Abstract
The critical role of Toll-like receptors (TLRs) in mammalian host defense has been extensively explored in recent years. The capacity of about 10 TLRs to recognize conserved patterns on many bacterial and viral pathogens is remarkable. With so few receptors, cross-reactivity with self-tissue components often occurs. Previous studies have frequently assigned detrimental roles to TLRs, in particular to TLR2 and TLR4, in immune and cardiovascular disease. Using human and murine systems, we have investigated the consequence of TLR3 signaling in vascular disease. We compared the responses of human atheroma-derived smooth muscle cells (AthSMC) and control aortic smooth muscle cells (AoSMC) to various TLR ligands. AthSMC exhibited a specific increase in TLR3 expression and TLR3-dependent functional responses. Intriguingly, exposure to dsRNA in vitro and in vivo induced increased expression of both pro- and anti-inflammatory genes in vascular cells and tissues. Therefore, we sought to assess the contribution of TLR3 signaling in vivo in mechanical and hypercholesterolemia-induced arterial injury. Surprisingly, neointima formation in a perivascular collar-induced injury model was reduced by the systemic administration of the dsRNA analog Poly(I:C) in a TLR3-dependent manner. Furthermore, genetic deletion of TLR3 dramatically enhanced the development of elastic lamina damage after collar-induced injury. Accordingly, deficiency of TLR3 accelerated the onset of atherosclerosis in hypercholesterolemic ApoE−/− mice. Collectively, our data describe a protective role for TLR signaling in the vessel wall.
Keywords: inflammation, innate immunity, intimal hyperplasia
Cardiovascular disease, which is caused by atherosclerosis and its thrombotic complications, is the world's leading killer (1). The therapeutic advantage of statins over other lipid-lowering strategies (2) supports a complex interaction between risk factors, including lipid metabolism and inflammation. The pathways that sustain disease are beginning to be understood, but those that might protect against disease are less clear. These gaps in our knowledge are hampering the generation of new preventative or therapeutic strategies.
In recent years, the interplay between “innate immunity”—which exists in all individuals before exposure to antigens—and “adaptive immunity”—which occurs after antigenic stimulation—has become more appreciated. Charles Janeway's realization that innate immunity triggers adaptive responses (immunology's “dirty” secret) has catalyzed research to analyze innate immune sensors and their effector pathways (3). Since then, a family of at least 13 Toll-like receptors (TLRs) that can recognize the conserved molecular patterns of all microbial classes—bacterial, parasitic, and viral—and protect the host from these pathogens has emerged. Whereas extracellular TLRs (TLR2, TLR4, TLR5) recognize bacterial wall components, endosomal TLRs recognize nucleic acid patterns belonging to viruses or bacteria, including dsRNA (TLR3), ssRNA (TLR7/8), and dsDNA with hypomethylated Cytosine-phosphate-Guanine (CpG) motifs (TLR9) (4).
Not surprisingly, with so few receptors, TLR ligand selectivity is relatively low, and so TLRs also recognize self-molecules generated during tissue damage and inflammation in the presence or absence of infection. There is increasing evidence that inappropriate activation of TLRs, for example, via endogenous ligands, may contribute to disease. This was first documented for systemic lupus erythematosus where TLR9 was implicated by Marshak-Rothstein (5). Subsequent work has documented a role for TLRs and potentially endogenous TLR ligands in inflammatory arthritis, experimental autoimmune encephalomyelitis (EAE), and atherosclerosis as judged by the effects of ablating MyD88 (6–8), the common TLR and interleukin-1 (IL1) signaling adapter, as well as ablating specific TLRs (7–10).
However, there is also increasing evidence for the antithesis—that TLR signaling might prevent the onset of autoimmune responses. The administration of agonists of TLR3, TLR4, TLR7, and TLR9 prevents spontaneous diabetes in nonobese diabetic mice (11), whereas TLR4 abrogation increases EAE (12). TLR3, TLR5, and TLR9 signaling exert protection in mouse models of colitis (13–15).
Although TLRs were first documented in cells of the innate immune system, there is increasing evidence that structural cells, such as endothelial cells, can also acquire TLR expression and respond to their ligands during pathological processes. In atherosclerosis, TLR2 expression has been reported at very early stages of disease on endothelial cells in atherosclerosis-susceptible regions of the aorta (16).
Here we describe the paradoxical findings that TLR3 is up-regulated in human atherosclerotic tissue-derived smooth muscle cells, leading to the augmented production of several cytokines and chemokines, many of which are proinflammatory. Surprisingly, in vivo analysis revealed that TLR3 is involved in protection of the integrity of the vessel wall against intimal and medial injury and the early stages of atherosclerosis. Deeper understanding of the nature of the protective mechanism may help guide therapy against cardiovascular diseases.
