Abstract
Common heart failure has a strong undefined heritable component. Two recent independent cardiovascular SNP array studies identified a common SNP at 1p36 in intron 2 of the HSPB7 gene as being associated with heart failure. HSPB7 resequencing identified other risk alleles but no functional gene variants. Here, we further show no effect of the HSPB7 SNP on cardiac HSPB7 mRNA levels or splicing, suggesting that the SNP marks the position of a functional variant in another gene. Accordingly, we used massively parallel platforms to resequence all coding exons of the adjacent CLCNKA gene, which encodes the Ka renal chloride channel (ClC-Ka). Of 51 exonic CLCNKA variants identified, one SNP (rs10927887, encoding Arg83Gly) was common, in linkage disequilibrium with the heart failure risk SNP in HSPB7, and associated with heart failure in two independent Caucasian referral populations (n = 2,606 and 1,168; combined P = 2.25 × 10−6). Individual genotyping of rs10927887 in the two study populations and a third independent heart failure cohort (combined n = 5,489) revealed an additive allele effect on heart failure risk that is independent of age, sex, and prior hypertension (odds ratio = 1.27 per allele copy; P = 8.3 × 10−7). Functional characterization of recombinant wild-type Arg83 and variant Gly83 ClC-Ka chloride channel currents revealed ≈50% loss-of-function of the variant channel. These findings identify a common, functionally significant genetic risk factor for Caucasian heart failure. The variant CLCNKA risk allele, telegraphed by linked variants in the adjacent HSPB7 gene, uncovers a previously overlooked genetic mechanism affecting the cardio-renal axis.
Keywords: cardiomyopathy, genetic association
The lifetime risk of developing heart failure is estimated at one in five (1, 2). Although rare familial cardiomyopathies can lead to heart failure that is almost entirely attributable to genetic factors, common heart failure has a smaller, poorly defined heritable component. Framingham Heart Study data show that parental heart failure confers a 70% greater disease risk than in individuals without a family history (3). Cardiac hypertrophy that predisposes to heart failure is also heritable, suggesting that underlying genetic variation contributes to interindividual variability in heart failure risk (4). A fraction of common heart failure may be due to unrecognized monogenic cardiomyopathy (5), but most cases are explained by multiple interacting environmental and as-yet unidentified genetic susceptibility factors. Previous efforts to identify genetic risk variants uncovered a rare combination of adrenergic receptor polymorphisms that increases heart failure susceptibility 10-fold among African Americans (6), although this particular combination of risk alleles has not yet been replicated (7, 8). A few population-based cohort studies have implicated genomic regions in heart failure risk (9–11) or mortality (12), but specific causative risk alleles are still unknown.
The Institute for Translational Medicine and Therapeutics/Broad Institute/Candidate-gene Association Resource (IBC) consortium developed a high-density SNP cardiovascular subgenome array to complement genome-wide platforms in heart disease (13). Using this array, we previously identified in two US Caucasian heart failure referral populations a heart failure risk locus on chromosome 1p36 (rs1739843), within the cardiovascular heat shock protein gene HSPB7 (14). A European consortium using the IBC array recently reported that the identical variant was their strongest genetic predictor of nonfamilial dilated cardiomyopathy (15). Thus, taken together, rs1739843 has been linked with heart failure in six independent populations on two continents, establishing it as a bona fide heart failure risk variant. rs1739843 occurs in intron 2 of HSPB7 and has no predicted affect on its protein sequence or function. Accordingly, we previously resequenced the entire HSPB7 gene to screen for nearby functional variants (16). Although these experiments further confirmed the rs1739843 association and revealed 11 additional heart failure-associated SNPs in tight linkage disequilibrium, the additional HSPB7 SNPs were also intronic or synonymous. As such, the causative gene variant(s) remain unknown, suggesting either that rs1739843 marks an expression quantitative trait locus (eQTL) that modifies HSPB7 expression (17) or that it marks the position of a genetically linked functional variant located outside of HSPB7.
Here we demonstrate that there is no association between rs1739843 genotype and HSPB7 mRNA expression levels or splicing in human myocardium, making an eQTL mechanism unlikely. For this reason, we resequenced the coding exons of the neighboring gene, CLCNKA, which is within the same linkage-disequilibrium block as HSPB7. Of 51 CLCNKA sequence variants identified, rs10927887 (encoding Arg83Gly) is positively associated with heart failure risk in three independent Caucasian heart failure populations. Functional analysis shows the Gly83 variant channel to exhibit markedly abnormal chloride currents, suggesting that this common loss of function variant in CLCNKA at 1p36 confers heart failure risk in Caucasians and accounts for the previously described association between some HSPB7 SNPs and heart failure.
