Abstract
MicroRNAs (miRNAs) are small noncoding RNAs able to regulate a broad range of protein-coding genes involved in many biological processes. miR-96 is a sensory organ-specific miRNA expressed in the mammalian cochlea during development. Mutations in miR-96 cause nonsyndromic progressive hearing loss in humans and mice. The mouse mutant diminuendo has a single base change in the seed region of the Mir96 gene leading to widespread changes in the expression of many genes. We have used this mutant to explore the role of miR-96 in the maturation of the auditory organ. We found that the physiological development of mutant sensory hair cells is arrested at around the day of birth, before their biophysical differentiation into inner and outer hair cells. Moreover, maturation of the hair cell stereocilia bundle and remodelling of auditory nerve connections within the cochlea fail to occur in miR-96 mutants. We conclude that miR-96 regulates the progression of the physiological and morphological differentiation of cochlear hair cells and, as such, coordinates one of the most distinctive functional refinements of the mammalian auditory system.
Keywords: deafness, mouse model, sensory system, currents, action potentials
In the mammalian cochlea, inner hair cells (IHCs) and outer hair cells (OHCs) transduce sound into electrical responses. IHCs are the primary sensory receptors that relay sound stimuli to the brain with high temporal precision via the release of neurotransmitter from their ribbon synapses onto type I spiral ganglion neurons (1). Synaptic ribbons are specialized organelles able to tether a large number of synaptic vesicles at the cell's active zones and are thought to allow sensory cells to mediate high rates of sustained synaptic transmission, coordinated release of multiple vesicles, and temporally precise transfer of information (2). OHCs provide electromechanical amplification of the cochlear partition to enhance the sensitivity and frequency selectivity of the mammalian cochlea via voltage-dependent electromotility, which is mediated by the motor protein prestin (3) and modulated by the inhibitory efferent cholinergic system (4). Before sound-induced responses begin at the onset of hearing, which occurs at around postnatal day 12 (P12) in most rodents, hair cells undergo a precise developmental program. Although hair cell maturation is known to be influenced by many proteins (5–9), we know little about the mechanisms underlying their biophysical and morphological development, especially those involved in the functional differentiation of IHCs and OHCs that occurs from around birth (10).
MicroRNAs (miRNAs) regulate posttranscriptional gene expression programs by decreasing the level of target mRNA in mammals (11) and are involved in tissue development, cell fate specification, morphogenesis, and a range of diseases (12–16). Members of the miR-183 family (miR-96, miR-182, and miR-183) are specific to sensory organs (17, 18) and are highly expressed in the inner ear (19, 20), eye (17), and nose (21). In the inner ear, they appear to be important for determining cell fate and development (22). miR-96 is expressed in developing cochlear hair cells up to at least P5 (23) and in the spiral ganglion up to P14 (19). Mutations in miR-96 have been associated with nonsyndromic progressive hearing loss in humans (15) and mice (23). We found that hair cell development in diminuendo mice with a Mir96 mutation is arrested at around the day of birth, a time when IHCs and OHCs still exhibit qualitatively similar biophysical properties. We have also shown that miR-96 is involved in the maturation of the hair cell stereocilia bundle and the remodelling of auditory nerve connections within the cochlea.
Results
Hair Cell Morphology Is Immature in Diminuendo Mutant Mice.