Results
Increased Expression of TLR3 by Atheroma-Derived Smooth Muscle Cells.
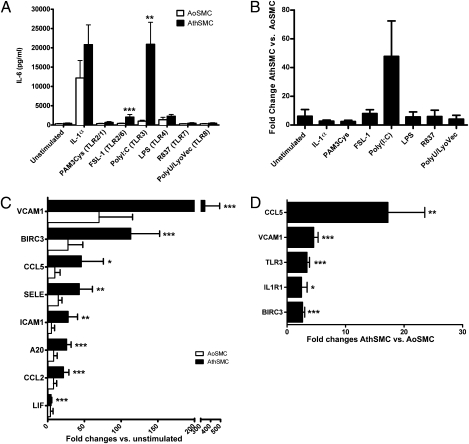
We screened responses to TLR-IL-1 family agonists in atheroma-derived smooth muscle cells (AthSMC) and in control aortic smooth muscle cells (AoSMC). SMC have been previously shown to respond to a variety of TLR agonists (17). However, AthSMC specifically displayed an enhanced expression of IL-6, IL-8, and CCL2/MCP-1 when stimulated with the dsRNA synthetic analog Poly(I:C) compared with AoSMC (Fig. 1 A and B; Fig. S1 A–D). To a lesser extent, the TLR2/TLR6 agonist FSL-1 induced an enhanced response in AthSMC vs. AoSMC in terms of IL-6 and IL-8 but not CCL-2/MCP-1 production. Other TLR agonists did not elicit enhanced responses in AthSMC vs. AoSMC. As the TLR3-dependent responses in AthSMC were particularly marked (over 40-fold compared with AoSMC; Fig. 1B), we explored further the gene expression of atherosclerosis-relevant genes by quantitative polymerase chain reaction (Q-PCR) array (SABiosciences). dsRNA stimulation significantly increased the expression of a selected set of genes involved in inflammatory cell recruitment (e.g., VCAM1 and CCL5/RANTES) and regulation of inflammation (e.g., A20/TNFAIP3 and BIRC3/cIAP2) in AthSMC, but not in AoSMC (Fig. 1C).
Fig. 1.
AthSMC exhibit enhanced expression and response to TLR3. (A) Concentration of IL-6 in the supernatants of SMC stimulated with various TLR agonists for 24 h are shown. Bars show mean ± SEM (n = 6 donors per group). AthSMC displayed enhanced expression of IL-6 when stimulated with Poly(I:C) and FSL-1 compared with AoSMC (**P < 0.01 and ***P < 0.001, respectively; rank ANCOVA). (B) The same data as in A are shown as fold change in IL-6 production between AthSMC and AoSMC following TLR agonist stimulation. Bars show mean ± SEM (n = 6 donors per group). (C) AthSMC and AoSMC were stimulated for 5 h with 25 μg/mL Poly(I:C) or left unstimulated. Genes induced by dsRNA in AoSMC (n = 3) and AthSMC (n = 7) were examined by quantitative PCR analysis using an Atherosclerosis RT2 Profiler PCR Array (SA Biosciences). Data are shown as mean ± SEM. Genes with a fold regulation greater than two are shown here. AthSMC displayed an enhanced expression of the indicated genes when stimulated with Poly(I:C) (*P < 0.05, **P < 0.01, ***P < 0.001; paired t test vs. unstimulated). (D) Atherosclerosis-related and TLR-pathway–related genes were assessed using quantitative PCR gene arrays (SA Biosciences) with cDNA from unstimulated AoSMC and AthSMC. Genes with a statistically significant up-regulation greater than twofold are displayed here. Data shown are mean fold changes of gene expression ± SEM of AthSMC (n = 9) vs. AoSMC (n = 4) (*P < 0.05, **P < 0.01, ***P < 0.001; AthSMC vs. AoSMC; paired t test). BIRC3, baculoviral inhibitor of apoptosis repeat-containing 3; CCL2, chemokine (C-C motif) ligand 2; CCL5, chemokine (C-C motif) ligand 5; ICAM1, intercellular adhesion molecule 1; LIF, leukemia inhibitory factor; SELE, E-selectin; VCAM1, vascular cell adhesion molecule 1; A20/TNFAIP3, tumor necrosis factor alpha-induced protein 3; IL-1R1, interleukin-1 receptor-1.