Results
rs1739843 Is Not Associated with Altered Myocardial HSPB7 Expression or Splicing.
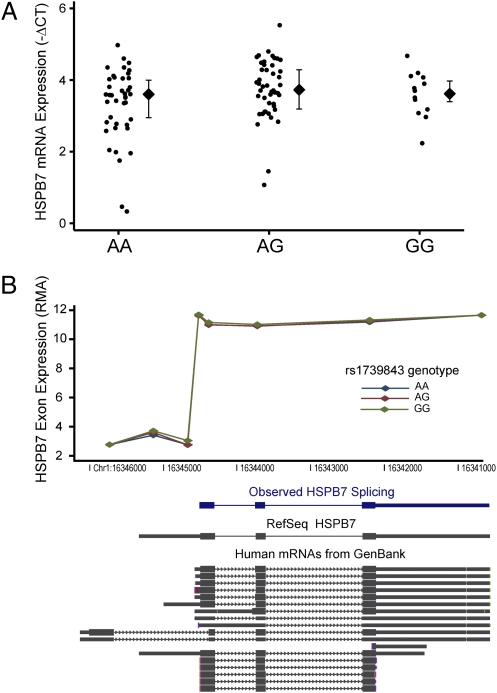
The rs1739843 intronic HSPB7 SNP has been associated with heart failure in multiple independent cohorts. Neither it nor other linked polymorphisms alter protein coding. To examine whether this SNP might instead confer HSPB7 dysfunction by altering transcription, we measured HSPB7 mRNA levels from human left-ventricular heart specimens of 111 Caucasian heart failure subjects genotyped for rs1739843. Using microarrays, we found no difference in HSPB7 expression across rs1739843 genotypes, and we verified this finding using RT–quantitative PCR (RT-qPCR) (Fig. 1A). HSPB7 exon expression revealed identical splicing patterns regardless of rs1739843 genotype (Fig. 1B). Thus, the association between rs1739843 and heart failure risk is unlikely to be caused by an eQTL mechanism or by alterations in HSPB7 splicing.
Fig. 1.
No association between rs1739843 genotype and HSPB7 mRNA expression or splicing in human myocardium. Data are from n = 111 left-ventricular free-wall specimens from genetically inferred Caucasians with heart failure. (A) HSPB7 RT-qPCR showed no differences in mRNA abundance across genotypes (P = 0.3; diamonds show median with interquartile range). (B) Exon arrays revealed a typical pattern of splicing observed in GenBank that was identical across rs1739843 genotypes (P = 0.8; diamonds show median expression of each exon probeset, spaced along Chr1).
A Common Nonsynonymous CLCNKA SNP Is Associated with Systolic Heart Failure.
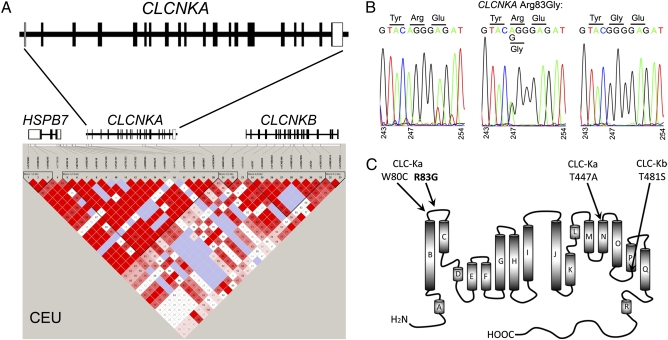
HapMap data reveal the HSPB7 locus on 1p36 to be an area of high linkage disequilibrium (Fig. 2A). Because the HSPB7 heart failure-associated SNPs do not alter protein coding or cardiac gene expression, we hypothesized that the HSPB7 risk variants marked heart failure risk alleles within the broader genetic region. Accordingly, we resequenced all 19 coding exons and intron–exon boundaries of the adjacent CLCNKA gene in 1,742 DNA samples from the Cincinnati Heart Failure Study (18, 19). (Detailed clinical characteristics of the study populations are in Table S1.) Polymorphism screening by pooled resequencing identified 51 nonprivate exonic CLCNKA SNPs (nine in dbSNP and 42 previously unreported; 11 synonymous and 40 nonsynonymous; Table S2). Only seven of these SNPs (five nonsynonymous) are common, defined as having an allele frequency of ≥0.05. Thus, the CLCNKA gene exhibits limited polymorphic variability.
Fig. 2.