The hair bundle morphology of IHCs and OHCs was investigated using SEM of P4 mouse cochleae. Hair cells from homozygous mutant (Dmdo/Dmdo) mice appeared more immature than those from WT (+/+) animals (Fig. 1 A and B). Mutant IHC stereocilia remained uniformly thin compared with the increased width of stereocilia in controls (Fig. 1 A and B, Upper). OHCs had extra rows of stereocilia in which microvilli had not been reabsorbed and bundles exhibited a rounder shape (Fig. 1 A and B, Lower). Heterozygous (Dmdo/+) mutant hair cells had a similar but less severe phenotype than homozygous mutants (see also ref. 23), suggesting that miR-96 levels are normally tightly regulated. Hair cell membrane capacitance (Cm) measurements, which give an estimate of the cell's surface area, showed that IHCs (Fig. 1C) and OHCs (Fig. 1D) from the apical turns of Dmdo/Dmdo and Dmdo/+ mice failed to grow after birth. In OHCs, this was verified by measuring their length at the same apical location at P4, with Dmdo/Dmdo cells (19.0 ± 1.3 μm, n = 329, 11 cochleae) being significantly shorter than control cells already at this early stage (21.4 ± 1.1 μm, n = 270, 9 cochleae; overall P < 0.001; Fig. 1E). The reduced OHC length may result from the down-regulation of Slc26a5 (prestin) expression previously reported in this mutant (23), because prestin is a major component of the OHC lateral wall and prestin KO mice have similarly short OHCs (3). In Dmdo/Dmdo mice, the normal length of the cochlear duct and the organization of hair cells into the usual three rows of OHCs and one row of IHCs (23) suggest that the initial development of the cochlea is likely to occur normally. However, the development of auditory hair cells stops prematurely at birth or soon afterward.
Fig. 1.
Hair cell morphology and cochlear physiology in diminuendo mice. (A and B) SEM from apical coil IHCs (Upper) and OHCs (Lower) from P4+/+ and Dmdo/Dmdo cochleae. The hair bundle structure in Dmdo/Dmdo hair cells is more immature than that in controls. (Scale bar: 3 μm.) (C and D) Cm of IHCs (C: 1 ≤ n ≤ 11, P1–P30) and OHCs (D: 1 ≤ n ≤ 8, P1–P18) increases with age in control but not Dmdo/Dmdo mice. Dashed gray lines in C and D give an indication of the range of expected normal growth in WT mice (25, 26). (E) Immature Dmdo/Dmdo OHCs (P4) are significantly shorter than control cells. +/+: 270 OHCs, 9 cochleae; Dmdo/+: 240 OHCs, 8 cochleae; Dmdo/Dmdo: 329 OHCs, 11 cochleae. (F) Mean ABR thresholds (mean ± SD) are raised in P15 Dmdo/+ (n = 9) compared with age-matched +/+ mice (n = 8).
Early Onset of Hearing Loss in Heterozygous Mutant Mice.
Homozygous (Dmdo/Dmdo) mutant mice do not have a Preyer reflex (ear flick in response to sound), whereas it is present in heterozygous mutants but is progressively reduced and eventually lost (23). Because the Preyer reflex is a suprathreshold response, we investigated auditory thresholds in Dmdo/+ mice using auditory brainstem responses (ABRs), which reflect the activity of IHCs and the afferent auditory pathway. ABR measurements repeated weekly from 3- to 8-wk-old mice showed stable raised thresholds. Even at P15, we found that Dmdo/+ mice showed severely elevated thresholds (Fig. 1F), indicating a very early-onset hearing deficit in these mutants. In addition, forward masking of ABR wave 1 amplitudes in Dmdo/+ heterozygotes and WT littermate controls at P15 showed that responses to a probe tone following exposure to a masker stimulus were smaller in size when the time intervals between the masker and the probe tone were shorter and that this reduction in amplitude was significantly greater (two-way ANOVA, P < 0.001) in the heterozygotes than in the WT mice (Fig. S1). This observation indicated that mutant synapses were less able to recover rapidly from an earlier stimulus than those in WT mice. The unexpectedly high thresholds for ABRs in heterozygotes may be related to a lack of synchrony, which is consistent with the reduced ability of the response to recover from a forward masker tone.
Hair Cell Functional Differentiation Requires miR-96.