To assess the mechanism of the increased response to the dsRNA analog Poly(I:C) of AthSMC, we compared the baseline gene expression of AthSMC and AoSMC in unstimulated conditions. TLR3 expression was 3.4-fold higher in AthSMC vs. AoSMC (Fig. 1D). Up-regulation of TLR3 protein was verified by FACS analysis (Fig. S1 E and F). Interestingly, genes that were found to be up-regulated by dsRNA stimulation, such as VCAM-1, BIRC3/c-IAP2, and CCL5/RANTES, were also significantly higher in AthSMC vs. AoSMC in unstimulated conditions, suggesting prior in vivo TLR stimulation. Hence the mechanism of the increased dsRNA responsiveness in AthSMC appears to be an up-regulation of TLR3.
We hypothesized that exposure of AthSMC to interferons (IFN) within the atherosclerotic plaque was a potential mechanism of up-regulation of TLR3. To test this hypothesis, we exposed both AoSMC and AthSMC to IFNγ and IFNα and quantified TLR3 gene expression by Q-PCR. IFNα, and to a lesser extent IFNγ, was able to increase the expression of TLR3 (Fig. S1 G and H). Interestingly, Poly(I:C) itself is able to up-regulate the expression of TLR3 selectively in AthSMC but not in AoSMC (Fig. S1 G and H), which is consistent with our previous findings. We have previously shown that a mixed cell population of cells isolated from human atheroma is able to spontaneously produce TNF, IL-1, and IFNγ (18). However, we were unable to detect IFNα. Earlier work by Weyand's group demonstrated IFNα production after TLR9 stimulation with synthetic unmethylated oligonucleotides containing a CpG DNA motif in human atherosclerotic plaques (19). We confirmed in our mixed-cell-type atheroma cell culture system that IFNα production could be induced by TLR9 stimulation (Fig. S1I).
Therefore, we demonstrate that SMC isolated from human carotid atheroma have an increased responsiveness to dsRNA due to increased TLR3 expression. Interferons produced in the atherosclerotic plaque contribute to this increased TLR3 expression.
Augmented Expression of Pro- and Anti-Inflammatory Genes Following in Vivo TLR3 Activation.
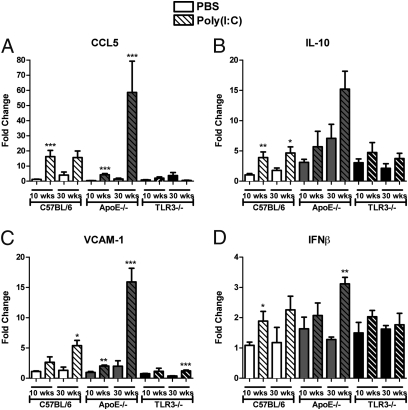
To investigate whether the increased TLR3 expression in AthSMC vs. AoSMC was due to the different arterial site (carotid vs. aorta) or to the presence or absence of atherosclerotic disease, we sought to examine the effect of i.p. Poly(I:C) stimulation in vivo in 10- and 30-wk-old C57BL/6, ApoE−/−, and TLR3−/− mice. In accordance with our in vitro data, stimulation with a single dose of Poly(I:C) induced the aortic expression of both proinflammatory genes including CCL5/RANTES, CCL2/MCP-1, and VCAM-1 (Fig. 2 A and C; Fig. S2A) and anti-inflammatory genes such as IFNβ, IL-10, and PD-L2 (Fig. 2 B and D; Fig. S2B). A20 induction was not observed in mice. This effect was more pronounced in the aorta of ApoE−/− mice with advanced disease compared with young ApoE−/− mice. The effect of Poly(I:C) stimulation was even more marked in carotid artery tissue, indicating that the human AthSMC responses might reflect both disease development and arterial site (Fig. S3). The expression of these genes was largely dependent on TLR3 and almost abrogated in TLR3−/− mice. Poly(I:C) up-regulated TLR3 gene expression in vivo in vascular tissues (Fig. S4), fitting with our in vitro human data. Expression of selected genes in aortas and secondary lymphoid organs of C57BL/6 mice that received chronic Poly(I:C) stimulation for 3 wk was also examined. In the aorta of Poly(I:C)-treated C57BL/6 mice, expression of CCL5, IFNβ, and IL10 was significantly increased (Fig. S5A). Expression of IFNβ mRNA was also induced in the spleen of Poly(I:C)-treated C57BL/6 mice (Fig. S5B). In addition, gene expression of PD-L1 and PD-L2 was induced by Poly(I:C) stimulation in the para-aortic lymph nodes (Fig. S5C). These results suggest that TLR3 activation induces gene expression of both potentially detrimental and protective genes.