Genetic characteristics of CLCNKA. (A) Structure (Upper) and linkage disequilibrium (Lower) at 1p36 for HapMap Caucasian Europeans (CEU). (B) Representative rs10927887 genotypes by Sanger sequencing and associated changes in amino acid coding. (C) Transmembrane structural diagram showing position of heart failure risk variant Arg83Gly (R83G) relative to Bartter's syndrome Cys80 mutation (W80C) and hypertension-associated Ala447 variant (T447A). Analogous position of common ClC-Kb variant T481S is also shown.
To determine whether CLCNKA polymorphisms were associated with heart failure, allele frequencies for the 51 SNPs were compared between 1,117 Caucasian heart failure cases and 625 unaffected controls. Nine SNPs (seven nonsynonymous) had significant associations with heart failure (P < 0.001, corresponding to a Bonferroni-adjusted P < 0.05). In all but one instance allele frequencies for these SNPs were <0.05, whereas that for the marker HSPB7 SNP was ≈0.5 (14, 16). However, the allele frequency of rs10927887 was ≈0.50 in controls and ≈0.57 in heart failure cases (P = 7.09 × 10−5; Table S2), which is similar to the marker HSPB7 SNP. This CLCNKA variant encodes a change from Arg to Gly at amino acid 83 (exon 3) of the renal ClC-Ka chloride channel (Fig. 2B).
Because the association between rs10927887 and heart failure was identified during comprehensive screening of all CLCNKA coding exons, we performed a technical replication of the findings by targeted resequencing of just CLCNKA exon 3 at greater sequencing depth (90-fold vs. 19-fold) in the same study population. Allele frequencies in controls and heart failure cases were similar to the screening study (≈0.50 in controls and ≈0.56 in heart failure cases; P = 8.7 × 10−4; Table 1, primary cohort). Sequence data from Caucasians in the 1000Genomes project show that rs10927887 is in strong linkage disequilibrium (R2 = 0.87) with the previously identified heart failure-associated intronic HSPB7 SNP, rs1739843 (14, 16). On the basis of these findings, we considered that rs1739843 on the IBC array tagged the position of the nonsynonymous CLCNKA variant encoded by rs10927887.
Table 1.
Association and subgroup analysis of CLCNKA rs10927887 G allele with nonfamilial heart failure
| G allele frequency |
|||
| Cohort | Controls | Cases | P |
| Primary cohort (n = 625 controls, 1,117 cases) | 0.4979 | 0.5565 | 8.7 × 10−4 |
| Ischemic cardiomyopathy (n = 691) | 0.5440 | 0.0172 | |
| Nonischemic cardiomyopathy (n = 426) | 0.5767 | 4.3 × 10−4 | |
| Secondary cohort: (n = 311 controls, 857 cases) | 0.4777 | 0.5600 | 4.4 × 10−4 |
| Ischemic cardiomyopathy (n = 422) | 0.5613 | 1.5 × 10−3 | |
| Nonischemic cardiomyopathy (n = 435) | 0.5587 | 2.3 × 10−3 | |
| Combined analysis (n = 936 controls, 1,974 cases) | 0.4912 | 0.5580 | 2.25 × 10−6 |
| Ischemic cardiomyopathy (n = 1113) | 0.5526 | 9.88 × 10−5 | |
| Nonischemic cardiomyopathy (n = 861) | 0.5697 | 2.82 × 10−6 | |
To validate the association between rs10927887 and heart failure, we resequenced CLCNKA exon 3 in an independent cohort of 857 Caucasian heart failure cases and 311 unaffected Caucasian controls from the Penn Heart Failure Study (20). Again, rs10927887 was overrepresented in heart failure (≈0.56 vs. ≈0.48 in controls; P = 4.4 × 10−4; Table 1, secondary cohort). Combined analysis of cases (n = 1,974) and controls (n = 936) from both study cohorts gives a P value for association of 2.25 × 10−6 (Table 1, combined analysis).
Our study design compares carefully selected nonaffected controls with systolic heart failure of any etiology (i.e., all-cause heart failure). To determine whether the rs10927887 association was stronger with ischemic or nonischemic heart failure, we performed subgroup analyses in the primary and secondary cohorts. The association was present for heart failure of either etiology in both groups, with combined analyses giving P values of 9.88 × 10−5 for ischemic heart failure and 2.82 × 10−6 for nonischemic heart failure.
Heart Failure Risk Conferred by rs10927887 Increases with Gene Dose.