In most rodents, after terminal mitosis at around embryonic day 14 (24), undifferentiated cells begin to acquire ion channels typical of immature auditory hair cells (10, 25). The onset of adult-like characteristics normally occurs at around P8 for OHCs (26) and at around P12 for IHCs (25). We found that the mutation affecting miR-96 prevented the normal biophysical differentiation into mature hair cells. The K+ currents characteristic of adult WT IHCs [large conductance Ca2+-activated K+ current (IK,f) and negatively activating K+ current (IK,n)] (25) (Fig. 2 A and C, +/+) were absent in Dmdo/Dmdo (Fig. 2 B and C) and reduced in Dmdo/+ (Fig. 2C) apical-coil cells. Mutant IHCs not only failed to become functionally mature but retained the very low level of K+ current expression that was present during embryonic and early postnatal stages [inward rectifier K+ current (IK1) (27), delayed rectifier K+ current (IK) (25) and small conductance Ca2+-activated K+ current (ISK2) (28)] (Fig. 2B). These currents normally get larger before becoming down-regulated at around the onset of hearing. The physiological consequence of these abnormalities was that adult mutant IHCs, instead of acquiring the fast graded voltage responses to stimulation present in mature cells (25) (Fig. 2D), retained the ability to fire spontaneous Ca2+-dependent action potentials (Fig. 2E), normally a characteristic of immature cells (25). The time course of K+ current expression appeared to stall at or just after the day of birth in mutant IHCs [Fig. 2F, delayed rectifier K+ current (IK); Fig. 2G, IK1]. As for the Cm (Fig. 1C), heterozygous IHCs showed an intermediate phenotype (Fig. 2 F and G). Immature basal coil Dmdo/Dmdo IHCs exhibited similar or slightly smaller currents than those of mutant apical cells. Because the maturation of K+ currents in basal cells is usually shifted forward by a few days (25), it is likely that their development in Dmdo/Dmdo mice stalls earlier than in apical cells. The abnormal characteristics of adult mutant IHCs were not attributable to cell deterioration because their basic biophysical properties, such as resting membrane potentials and resting conductance, were similar to those of immature cells (Fig. S2). OHCs normally follow a different developmental program from IHCs (10), but their maturation also appeared to stall at a very immature stage of development in homozygotes (Fig. S3). These findings suggest that in the diminuendo mutant, the biophysical properties of IHCs and OHCs do not develop further than the late embryonic/early postnatal stage, resulting in a coordinated general halt in their physiological development.
Fig. 2.
Potassium currents and voltage responses in IHCs from diminuendo mice. (A and B) Examples of K+ currents recorded from +/+ (black) and Dmdo/Dmdo (red) adult IHCs (P22), respectively. Currents were elicited by depolarizing voltage steps in 10-mV nominal increments from the holding potential of −64 mV to the various test potentials shown by some of the traces. IK (25); IK,f, (49); IK,n (25, 50); IK1 (27); ISK2 (28). (C) K+ currents characteristic of adult IHCs are smaller in Dmdo/+ cells (blue) and absent in Dmdo/Dmdo cells. The size of the isolated IK,n in adult Dmdo/+ IHCs could not be accurately assessed because it was contaminated by the persistence of the immature-type current (27). Voltage responses in control (D, induced by depolarizing current injections) and homozygous mutant (E, spontaneous Ca2+-dependent action potentials) adult IHCs. Development of the outward K+ current was measured at 0 mV (F, IK) and that of the inward rectifier K+ current was measured at −124 mV (G, IK1) in all three genotypes. Dashed gray lines give an indication of the range of expected normal growth in WT mice (25, 27). The vertical dashed line in G indicates that the size of IK1 is normally rapidly down-regulated from P12 (27).
miR-96 Is Required for the Normal Maturation of the IHC Exocytotic Ca2+ Dependence.
The presynaptic function of IHCs in Dmdo mice was assessed by measuring the change in cell membrane capacitance (ΔCm) following stimulation, which is normally interpreted as a sign of vesicle fusion. At the onset of hearing, the synaptic machinery of IHCs becomes more sensitive to Ca2+, causing docked vesicles to be released linearly with increases in Ca2+ current (ICa) (29, 30), a process that requires synaptotagmin IV (31). We found that the mutation in Mir96 interfered with the normal IHC synaptic development. Adult Dmdo/Dmdo IHCs (n = 7) had a larger maximal ICa but a smaller corresponding ΔCm (P < 0.001) compared with control littermates (n = 8; Fig. 3 A and B). As observed for the K+ currents (Fig. 2), the size of ICa and ΔCm in Dmdo/Dmdo IHCs did not follow normal maturation but, instead, remained approximately constant at values consistent with those measured around birth (29, 30) (Fig. 3 C and D). The consequence of the immature ICa and ΔCm in adult Dmdo/Dmdo IHCs was that the exocytotic Ca2+ dependence, defined as the change in ΔCm as a function of ICa and measured using the synaptic transfer function (29–31), was significantly less linear (power of ∼3.4 ± 0.4, n = 8) than that in heterozygous (power of 1.9 ± 0.2, n = 7; P < 0.01) and control (power of 1.2 ± 0.2, n = 7; P < 0.001; Fig. 3E) IHCs. It was, however, comparable to that of immature IHCs (29–31) (Fig. S4). The smaller Ca2+ current in immature Dmdo/Dmdo IHCs, compared with that of control cells (Fig. 3D), was confirmed by a reduced Ca2+ channel expression by RT-PCR of Cacna1d using similar age range cochleae (Fig. S5).
