CK2 inhibitors of differing chemical classes all are greatly reduced with IP6K2 deletion. CK2 acts by phosphorylating IP6K2 to decrease its stability (Fig. 4G). Under normal circumstances IP6K2 turns over with a half-life of about 2 h. Enzyme stability is notably enhanced by inhibition of CK2. CK2 phosphorylates IP6K2 at serines 347 and 356 whose mutation leads to marked increases in IP6K2 stability.
The amino acid sequence in IP6K2 that is phosphorylated by CK2 is not present in IP6K1 or IP6K3. IP6K1 can be phosphorylated by CK2 in vitro (3). Mass spectrometric analysis of IP6K1 overexpressed in HEK293 cells reveals multiple phosphorylation sites, none of which represent CK2 consensus sites. Reactivity of IP6K3 to CK2 has not been reported.
The apoptotic actions of IP6K2 are dependent upon IP7 generation. In several studies, kinase-dead IP6K2 fails to enhance apoptosis (12, 14) and the proapoptotic actions of p53 (15).
Elegant studies of Pandolfi and coworkers (22) established an analogous pathway whereby CK2 phosphorylates and destabilizes the tumor suppressor protein lost or altered in PML. PML mutants that resist CK2 phosphorylation display augmented tumor suppression. Moreover, in lung cancer derived cell lines and primary tumors CK2 catalytic activity and PML protein levels are inversely correlated.
CK1, like CK2, phosphorylates a wide range of substrates (25). However, the two enzymes differ substantially in catalytic mechanisms and substrate specificity. Scansite analysis reveals IP6K2 as a substrate for CK2 but not for CK1. Moreover, TBB, which inhibits CK2 much more potently than CK1, abolishes the physiologic phosphorylation of IP6K2. We also observe similar effects with the more specific CK2 inhibitor TBCA (23). Thus, CK1 does not appear to exert any noticeable effect on IP6K2 function.
Lindner and coworkers (26) noted that IP6K2 can bind tumor necrosis factor receptor associated factor (TRAF2) in OVCAR-3 ovarian carcinoma cells thereby influencing NFκβ via transforming growth factor β activated kinase. The binding site on IP6K2 includes its PEST sequence, and mutations of both serines 347 and 359, but not of either singly, impair binding with less cell death. In our study serine 359 was not phosphorylated by CK2, making it difficult to compare our findings with Lindner’s group.
IP6K2 may be important for organ development. Very recently, Wente and coworkers (27) obtained evidence that IP6K2 influences development of zebrafish and mammals by stimulating the hedgehog pathway. Thus, IP6K2 deletion disturbs overall development and hedgehog signaling, which is increased by overexpression of IP6K2.
Our findings may have clinical implications. CK2 is overexpressed in a large number of tumors (18), and CK2 inhibitors have potential antitumor activity (18, 24, 28). Conceivably, the beneficial effects of CK2 inhibitors derive from stabilization of IP6K2 to augment its apoptotic effects. Such drugs may also act by preventing the destabilizing actions of CK2 upon PML (22). IP6K2 actions can be facilitated by anticancer drugs such as cisplatin and novobiocin that block its binding to HSP90, as this binding inhibits catalytic activity of IP6K2 (14). One might obtain synergistic antitumor activity by combining drugs that block HSP90-IP6K2 binding together with CK2 inhibitors that stabilize IP6K2. In support of this notion, cisplatin induced cell death is potentiated by the CK2 inhibitor TBB (24).
CK2 promotes cell survival in part by activating the Akt and Wnt signaling pathway (29, 30). Inositol pyrophosphates inhibit Akt which activates GSK3β (9), an important component of the Wnt pathway. In this signaling cascade, IP7 competes with phosphatidylinositol (3,4,5)-trisphosphate for binding to the Pleckstrin homology domain of Akt thereby reducing phosphorylation and activation of Akt (9). As Akt physiologically inhibits GSK3β, IP7 signaling stimulates GSK3β. GSK3β phosphorylates and facilitates degradation of β-catenin, an essential component of the Wnt signaling pathway. Interestingly, CK2 phosphorylates both Akt and β-catenin to activate Wnt signaling (30, 31). Thus, CK2 may regulate Akt/GSK3β/β-catenin signaling directly by phosphorylating Akt and β-catenin and indirectly by enhancing IP7 levels via IP6K2 stabilization.
Among the three IP6Ks, IP6K2 appears to influence cell death selectively. Although overexpression of IP6K1, IP6K2, and IP6K3 all enhance sensitivity of cells to lethal stimuli, cell survival is uniquely augmented by deletion of IP6K2 but not the other two isoforms (12). IP6K1 does exert antitrophic effects by physiologically inhibiting Akt (9). Our principal experiments on cell death employed HCT116 cells in which essentially all IP7 formation is mediated by IP6K2 so that the other isoforms do not contribute to the apoptotic phenotype.