For a more detailed assessment of the association between rs10927887 and heart failure risk, we individually genotyped Caucasian heart failure cases and controls from the primary Cincinnati Heart Failure Study cohort, an expanded secondary Penn Heart Failure Study cohort, and from a third independent heart failure cohort (total n = 5,489 subjects; Table 2). In age- and gender-adjusted models, the CLCNKA Gly83 allele was associated with all-cause heart failure in all three cohorts, with an overall 1.26-fold increase in risk per allele copy (P = 3.8 × 10−6; Table 2). Further adjusting for age, gender, and hypertension did not alter the association between Gly83 and heart failure risk (odds ratio 1.27, P = 8.3 × 10−7; Table 2). Analysis of multiple race-informative genetic markers showed no evidence for population stratification (λ = 1.010 and 1.055). These findings indicate that risk of heart failure is increased by ≈27% and 54% in Gly83 heterozygotes and homozygotes, respectively, independent of age, sex, or hypertension.
Table 2.
Odds of advanced heart failure associated with each copy of the of CLCNKA risk allele in three study cohorts
| Age- and sex-adjusted |
Age-, sex-, and HTN-adjusted |
|||
| Study cohort | OR (95% CI) | P | OR (95% CI) | P |
| Primary cohort (603 controls, 1,113 cases) | 1.21 (1.04–1.39) | 0.011 | 1.28 (1.05–1.55) | 0.012 |
| Secondary cohort (1,887 controls, 755 cases) | 1.26 (1.11–1.43) | 1.9 × 10−4 | 1.26 (1.11–1.44) | 2.0 × 10−4 |
| Tertiary cohort (316 controls, 785 cases) | 1.31 (1.08–1.58) | 0.0026 | 1.29 (1.06–1.57) | 0.0048 |
| Metaanalysis (2,806 controls, 2,653 cases) | 1.26 (1.14–1.38) | 3.8 × 10−6 | 1.27(1.16–1.40) | 8.3 × 10−7 |
HTN, hypertension; OR, odds ratio per copy of the risk allele (G, encoding Gly) at rs10927887 in additive genetic models; CI, confidence interval. P values are two-sided for primary cohort and metaanalysis and one-sided for secondary and tertiary cohorts.
rs10927887 Confers Loss of Function of Renal Chloride Channel CLC-Ka.
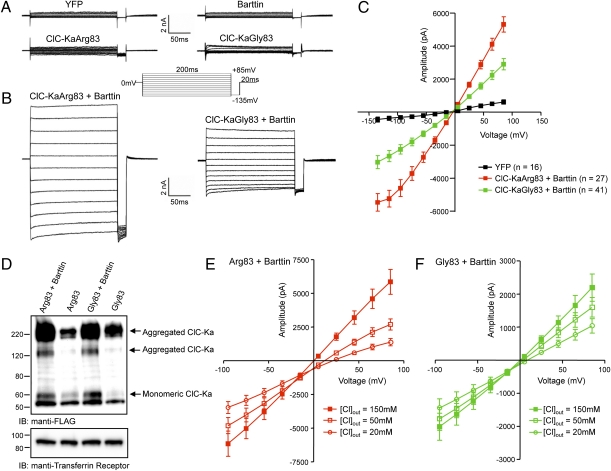
rs10927887 encodes a substitution of Gly for normal Arg at amino acid position 83 of the ClC-Ka renal chloride channel. We examined the functional implications of this gene variant by comparative analysis of recombinantly expressed Arg83 and Gly83 ClC-Ka channels in tsA201cells (21). Because barttin is necessary for ClC-K functional expression (22, 23), inward and outward currents in cells expressing ClC-Ka Arg83 or Gly83 alone or barttin alone were no different from nontransfected or YFP-expressing cells (Fig. 3A). However, coexpression of ClC-Ka Arg83 with barttin evoked large inward and outward currents (Fig. 3B) having nearly linear current–voltage relations and a reversal potential (in symmetrical Cl−) of −0.2 ± 0.9 mV (Fig. 3C). By comparison, currents evoked by ClC-Ka Gly83 had diminished amplitudes at all test potentials (P < 0.001; Fig. 3C). Immunoblot analysis revealed similar expression levels of Arg83 and Gly83 ClC-Ka, suggesting that reduced currents are an intrinsic property of the Gly83 ClC-Ka variant (Fig. 3D). The mean ± SEM (n = 10) reversal potential of the ClC-KaArg83–encoded currents shifted to 12.2 ± 0.7 mV and 23.2 ± 1.8 mV when the extracellular Cl− concentration was reduced to 50 mM and 20 mM, respectively (Fig. 3E). In contrast, the mean ± SEM (n = 13) reversal potential of the ClC-KaGly83–encoded currents (n = 13) was less affected by changes in extracellular Cl−, shifting to 4.0 ± 2.7 mV and 10.0 ± 3.7 mV in 50 mM and 20 mM extracellular Cl−, respectively (Fig. 3F). These values are significantly lower than those determined for the ClC-KaArg83–encoded currents at both 50 mM (P = 0.017) and 20 mM (P = 0.008) extracellular Cl−. Overall these findings indicate that the heart failure-associated Gly83 variant substantially impairs ClC-Ka function.