Fig. 3.
Exocytotic Ca2+ dependence and synaptic morphology in diminuendo IHCs. (A and B) ICa and ΔCm in adult control (+/+, P21) and homozygous mutant (Dmdo/Dmdo, P15) IHCs. Recordings were obtained in response to 100-ms voltage steps from −81 mV in 10-mV increments. Only maximal responses are shown in A. (C and D) Maximal peak ICa and ΔCm values, respectively, from immature and adult control and mutant IHCs. (E) Adult mutant IHCs showed a steeper intrinsic Ca2+ dependence of exocytosis. (Right) Synaptic transfer curves obtained by plotting ΔCm against the corresponding ICa between −71 mV and −11 mV (31). Fits are according to Eq. 1 (SI Materials and Methods). (Left) Average ΔCm traces obtained from all IHCs investigated. (F and G) Transmission electron microscopy showing the cross-sectional profiles of presynaptic dense bodies (arrows) from a control IHC and a Dmdo/Dmdo adult IHC, respectively. aff, afferent endings. (Scale bar: 100 nm.) (H and I) Immunostaining of neurofilament showing a more irregular wiring pattern of fibers below adult Dmdo/Dmdo IHCs compared with that observed in controls. TC, tunnel of Corti. (Scale bar: 50 μm.)
Hair Cell Synaptic Morphology and Innervation Pattern Remain Immature in Diminuendo Mutant Mice.
The immature biophysical properties of adult mutant IHC exocytosis were coupled with morphological abnormalities in the characteristic ribbon synapses (2). Although ribbon synapses of control IHCs showed the normal ellipsoid shape (Fig. 3F), mutant cells had spherical synaptic ribbons (Fig. 3G), which are normally observed in early postnatal hair cells (32). Auditory afferent endings contacting adult mutant IHCs were also affected because they showed extensive disorganization compared with controls (Fig. 3 H and I). During the first week of postnatal development, afferent endings onto IHCs, which show extensive branching up to this point, normally undergo pruning that results in the typical one-to-one conformation observed in adult cells (33). The disorganized afferent endings in Dmdo/Dmdo mice suggest that fibers had not undergone such pruning, further supporting the requirement of WT miR-96 for the maturation of the mammalian cochlea. Because miR-96 has been found in the spiral ganglion (19) as well as in hair cells (23), the defects in afferent synapse formation and pruning might be attributed to effects of the miR-96 mutation in hair cells and/or peripheral sensory neurons.