Fig. 2.
Aortic gene expression of both pro- and anti-inflammatory factors is induced by Poly(I:C) stimulation. Ten- and 30-wk-old C57BL/6, ApoE−/−, and TLR3−/− mice were stimulated with PBS or 250 μg Poly(I:C) in PBS. Twenty-four hours poststimulation, mice were killed, aortas harvested, and RNA extracted. Gene expression of CCL5 (A), IL-10 (B), VCAM-1 (C), and IFNβ (D) was examined by quantitative RT-PCR. Bars show overall mean ± SEM [n = 3–5 mice/group; *P < 0.05, **P < 0.01, ***P < 0.001; PBS vs. Poly(I:C); unpaired Student's t test].
Therapeutic Effect of Poly(I:C) and TLR3 on Neointima Formation in Response to Arterial Injury.
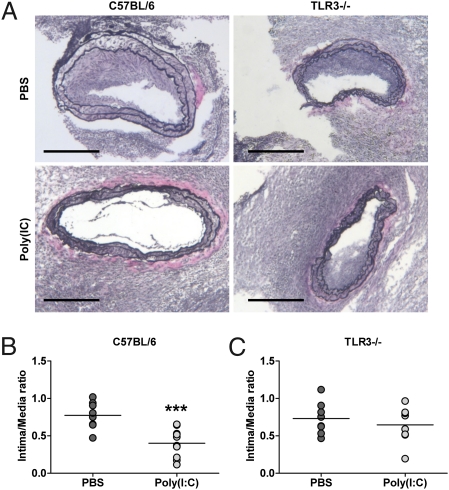
To determine the role of TLR3 activation on injury-induced neointima formation, we used a well-characterized arterial injury model involving the placement of a perivascular collar, based on an earlier rabbit model first described by Salvador Moncada (20). After collar placement, C57BL/6 and TLR3−/− mice (21) were treated either with Poly(I:C) or vehicle alone three times a week for 3 wk. No difference between the end weight of all groups of mice was observed. Neointima formation upon collar placement, as assessed by intima/media ratio, was significantly reduced in Poly(I:C)-treated C57BL/6 mice compared with vehicle-treated mice (P < 0.001) (Fig. 3B). Protection against neointima formation after Poly(I:C) treatment was ablated in TLR3−/− mice (P > 0.05) (Fig. 3C), indicating that the protective effect of the dsRNA analog was mediated by TLR3.
Fig. 3.
TLR3 activation protects against neointima formation in response to carotid collar injury. (A) Representative photomicrographs of injured carotid arteries from C57BL/6 and TLR3−/− mice treated with PBS or Poly(I:C) stained for elastin and counterstained with hematoxylin. (Scale bars: 200 μm.) (B and C) Intima/media ratio (IMR) of carotid arteries 21 d after injury from C57BL/6 (B) and TLR3−/− (C) mice treated with PBS or Poly(I:C). Each dot represents the mean IMR per individual mouse. Line represents the mean IMR per group [n = 8–11; ***P < 0.001; PBS vs. Poly(I:C); unpaired Student's t test].
Genetic Deletion of TLR3 Enhances Elastic Lamina Damage upon Arterial Injury.
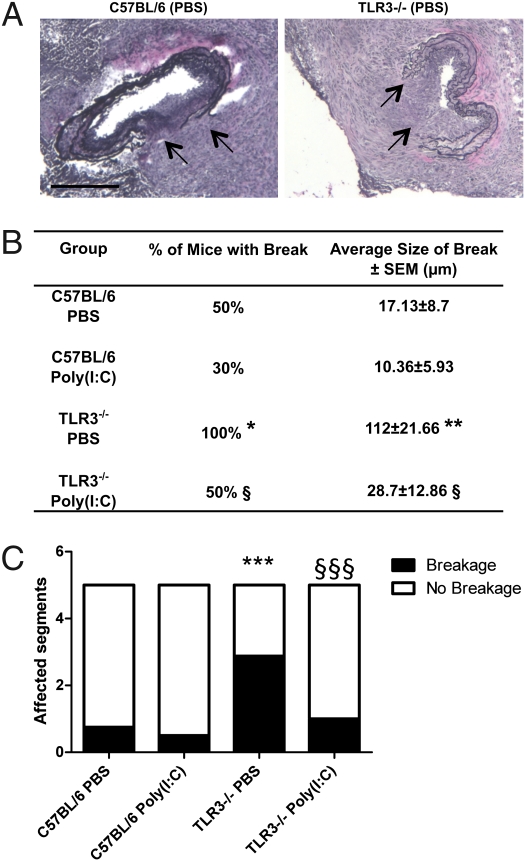
Upon collar placement, short interruptions of the elastic lamina were occasionally observed in elastin Van Gieson-stained tissue sections in C57BL/6 mice (Fig. 4A). In contrast, all TLR3−/− mice developed large interruptions of the elastic laminas after collar placement (Fig. 4). The interruptions in the elastic lamina were located immediately beneath the neointimal lesion and were not present in contralateral arteries. In comparison with C57BL/6 mice, the interruptions to the elastic laminas were more frequent (Fig. 4B) and had a larger cross-sectional width (Fig. 4 A and B) and longitudinal span across the carotid artery in TLR3−/− mice (Fig. 4C). These findings indicate an important role for TLR3 in vasculoprotective mechanisms in the medial layer of the arterial wall. Systemic administration of Poly(I:C) reduced the frequency, depth and width of the elastic lamina breaks in TLR3−/− mice (Fig. 4 B and C).