Fig. 3.
Functional characterization of ClC-Ka Arg and Gly-83. (A) Whole-cell Cl− currents, evoked in response to voltage steps ranging from −165 mV to +85 mV (in 10-mV increments) from a holding potential of 0 mV recorded from tsA201 cells expressing YFP, barttin, ClC-KaArg83 (WT), or ClCKaGly83 (mutant); the voltage clamp paradigm is illustrated adjacent to the current records. (B) Representative whole-cell currents recorded from YFP + barttin + ClC-KaArg83 (WT) and from YFP + barttin + ClC-KaGly83 (mutant)-expressing cells. (C) Mean ± SEM peak inward and outward currents determined from records similar to those illustrated in B plotted as a function of test potential; n = numbers of cells. (D) Representative anti-FLAG immunoblots of tsA201 cells transiently expressing FLAG-tagged ClC-KaArg83 or ClC-KaGly83 alone and in the presence of barttin. Anti-transferrin receptor is loading control. (E and F) Reversal potentials of ClC-Ka Arg83 (E) and Gly83 (F) currents as a function of extracellular Cl− concentration.
Discussion
Here, we performed exomic sequencing within the heart failure risk locus on chromosome 1p36 and identified rs10927887 encoding ClC-Ka Gly83 as a common risk allele for heart failure among Caucasians. These findings also provide genetic evidence supporting clinical observations that individual differences in salt-sensitivity can predispose to cardiovascular complications (24, 25). As expected for a common polymorphism, the increase in heart failure risk conferred by the variant allele is modest (≈27% per copy of the risk allele). However, approximately one fourth of all Caucasians are homozygous for the rs10927887 variant allele, with an expected 54% increase in heart failure risk, indicating a potentially broad impact at the population level.
In analyzing the functional consequences of the CLCNKA heart failure risk polymorphism, we determined that the Gly83 renal ClC-Ka chloride channel variant reduces chloride currents. Intrinsic renal mechanisms have been largely overlooked in assessing genetic risk for heart failure, and this aspect of our work reinforces a strength of genetic studies directed by array-based SNP genotype–phenotype associations, their mechanistic agnosticism. Although it seems counterintuitive that a genetic variant that diminishes the ability of kidney cells to retain NaCl would increase risk for heart failure, nature has provided a possible explanation: the Gly83 renal ClC-Ka variant is located near, and has a similar loss of function profile to, a previously described Cys80 ClC-Ka mutation that in combination with disruption of the related CLCNKB gene caused a Bartter's-like syndrome in one affected individual (26). Bartter's syndrome is a multifactorial inherited salt-wasting disorder produced by loss-of-function mutations in NCCT, NKCC2, ROMK, ClC-Kb, BSND, or barttin (i.e., factors determining renal NaCl reabsorption) (27). The hallmark abnormality in all forms of Bartter's syndrome is hyperreninemia independent of volume status (28, 29). Hyperreninemia likewise mediates heart failure in the cardiorenal syndrome (30) and was an independent risk factor for heart failure in the Heart Outcomes Prevention Evaluation Study (31). Thus, the critical physiologic abnormality conferred by loss of ClC-Ka function with the Gly83 variant may be increased renin, predisposing affected subjects to heart failure when a second “hit,” such as infarction or mechanical overload, damages the heart. Indeed, unexplained cardiomyopathies are a rare but recognized complication of Bartter's syndrome (32–34), and pharmacological blockade of renin–angiotensin–aldosterone system components is first-line therapy for heart failure (35).
Because the seminal findings localizing heart failure risk to chromosome 1p36 derived from a microarray study of Caucasian heart failure (14), chromosome walking in the present study also focused on Caucasian disease. However, HapMap data indicate that the ClC-Ka Gly83 allele is ≈40% more prevalent in African-derived than European-derived individuals (≈70% vs. ≈50%, respectively). African Americans are known to have an increased burden of heart failure compared with Caucasians [prevalence of 3% vs. 2%, respectively (36)] and a greater risk for heart failure progression with mild or moderate ventricular systolic dysfunction (37). It is interesting to consider that hyperreninemic hyperaldosteronism induced by the Gly83 ClC-Ka polymorphism may be one of the genetic factors that contribute to disproportionate heart failure risk among African Americans. Our studies are limited to Caucasians, and large-scale genetic association studies in African American populations will be necessary to address this hypothesis.