IP6K2 levels are higher in brain than other mammalian organs (7). Accordingly, activation of IP6K2 may play a role in neuronal death in stroke and neurodegenerative disease so that centrally active IP6K2 inhibitors might be beneficial.
Materials and Methods
Reagents.
[32P]orthophosphates were from Perkin Elmer; CK2 and λ phosphatase were from NEB, TBB and TBCA were from EMD Biosciences; all the other biochemical reagents were from Sigma unless otherwise mentioned. Antibodies: α-Myc from Roche; α-PARP from Cell Signaling; and IP6K2 polyclonal antibody was developed in our lab.
Experimental Procedures.
Cell lines and culture conditions.
HEK293, HEK293T, and HeLa cells were cultured in DMEM supplemented with 10% FBS. Transient transfections were carried out using Polyfect (Qiagen). U2OS and HCT116 cell culture were done following standard procedures (15).
Orthophosphate labeling of HEK293 cells.
HEK293 cells were labeled for 4 h with [32P]orthophosphate 48 h post transfection of control or Myc-IP6K2 plasmids. Cells were lysed in the presence of a phosphatase inhibitor cocktail, and immunoprecipitated with α-Myc antibody for 2 h. Beads were washed 3x with wash buffer, boiled, and run on SDS-PAGE. Proteins were transferred onto nitrocellulose membranes and phosphorylation was detected by autoradiography. Total protein was detected by immunoblotting with α-Myc antibody.
To remove radiolabeled phosphates from immunoprecipitated Myc-IP6K2 λphosphatase treatment of immunoprecipitated proteins was carried out in the presence of 1X buffer and the enzyme at 37 °C for 1 h. Beads were processed as described above.
CK2 phosphorylation of IP6K2 in vitro.
Immunoprecipitated Myc-IP6K2 after λphosphatase treatment was used as a substrate for CK2 in the presence of 10 Units of the enzyme, 250 μM Mg-ATP, and 1 μCi [32P]γATP at 37 °C for 30 min. Proteins were run on SDS-PAGE and processed for phosphorylation and total protein detection as described earlier.
To detect CK2 phosphorylation at S347 site by the phospho-specific IP6K2 antibody, recombinant bacterially purified 6XHis-IP6K2 was used as a CK2 substrate. CK2 kinase reaction, SDS-PAGE, and Western transfer were carried out as described above. Phosphorylation of IP6K2 was detected using the antibody at 1∶1,000 dilutions.
CK2 inhibitor treatment for cell death induction.
Varying concentrations of CK2 inhibitors TBB, TBCA, or resorufin were incubated with WT and IP6K2Δ HCT116 cells for indicated time periods. Cell viability was assessed by MTT assay (14, 15). Each experiment was repeated at least three times.
To detect cleaved PARP protein as an indicator of apoptotic cell death (15), cells were lysed after indicated treatments. Equal amount of proteins were loaded on SDS-PAGE and were blotted with α-PARP antibody. α-GAPDH or α-β Tubulin were used as loading controls.
Cycloheximide treatment.
Cycloheximide (20 μg/mL) was added for indicated time periods to cells overexpressing IP6K2. Cells were lysed and equal amounts of proteins were loaded on SDS-PAGE.
Immunoprecipitation of endogenous IP6K2 from HCT116 cells.
Endogenous IP6K2 was immunoprecipitated from HCT116 cells after TBB treatment for indicated time periods. Immunoprecipitation was done using α-IP6K2 antibody using standard procedure (15).
Detection of intracellular inositol phosphates.
U2OS cells overexpressing tetracycline inducible Myc-IP6K2 were used for the experiment. Cells were plated at 60% density and incubated with 100 μCi [3H]myoinositol for 3 d in presence of 1 μg/mL tetracycline. Cells were processed for inositol phosphate detection by HPLC (9).
Ubiquitination of IP6K2.
Myc-IP6K2 and HA-ubiquitin were cooverexpressed in HEK293T cells. Forty-eight h posttransfection, cells were treated with DMSO, TBB (50 μM) or TBCA (10 μM) for 3 h. Cells were harvested, lysed, and equal amount of proteins were loaded on SDS-PAGE. Myc-IP6K2 was immunoprecipitated and ubiquitination detected by α-HA antibody.
To detect ubiquitinated IP6K2, tetracycline inducible U2OS cells expressing Myc-IP6K2 were treated with 20 μM MG132 for 3 h after 24 h tetracycline induction. IP6K2 was immunoprecipitated using a α-Myc antibody and was run on SDS-PAGE. Ubiquitinated IP6K2 was detected with a α-Myc antibody.
Generation of IP6K2 phospho-specific antibody.