Fig. 4.
TLR3 activation protects against elastic lamina interruptions during carotid collar injury. (A) Representative photomicrographs of injured carotid arteries from C57BL/6 and TLR3−/− mice treated with PBS stained for elastin and counterstained with hematoxylin. Arrows denote area of breakage of the elastic laminae. (Scale bars: 200 μm.) (B) Table detailing number of mice in which a breakage in the elastic lamina was observed [n = 8–10 mice/group; *P < 0.05 C57BL/6 PBS vs. TLR3−/− PBS; §P < 0.05 TLR3−/− PBS vs. TLR3−/− Poly(I:C); χ2 test] and the average size of observed breaks [n = 8–10 mice/group; **P < 0.01 C57BL/6 PBS vs. TLR3−/− PBS; §P < 0.05 TLR3−/− PBS vs. TLR3−/− Poly(I:C); Mann–Whitney U test]. (C) Graph showing the average number of segments of the injured carotid artery of the five examined for each mouse that were affected or unaffected by elastic lamina breakage [n = 8–10 mice/group; ***P < 0.001 C57BL/6 PBS vs. TLR3−/− PBS; §§§P < 0.001 TLR3−/− PBS vs. TLR3−/− Poly(I:C); χ2 test].
TLR3 Deficiency Accelerates Early Atherosclerosis in Hyperlipidemic Mice.
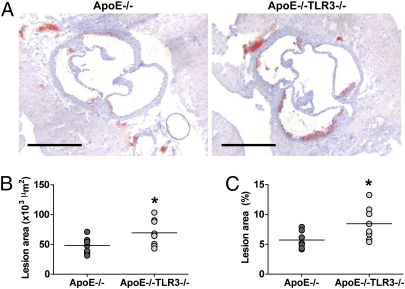
To assess the effect of TLR3 deficiency on atherosclerotic lesion development, ApoE−/− mice were crossed with TLR3−/− mice to generate ApoE−/−TLR3−/− mice. ApoE−/− and ApoE−/−TLR3−/− mice were fed a normal chow diet and were culled at 15 or 30 wk of age. No difference in body weight or total serum cholesterol levels was observed between ApoE−/− and ApoE−/−TLR3−/− mice at either time point examined (Table S1). Fifteen-week ApoE−/−TLR3−/− mice displayed a greater than 40% increase in aortic root atherosclerotic lesion formation compared with ApoE−/− mice with both absolute lesion size and lesion area fraction being significantly increased (P < 0.05; Fig. 5). However, no difference in absolute aortic root lesion size or lesion area fraction at the aortic root was observed between ApoE−/− and ApoE−/−TLR3−/− mice aged 30 wk (Fig. S6).
Fig. 5.
TLR3 deficiency accelerates early atherosclerotic lesion development in the aortic root. (A) Representative photomicrographs of aortic roots from 15-wk ApoE−/− and ApoE−/−TLR3−/− mice stained with Oil Red O and hematoxylin. (Scale bars: 500 μm.) (B) Cross-sectional aortic root lesion area (×103 μm2) in 15-wk ApoE−/− and ApoE−/−TLR3−/− mice. (C) Cross-sectional aortic root lesion area (%) in 15-wk ApoE−/− and ApoE−/−TLR3−/− mice. (B and C) Each circle represents the mean lesional area per individual mouse. Line represents the mean lesional area per group (n = 7–8; *P < 0.05; unpaired Student's t test).