Our findings suggest that the association with heart failure of the seminal 1p36 SNP in intron 2 of HSPB7 is at least in part due to the linked ClC-Ka Gly-83 polymorphism. Although the functional data are striking and the reported associations of unexplained cardiomyopathy with Bartter's syndrome are suggestive, these clinical data do not establish either causality of the risk allele or define the responsible mechanism. Such efforts will require structure–function studies to define how Gly83 alters the biophysical properties of ClC-Ka and development of novel experimental systems to assess the effects of ClC-Ka Gly83 on cardio-renal physiology. Furthermore, if the proposed concepts linking CLCNKA to heart failure are correct, it is likely that other gene variants with similar effects on the cardio-renal axis can also modify heart failure risk. We consider that the possibilities for such risk variants include rare or private nonsynonymous loss-of-function variants in CLCNKA (26, 38), variants of the related CLCNKB gene that confer similar channel dysfunction, or variants of other genes that affect renal salt handling and therefore the renin–angiotensin set point. Thus, on the basis of the current genetic and functional data, we postulate that multiple genetic events inducing a common cardio-renal physiological response can modify heart failure risk. Indeed, multiple or combinatorial interactions are likely. Because ClC-Ka Gly83 is both common and functional, its relative importance as a heart failure risk modifier gene seems clear.
Methods
Study Subjects.
Human study protocols were approved by the institutional review boards of the University of Cincinnati, Cincinnati, OH; the University of Pennsylvania, Philadelphia, PA; Case Western Reserve University, Cleveland, OH; or the University of Wisconsin, Madison, WI. All subjects provided written informed consent. Flash-frozen left ventricular myocardial samples from heart failure patients were obtained at time of cardiac surgery as previously described (39, 40). Subjects with advanced systolic heart failure were recruited into one of two National Heart, Lung, and Blood Institute-funded longitudinal studies of heart failure genomics (P50 HL77101 and R01 HL88577) from patients presenting to the heart failure referral program at the University of Cincinnati (16, 19) or the University of Pennsylvania (20), according to prespecified criteria. The same infrastructure was used to recruit nonaffected controls. Additional cases and controls were analyzed from Case Western Reserve University, University of Wisconsin at Madison, and the PennCATH study (41) to constitute a tertiary study cohort.
Assessing Myocardial HSPB7 Expression and Splicing by rs1739843 Genotype.
DNA and RNA were isolated directly from left-ventricular myocardium using Gentra and miRNeasy kits. Genotypes at rs1739843 were obtained using the IBC array, and multidimensional scaling of all IBC genotypes was used to identify a subgroup of 111 genetically inferred Caucasians. Gene expression and splicing were assessed using Affymetrix ST1.0 arrays. Expression was further assessed using RT-qPCR with ABI Taqman assays on demand for HSPB7 (Hs00205296_m1) with RPLP0 (Hs99999902_m) as a housekeeping gene. All samples were run in duplicate, and expression was quantified as the average change in cycle threshold (-ΔCT). Expression was compared across rs1739843 genotypes using the Kruskal-Wallis test.
Resequencing and Genotyping.
In the primary and secondary cohorts, pooled DNA library preparation and Illumina Genome Analyzer II resequencing of CLCNKA exons and exon–intron boundaries were performed as previously described (16). Exon-spanning primer pairs (Table S3) were developed for all 19 CLCNKA coding exons. Average sequence coverage depth was 19-fold for initial CLCNKA whole exon screening of the primary Cincinnati Heart Failure Study cohort and 91-fold for targeted exon 3 resequencing of the primary (90.4-fold) and secondary Penn Heart Failure Study cohorts (92.7-fold). Individual genotypes for the Cincinnati Heart Failure Study cohort were determined by dye terminator sequencing of exon 3 using an ABI3730xl capillary sequencer. Each sequence pherogram was blindly and independently examined by two researchers (S.J.M. and G.W.D.). Success rate was 98.9% and conforming to predictions of Hardy-Weinberg equilibrium (P = 0.44 controls, P = 0.75 heart failure). Because rs10927887 genotypes are available as part of the 1000Genomes project (42), existing IBC genotypes (14) were imputed with 1000Genomes data using the software package MACH (43) (http://www.sph.umich.edu/csg/abecasis/mach/) to obtain rs10927887 genotypes for the Penn Heart Failure Study, Case Western Reserve, University of Wisconsin, and PennCATH (41). Imputed genotypes showed good quality with imputation R2 > 0.92; frequency of the G allele was similar to 1000Genomes CEU (0.53).