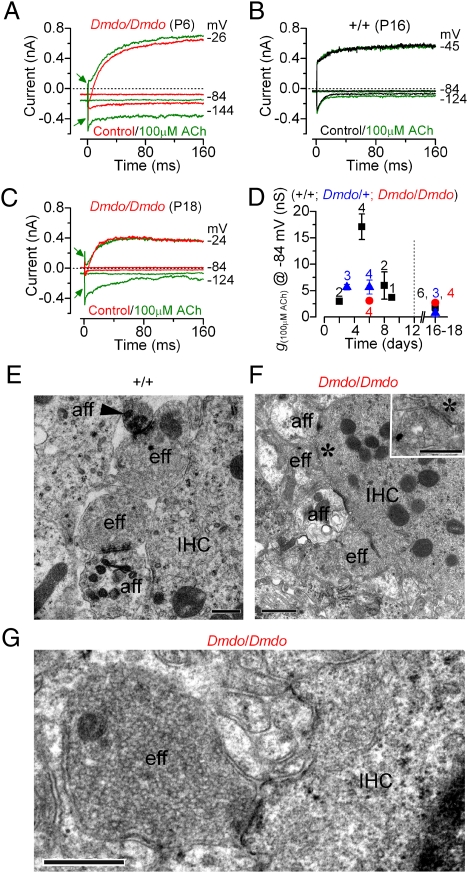
The olivocochlear efferent fibers of the auditory nerve modulate the activity of hair cells by releasing the neurotransmitter ACh. In the mature animal, the principal targets of efferent fibers are OHCs (4). During cochlear development, however, efferent endings make transient axosomatic synaptic contact with immature IHCs. The few efferent fibers that remain below adult IHCs make axodendritic contacts with the afferent terminals (33) (Fig. 4E). In immature IHCs, the ACh-activated current, which is mediated by Ca2+ entering hair cells through α9α10-nicotinic acetylcholine receptors (nAChRs) that activates SK2 channels, is initially detected in apical IHCs from just after birth (28). Membrane currents were recorded from immature and adult IHCs (Fig. 4 A–C: +/+, black traces; Dmdo/Dmdo, red traces) before and during the extracellular application of 100 μM ACh. In the presence of ACh, depolarizing and hyperpolarizing voltage steps from the holding potential of −84 mV elicited an instantaneous current (green traces), which is carried by SK2 channels and nAChRs (28), in all mutants (Fig. 4 A and C) and in immature WT IHCs. ACh responses were smaller in mutants (Fig. 4D), indicating an early halt in the normal IHC development in these animals. Although control IHCs were no longer sensitive to ACh at adult stages (Fig. 4 B and D), mutant cells remained responsive at a level similar to that observed in early postnatal cells (Fig. 4 C and D). ACh responses were also recorded in immature basal-coil Dmdo/Dmdo IHCs, although they were about threefold smaller than in apical mutant cells. This further supports our hypothesis that the development of basal cells is likely to stall a few days earlier, at late embryonic stages. The continued presence of ACh receptors in mutant IHCs was associated with efferent fibers directly contacting their synaptic region (Fig. 4F, Dmdo/Dmdo IHC) instead of terminating onto the afferent endings (Fig. 4E, control IHC). The efferent terminals in mutants were normally full of vesicles (Fig. 4G) similar to those contacting afferent terminals of WT IHCs, indicating that the efferent system is likely to be functional in adult mutant mice. Despite ACh responses being mediated by the same channels in both IHCs and OHCs, mutant OHCs did not respond to ACh and were contacted by very few small efferent endings (Fig. S6). Chrna9 (α9-nAChR) was down-regulated and Chrna10 (α10-nAChR) was up-regulated in the Dmdo/Dmdo organ of Corti (Fig. S5), suggesting that there may be an imbalance in the components of the mutant ACh receptors.
Fig. 4.
Efferent modulation of diminuendo IHCs. (A–C) Membrane currents recorded from immature (A: Dmdo/Dmdo, red) and adult (B: +/+, black; C: Dmdo/Dmdo, red) IHCs before and during superfusion of ACh (green traces). The instantaneous current elicited by ACh is indicated by arrows. (D) Developmental change in the steady-state slope conductance measured near −84 mV in the presence of 100 μM ACh. (E) Transmission electron microscopy showing the synaptic region of an adult control IHC with efferent (eff) endings contacting radial afferent fibers (aff + arrowhead). Pre- and postsynaptic membrane densities are visible between efferents and afferents. (F) Afferent and efferent terminals onto adult Dmdo/Dmdo IHCs. (Inset) High-magnification view of the efferent terminal (*) shows vesicles and pre- and postsynaptic densities. (G) Efferent ending from another adult Dmdo/Dmdo IHC showing a large number of vesicles. (Scale bars: E, G, and Inset in F, 0.5 μm; F, 1 μm.)