Composition of atherosclerotic lesions in ApoE−/− and ApoE−/−TLR3−/− mice was assessed by performing CD68 staining to visualize plaque macrophages and Masson trichrome staining to identify collagen. Although a trend toward increased lesional macrophage content in 15-wk ApoE−/−TLR3−/− vs. ApoE−/− mice was observed, statistical significance was not reached (P > 0.05; Fig. S7B). However, when differences in lesion area were taken into account, no difference in lesion macrophage content between ApoE−/− and ApoE−/−TLR3−/− mice was observed (Fig. S7C). Furthermore, both absolute lesional CD68-positive area and lesion area fraction staining positive for CD68 were similar in 30-wk ApoE−/− and ApoE−/−TLR3−/− mice (Fig. S7 D and E). In 15-wk ApoE−/− and ApoE−/−TLR3−/− aortic root lesions, limited collagen was observed and there was no difference in lesional collagen content between the two strains (Fig. S8 B and C). In addition, no difference in absolute lesion collagen content or lesion area fraction collagen content was observed between 30-wk ApoE−/− and ApoE−/−TLR3−/− mice (Fig. S8 D and E).
Discussion
TLRs are among the oldest components of the innate immune system, their homologs existing in Drosophila (22). The list of endogenous TLR ligands is growing. Endogenous TLR ligands appear to promote disease, e.g., immunoglobulin/DNA complexes in lupus via TLR9 (5), extracellular matrix proteins such as tenascin C in arthritis via TLR4 (23), and modified LDL in atherosclerosis via TLR4 (24). Our own work on a unique human carotid atherosclerosis cell extraction and culture system revealed that TLR2 is markedly proatherogenic. TLR2 blockade reduces both cytokines and destructive matrix metalloproteinase enzymes, suggesting that a yet-to-be-identified TLR2 agonist may be present in human atherosclerotic plaques and that it may be a useful therapeutic target (25).
Here, we report that vascular SMC isolated from human atherosclerotic tissue highly express TLR3 and are primed for TLR3-dependent gene expression via dsRNA. Intriguingly, TLR3 was capable of inducing both proinflammatory and anti-inflammatory responses in the vessel wall in vitro and in vivo. Surprisingly, the net effect of TLR3 signaling is protective in in vivo models of mechanical as well as hypercholesterolemic arterial injury. This demonstrates an unexpected TLR-mediated protection in this major human disease process.
Heterogeneity of TLR expression in arterial vessels has been reported previously, yet the functional significance of TLR expression was not studied in the context of disease (26, 27). We took advantage of comparing SMC from a disease site (AthSMC) with control aortic SMC (AoSMC), and we noted a dramatic and functional increase of TLR3 expression in AthSMC. Augmented expression of TLR3 has been previously found in diseased tissue in other models, e.g., rheumatoid arthritis (28) and sepsis (29). TLR3 expression in SMC was up-regulated by type I and II interferons and Poly(I:C), a synthetic analog of dsRNA. Poly(I:C) was also able to up-regulate TLR3 expression in vascular tissue in vivo, mirroring the in vitro data in cultured SMC. As viral genome-dependent induction of TLR3 is blocked by neutralizing type I interferons or their receptor (30), Poly(I:C)-induced up-regulation of TLR3 in our systems may also be due to autocrine IFN signaling.
The effect of the dsRNA analog on AthSMC was dramatic compared with that on AoSMC, and it induced expression of genes involved in cell recruitment and inflammation (e.g., VCAM-1, CCL2/MCP-1, and CCL5/RANTES). It is noteworthy that anti-inflammatory and anti-apoptotic genes (e.g., A20/TNFAIP3 and BIRC3/cIAP2) were also up-regulated. Similarly, Poly(I:C) administration induced both proinflammatory (e.g., CCL5/RANTES, CCL2/MCP-1, and VCAM-1) and anti-inflammatory genes (e.g., IL-10 and PD-L2) in the vascular tissues of both ApoE −/− and wild-type mice. In keeping with the human SMC in vitro studies, the effect of TLR3 was more dramatic in aortas of mice with atherosclerotic disease.
Following these observations, an important question became, what is the net effect of TLR3 signaling in vivo? Systemic delivery of dsRNA prevented neointima formation after placement of a perivascular collar in a TLR3-dependent manner. Moreover, large interruptions in the elastic lamina were induced by the collar in all TLR3−/− mice, revealing an endogenous protective role for TLR3 in vessel wall integrity upon mechanical injury. Treatment with Poly(I:C) reduced the occurrence of the elastic lamina breaks in TLR3−/− mice, suggesting that, in the absence of TLR3, other sensors of dsRNA such as melanoma differentiation-associated protein 5 (MDA5) can mediate protection (4). Finally, TLR3 deficiency resulted in the accelerated onset of atherosclerosis in ApoE−/− mice, implying a role for TLR3 in protection from hypercholesterolemic arterial injury. Collectively, our data indicate a role for TLR3 in vessel wall integrity.