Functional Analysis of ClC-Ka Arg83 and Gly83.
tsA201 cells were transiently transfected using Lipofectamine with a plasmid (pBK-CMV-YFP) encoding enhanced YFP, pcDNA3.1-barttin, pCMV-Sport6-CLCNKA WT, or pCMV-Sport6-CLCNKAR83G, individually or in combination as described. The inclusion of the plasmid encoding YFP allowed transfected cells to be identified before electrophysiological recordings. Approximately 15 h after transfection, cells were washed and allowed to recover for 20–24 h before current recordings were obtained. Whole cell recordings were obtained at room temperature (22–25 °C). Data were collected using a Dagan Model 3900A patch-clamp amplifier; experimental parameters were controlled with Dell personal computer through a Digidata 1322 interface using pCLAMP9 (Axon Instruments). ClC-Ka currents were evoked during 400-ms voltage steps to test potentials between −165 and +85 mV in 10-mV increments. Data were compiled and analyzed using CLAMPFIT (Axon) and Excel (Microsoft) and presented as means ± SEM.
Western blot analyses were performed on 20-μg/lane protein lysates prepared from tsA201 cells transfected as above, except with FLAG epitope-tagged ClC-Ka. After blocking, membranes were incubated with the mouse monoclonal anti-FLAG antibody (Sigma) at 4 °C overnight. To ensure equal protein loading of lanes, membranes were also probed with a mouse monoclonal anti-transferrin receptor (Invitrogen) antibody. After washing, membranes were incubated with a rabbit anti-mouse horseradish peroxidase-conjugated secondary antibody (Bethyl Laboratories) followed by SuperSignal West Dura Extended Duration substrate (Pierce). Signals were detected using a Molecular Imager Chemidoc XRS system running Quantity One software version 4.6 (Bio-Rad).
Statistical Analysis.
For pooled resequencing, allele frequencies between study groups were compared using Fisher's exact test. The P value threshold for significance in the primary heart failure case–control analysis was <0.001 using a Bonferroni correction for multiple testing (n = 51 exonic SNPs at an α level of 0.05). Only one common SNP met criteria for association and was evaluated in the replication study. After comparison of all-cause heart failure with controls, cases were stratified into ischemic and nonischemic groups. For individual CLCNKA genotypes, SNP association was tested using logistic regression with the genotype score or the imputation dosage score included as a covariate, adjusting for age, gender, and hypertension. Metaanalysis was carried out using the software package METAL (http://www.sph.umich.edu/csg/abecasis/Metal/). Comparison of whole-cell chloride currents used Student t test, with P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Cardiac Translational Implementation Program Grants RC2 HL102222, HL088577, HL034161, and ULI RR024992.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017494108/-/DCSupplemental.
References
- 1.Lloyd-Jones D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 3.Lee DS, et al. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 4.Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: The Framingham Heart Study. Hypertension. 1997;30:1025–1028. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- 5.Richard P, Villard E, Charron P, Isnard R. The genetic bases of cardiomyopathies. J Am Coll Cardiol. 2006;48(9 Suppl A):A79–A89. [Google Scholar]
- 6.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 7.Canham RM, et al. Alpha2cDel322-325 and beta1Arg389 adrenergic polymorphisms are not associated with reduced left ventricular ejection fraction or increased left ventricular volume. J Am Coll Cardiol. 2007;49:274–276. doi: 10.1016/j.jacc.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Nonen S, et al. No positive association between adrenergic receptor variants of alpha2cDel322-325, beta1Ser49, beta1Arg389 and the risk for heart failure in the Japanese population. Br J Clin Pharmacol. 2005;60:414–417. doi: 10.1111/j.1365-2125.2005.02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson MG, et al. Framingham Heart Study 100K project: Genome-wide associations for cardiovascular disease outcomes. BMC Med Genet. 2007;8(Suppl 1):S5. doi: 10.1186/1471-2350-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasan RS, et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA. 2009;302:168–178. doi: 10.1001/jama.2009.978-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith NL, et al. Association of genome-wide variation with the risk of incident heart failure in adults of European and African ancestry: A prospective meta-analysis from the cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 2010;3:256–266. doi: 10.1161/CIRCGENETICS.109.895763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison AC, et al. Genomic variation associated with mortality among adults of European and African ancestry with heart failure: The cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. 