Phospho-specific antibodies against S347 and S356 sites were generated in rabbits by 21st Century Biochemicals using the phospho-peptides VLD[pS]DAEDLED and AEDLEDL[pS]EESA respectively. The second peptide did not generate an acceptable antibody titer. Antibodies against S347 site showed robust responses and were affinity purified.
Identification of phosphorylation sites in IP6K2 by mass spectrometry.
Phosphorylation sites at IP6K2 were identified by LC/MS/MS at the Taplin Mass Spectrometry facility at Harvard Medical School. GST-IP6K2 was transfected in HEK293 cells. The protein was pulled down and run on SDS-PAGE. The coomassie stained band was cut and analyzed.
Statistical analysis.
All results are presented as the mean and standard error of at least three independent experiments. Statistical significance was calculated by Student’s t-test using the “Sigmaplot software” (***p < 0.001, **p < 0.01, *p < 0.05).
Acknowledgments.
We thank Katherine Sixt for providing reagents and Molee Chakraborty for technical help. This work was supported by Public Health Service Grant DA-000266 and Research Scientist Award DA00074 (to S.H.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Hand CE, Honek JF. Phosphate transfer from inositol pyrophosphates InsP5PP and InsP4(PP)2: a semi-empirical investigation. Bioorg Med Chem Lett. 2007;17:183–188. doi: 10.1016/j.bmcl.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 3.Barker CJ, Illies C, Gaboardi GC, Berggren PO. Inositol pyrophosphates: structure, enzymology and function. Cell Mol Life Sci. 2009;66:3851–3871. doi: 10.1007/s00018-009-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shears SB. Diphosphoinositol polyphosphates: metabolic messengers? Mol Pharmacol. 2009;76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulugu S, et al. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 6.Lin H, et al. Structural analysis and detection of biological inositol pyrophosphates reveal that the family of VIP/diphosphoinositol pentakisphosphate kinases are 1/3-kinases. J Biol Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saiardi A, Erdjumant-Bromage H, Snowman AM, Tempst P, Snyder SH. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 8.Saiardi A, Nagata E, Luo HR, Snowman AM, Snyder SH. Identification and characterization of a novel inositol hexakisphosphate kinase. J Biol Chem. 2001;276:39179–39185. doi: 10.1074/jbc.M106842200. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty A, et al. Inositol pyrophosphates inhibit Akt signaling, regulate insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-beta in ovarian carcinoma cells. J Biol Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata E, et al. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J Biol Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 13.Morrison BH, et al. Gene deletion of inositol hexakisphosphate kinase 2 predisposes to aerodigestive tract carcinoma. Oncogene. 2009;28:2383–2392. doi: 10.1038/onc.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty A, et al. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc Natl Acad Sci USA. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koldobskiy MA, et al. p53 mediated apoptosis requires inositol hexakisphosphate kinase-2. Proc Natl Acad Sci USA. 2010;107:20947–20951. doi: 10.1073/pnas.1015671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 17.Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 18.Ruzzene M, Pinna LA. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta. 2010;1804:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: implications for cancer therapy. Curr Cancer Drug Tar. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- 20.Ljubimov AV, et al. Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophth Vis Sci. 2004;45:4583–4591. doi: 10.1167/iovs.04-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Maira G, et al. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007;26:6915–6926. doi: 10.1038/sj.onc.1210495. [DOI] [PubMed] [Google Scholar]
- 22.Scaglioni PP, et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126:269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 23.Pagano MA, et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 24.Fritz G, Issinger OG, Olsen BB. Selectivity analysis of protein kinase CK2 inhibitors DMAT, TBB and resorufin in cisplatin-induced stress responses. Int J Oncol. 2009;35:1151–1157. doi: 10.3892/ijo_00000431. [DOI] [PubMed] [Google Scholar]
- 25.Knippschild U, et al. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Morrison BH, et al. Effect of inositol hexakisphosphate kinase 2 on transforming growth factor beta-activated kinase 1 and NF-kappaB activation. J Biol Chem. 2007;282:15349–15356. doi: 10.1074/jbc.M700156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarmah B, Wente SR. Inositol hexakisphosphate kinase-2 acts as an effector of the vertebrate Hedgehog pathway. Proc Natl Acad Sci USA. 2010;107:19921–19926. doi: 10.1073/pnas.1007256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarno S, et al. Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol Ther. 2002;93:159–168. doi: 10.1016/s0163-7258(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 29.Di Maira G, et al. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 30.Song DH, et al. CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem. 2003;278:24018–24025. doi: 10.1074/jbc.M212260200. [DOI] [PubMed] [Google Scholar]
- 31.Ponce D, et al. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of beta-catenin transcriptional activity. J Cell Physiol. 2010 doi: 10.1002/jcp.22527. DOI: 10.1002/jcp.22527. [DOI] [PubMed] [Google Scholar]