Moreover, by showing acceleration of atherosclerosis development and enhanced elastic lamina damage in the absence of TLR3 and an exogenous viral stimulus, we implicate endogenous agonists—yet to be discovered—in vascular protection. TLR3 senses dsRNA, a by-product of viral replication, in the endosome. However, TLR3 has been increasingly linked to tissue damage. Endogenous RNA released by damaged tissue or necrotic cells is able to induce TLR3 expression and signaling (31), whereas the alarmin high-mobility group protein B1 sensitizes TLR3 to the recognition of RNA (32). Interestingly, stathmin, a protein with a regulatory function on microtubule assembly that is up-regulated in brain injury, has been described as a candidate TLR3 agonist, linked to the induction of a neuroprotective gene profile (33). However, no study has assigned a clear protective role to TLR3 endogenous agonists in disease.
In the past, there has been speculation about the pathogenic role of viruses in atherosclerotic type lesions, e.g., in chicken (34). The pathogenic viral mechanisms reported include cytolytic (35) and immunomediated effects (36). Our study shows that such mechanisms are clearly distinct from the effect of dsRNA and its sensor(s), which, on their own, exert protection. The molecular mechanism(s) of TLR3/Poly(I:C)-induced protection are not yet fully unraveled. The dsRNA motif induces IFNβ via TLR3 and MDA5. IFNβ is therapeutic in some, but not in all, patients with multiple sclerosis. The mechanism of such heterogeneity in therapeutic response has been partially elucidated by Lawrence Steinman, who showed the effectiveness of IFNβ in Th1/IFNγ-dependent, but not in Th17/IL-17-dependent, EAE (37). Whether the vasculoprotection provided by TLR3 is dependent on the production of type I IFNs is uncertain because IFNβ therapy has shown conflicting results in animal models of atherosclerosis (38, 39). The production of protective mediators, including IL-10, following TLR3 activation has been reported (40). In our study, TLR3 increased expression of IL-10 in vascular tissues, suggesting that IL-10, a cytokine beneficial in various disease models, including atherosclerosis, could mediate protection. The B7 family members PDL1 and PDL2, which are augmented after TLR3 stimulation, may also contribute to vascular protection (41, 42).
This study enhances our knowledge of the complex role of TLRs in health and disease and points to therapeutic opportunities. Although it is unlikely that Poly(I:C) itself is a candidate due to unsuitable pharmacology, unraveling the pathways of protection might permit the development of future therapeutics for the treatment of cardiovascular disease. In contrast to current concepts, TLRs are not always detrimental in vascular disease (7, 10, 25), but they can be relevant in repair mechanisms within the vessel wall. It also suggests an alternative paradigm: might we do better therapeutically by enhancing natural homeostatic regulatory pathways instead of blocking putative pathogenic ones?
Materials and Methods
Ex Vivo Culture of Cells Isolated from Human Atherosclerotic Plaques.
Carotid endarterectomies from patients undergoing surgery for carotid disease were obtained from Charing Cross Hospital, London. The protocol was approved by the Research Ethics Committee (RREC2989). All patients gave written informed consent, according to the Human Tissue Act 2004 (United Kingdom). Single-cell suspensions of mixed cell types were obtained via enzymatic digestion and cultured for 24 h as previously described (18).
Isolation and Culture of AthSMC.
AthSMC were isolated from the mixed atheroma cells via magnetic cell sorting (Miltenyi), using anti-CD45 (pan-leukocyte marker) and anti-CD31 (endothelial cells) antibodies coupled to microbeads. SMC-expressing SMC-α actin composed >90% of the cells. Control AoSMC were purchased from Promocell. Both SMC types were grown in SMC medium (Promocell) and used at passage 3 consistently in all experiments. The protocols of stimulation with TLR ligands are described in SI Materials and Methods.
Mice.
C57BL/6 mice were purchased from Charles River. Apolipoprotein E-deficient (ApoE−/−) mice on a C57BL/6 background were bred in house. Toll-like receptor 3-deficient (TLR3−/−) mice fully backcrossed onto a C57BL/6 background were a generous gift from Richard Flavell (Yale University, New Haven, CT) (21). All mice in this study were male. Animals were housed in specific pathogen-free conditions, and all experimental animal procedures were approved by the Kennedy Institute of Rheumatology Ethics Committee and performed according to UK Home Office guidelines.