2010;3:248–255. doi: 10.1161/CIRCGENETICS.109.895995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating BJ, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappola TP, et al. Common variants in HSPB7 and FRMD4B associated with advanced heart failure. Circ Cardiovasc Genet. 2010;3:147–154. doi: 10.1161/CIRCGENETICS.109.898395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark K, et al. Genetic association study identifies HSPB7 as a risk gene for idiopathic dilated cardiomyopathy. PLoS Genet. 2010;6:e1001167. doi: 10.1371/journal.pgen.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matkovich SJ, et al. Cardiac signaling genes exhibit unexpected sequence diversity in sporadic cardiomyopathy, revealing HSPB7 polymorphisms associated with disease. J Clin Invest. 2010;120:280–289. doi: 10.1172/JCI39085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moffatt MF, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 18.Cresci S, et al. Clinical and genetic modifiers of long-term survival in heart failure. J Am Coll Cardiol. 2009;54:432–444. doi: 10.1016/j.jacc.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liggett SB, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ky B, et al. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120:310–317. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baroudi G, et al. Novel mechanism for Brugada syndrome: Defective surface localization of an SCN5A mutant (R1432G) Circ Res. 2001;88:E78–E83. doi: 10.1161/hh1201.093270. [DOI] [PubMed] [Google Scholar]
- 22.Waldegger S, et al. Barttin increases surface expression and changes current properties of ClC-K channels. Pflugers Arch. 2002;444:411–418. doi: 10.1007/s00424-002-0819-8. [DOI] [PubMed] [Google Scholar]
- 23.Scholl U, et al. Barttin modulates trafficking and function of ClC-K channels. Proc Natl Acad Sci USA. 2006;103:11411–11416. doi: 10.1073/pnas.0601631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto A, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 26.Schlingmann KP, et al. Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med. 2004;350:1314–1319. doi: 10.1056/NEJMoa032843. [DOI] [PubMed] [Google Scholar]
- 27.Seyberth HW. An improved terminology and classification of Bartter-like syndromes. Nat Clin Pract Nephrol. 2008;4:560–567. doi: 10.1038/ncpneph0912. [DOI] [PubMed] [Google Scholar]
- 28.Modlinger RS, Nicolis GL, Krakoff LR, Gabrilove JL. Some observations on the pathogenesis of Bartter's syndrome. N Engl J Med. 1973;289:1022–1024. doi: 10.1056/NEJM197311082891909. [DOI] [PubMed] [Google Scholar]
- 29.Gill JR, Jr., et al. Bartter's syndrome: A disorder characterized by high urinary prostaglandins and a dependence of hyperreninemia on prostaglandin synthesis. Am J Med. 1976;61:43–51. doi: 10.1016/0002-9343(76)90029-2. [DOI] [PubMed] [Google Scholar]
- 30.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, et al. Plasma renin activity is associated with increased cardiovascular events and mortality in the HOPE study. Circulation. 2009;120:S453. (abstr) [Google Scholar]
- 32.Potts JL, Dalakos TG, Streeten DH, Jones D. Cardiomyopathy in an adult with Bartter's syndrome and hypokalemia. Hemodynamic, angiographic and metabolic studies. Am J Cardiol. 1977;40:995–999. doi: 10.1016/0002-9149(77)90051-0. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz U, et al. Evidence for cardiovascular remodeling in a patient with Bartter's syndrome. Clin Investig. 1994;72:874–877. doi: 10.1007/BF00190744. [DOI] [PubMed] [Google Scholar]
- 34.Laine J, Jalanko H, Alakulppi N, Holmberg C. A new tubular disorder with hypokalaemic metabolic alkalosis, severe hypermagnesuric hypomagnesaemia, hypercalciuria and cardiomyopathy. Nephrol Dial Transplant. 2005;20:1241–1245. doi: 10.1093/ndt/gfh760. [DOI] [PubMed] [Google Scholar]
- 35.Heart Failure Society Of America. HFSA 2006 comprehensive heart failure practice guideline. J Card Fail. 2006;12:e1–e2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96(7B):3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 37.Dries DL, et al. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 38.Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8:e1000294. doi: 10.1371/journal.pbio.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannenhalli S, et al. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114:1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]
- 40.Margulies KB, et al. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ Res. 2005;96:592–599. doi: 10.1161/01.RES.0000159390.03503.c3. [DOI] [PubMed] [Google Scholar]
- 41.Kathiresan S, et al. Myocardial Infarction Genetics Consortium; Wellcome Trust Case Control Consortium. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durbin RM, et al. 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Willer CJ, Sanna S, Abecasis GR. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.