Perivascular Collar Injury.
At 22 wk of age, male C57BL/6 and TLR3−/− mice were anesthetized with isofluorane by inhalation, the left carotid artery dissected and a nonocclusive tygon collar (length: 2.5 mm; internal bore diameter: 510 μm; Cole-Parmer) placed around the carotid artery. Mice received 250 μg Poly(I:C) (Sigma) or PBS intraperitoneally on alternate days for a total of eight doses starting 4 d after surgery. Twenty-one days following collar placement, mice were euthanized, and neointima development and the presence of elastic lamina breaks were assessed as described in SI Materials and Methods.
Analysis of Atherosclerosis Development.
ApoE−/−TLR3−/− mice were generated by crossing ApoE−/− mice with TLR3−/− mice. ApoE−/−TLR3−/− double knockout mice were fertile and exhibited no overt phenotype. Mice were fed a standard chow diet and euthanized at either 15 or 30 wk of age as described in SI Materials and Methods. Aortic root lesion area was assessed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We received funding from the European Commission under the Sixth Framework Programme [Contract LSHM-CT-2006-037400; IMMune receptors in ATHerosclerosis (IMMUNATH)] and the Seventh Framework Programme (FP7/2007-2013; Contract 201668; AtheroRemo), the Graham-Dixon Charitable Trust, and Arthritis Research UK. We are grateful for support from the National Institute of Health Research Biomedical Research Centre funding scheme.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018515108/-/DCSupplemental.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Montecucco F, Mach F. Update on statin-mediated anti-inflammatory activities in atherosclerosis. Semin Immunopathol. 2009;31:127–142. doi: 10.1007/s00281-009-0150-y. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janeway CA., Jr Innate immunity: The virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 6.Björkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 7.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prinz M, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197:537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aumeunier A, et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS ONE. 2010;5:e11484. doi: 10.1371/journal.pone.0011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marta M, Andersson A, Isaksson M, Kämpe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–575. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 13.Rachmilewitz D, et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428–1441. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- 14.Vijay-Kumar M, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijay-Kumar M, et al. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856–864. doi: 10.1002/ibd.20142. [DOI] [PubMed] [Google Scholar]
- 16.Mullick AE, et al. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, et al. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H2334–H2343. doi: 10.1152/ajpheart.00252.2006. [DOI] [PubMed] [Google Scholar]
- 18.Monaco C, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niessner A, et al. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 20.Booth RF, et al. Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis. 1989;76:257–268. doi: 10.1016/0021-9150(89)90109-3. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 22.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 23.Midwood K, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 24.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaco C, et al. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009;120:2462–2469. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
- 26.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad U, et al. IFN-gamma primes intact human coronary arteries and cultured coronary smooth muscle cells to double-stranded RNA- and self-RNA-induced inflammatory responses by upregulating TLR3 and melanoma differentiation-associated gene 5. J Immunol. 2010;185:1283–1294. doi: 10.4049/jimmunol.0902283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roelofs MF, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 29.Cavassani KA, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 31.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 32.Yanai H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 33.Bsibsi M, et al. The microtubule regulator stathmin is an endogenous protein agonist for TLR3. J Immunol. 2010;184:6929–6937. doi: 10.4049/jimmunol.0902419. [DOI] [PubMed] [Google Scholar]
- 34.Fabricant CG, Fabricant J, Litrenta MM, Minick CR. Virus-induced atherosclerosis. J Exp Med. 1978;148:335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naghavi M, et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 36.Krebs P, et al. Chronic immune reactivity against persisting microbial antigen in the vasculature exacerbates atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 2007;27:2206–2213. doi: 10.1161/ATVBAHA.107.141846. [DOI] [PubMed] [Google Scholar]
- 37.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang LN, et al. Interferon-beta attenuates angiotensin II-accelerated atherosclerosis and vascular remodeling in apolipoprotein E deficient mice. Atherosclerosis. 2008;197:204–211. doi: 10.1016/j.atherosclerosis.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Goossens P, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Bsibsi M, et al. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 41.Koga N, et al. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler Thromb Vasc Biol. 2004;24:2057–2062. doi: 10.1161/01.ATV.0000145015.23656.e4. [DOI] [PubMed] [Google Scholar]
- 42.Gröschel S, et al. TLR-mediated induction of negative regulatory ligands on dendritic cells. J Mol Med. 2008;86:443–455. doi: 10.1007/s00109-008-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.