Discussion
Here we describe the function of miR-96 in cochlear development. The striking feature of our findings in diminuendo mice is that the mutation in Mir96 causes a comprehensive brake on the functional maturation of auditory sensory hair cells. Hair cell fate is specified, and they appear to differentiate normally during embryonic development. At around birth, however, mutant hair cells develop no further and retain their immature phenotype (Fig. S7). This coherent brake in the maturation of a specific cell type is unusual. Other mutations involving proteins associated with cochlear function and/or maturation either cause the loss of hair cells or specific structural or functional deficits during defined periods of development, ranging from embryonic to postnatal stages (5). In mutants in which hair cells remain viable, there is generally a failure in the down-regulation of immature hair cell biophysical characteristics and in the acquisition of adult properties (6–9). The data presented here for miR-96 exemplify a coherent developmental brake that prevents the general biophysical and morphological differentiation between IHCs and OHCs that normally occurs from around birth.
Role of miR-96 in the Mammalian Cochlea.
Cochlear hair cells are specified during embryonic development (34), and from about embryonic day 14–16, IHCs and OHCs in the mouse are recognizable by their different hair bundle morphology and location within the sensory epithelium (24, 33). However, throughout embryonic development, their biophysical characteristics are qualitatively indistinguishable (10). At around birth, hair cells begin to diverge in terms of their electrophysiological properties, cell size, and innervation pattern (10, 33). The onset of functional maturity in OHCs and IHCs occurs at about P8 and P12, respectively, with the acquisition and/or elimination of different ion channels (25, 26). Postnatal hair cell maturation also includes expression of specialized membrane proteins such as prestin (3), changes in the structural and functional characteristics of ribbon synapses (30–32), and a major refinement in the neuronal connections within the mammalian cochlea (33). We have shown that the mutation in Mir96 prevents, from around birth, any further progression in the development of the hair cell stereocilia bundle morphology, cell length, ribbon synapses, and innervation pattern. Furthermore, hair cells retain their embryonic/early postnatal potassium conductances, calcium-dependent spiking activity, nonlinear calcium dependence of synaptic exocytosis, and sensitivity to the efferent neurotransmitter ACh. A major consequence of this phenotype is that IHCs and OHCs do not functionally differentiate from one another. Remarkably, the other two members of the miR-183 family (miR-182 and miR-183), known to be highly expressed in the inner ear (19, 20) and still present in diminuendo mutants (23), did not compensate for the mutant miR-96. This suggests that in contrast to the largely overlapping roles in zebrafish (22), the three members of the miR-183 family in the mouse have functionally distinct roles. Alternatively, the physiological abnormalities observed in diminuendo mutant mice could be attributable to a gain-of-function through the acquisition of different mutant miR-96 targets. However, since impairment is associated with three different base changes in the miR-96 seed region (one mouse and two human), this seems to be less likely. This is also suggested by the fact that although miR-96 modulates a broad range of target genes (23), the diverse phenotype of diminuendo mice is structurally and functionally coherent in the sense that it represents a coordinated brake on development. The importance of miR-96 is emphasized by the semidominant inheritance of Dmdo, suggesting that there is tight regulation over the expression level of each allele. This is evident from the intermediate phenotype observed in heterozygous mutant mice for all features we investigated, with the exception of the unexpectedly poor ABR thresholds in young heterozygotes. Thus, miR-96 is a crucial regulator of some of the most distinctive functional refinements of the mammalian auditory system, and the data offer an explanation for the association between mutations in Mir96 and human nonsyndromic progressive hearing loss (15).
Cochlear Development: Genetic Program or Sensory-Independent Electrical Activity?
One important question in sensory development concerns the relation between intrinsic genetic programs and the influence of sensory-independent electrical activity that occurs during immature stages (35), which has been shown to regulate a variety of cellular responses, including gene expression (36). Immature cochlear hair cells generate Ca2+-dependent action potentials (25, 37–39) that are thought to be required for the normal expression of the BK current IK,f (7), the linear exocytotic Ca2+ dependence (37), and the refinement of synaptic connections before the onset of sensory-induced activity (40). All these aspects of cochlear development were repressed in mutant diminuendo mice despite the persistence of action potential activity in hair cells. We conclude that action potential activity alone is not sufficient to drive hair cell maturation and that other processes are required in which miR-96 is likely to play a pivotal role. It is conceivable that miR-96 coordinates a crucial “checkpoint” in early hair cell development (at around birth) that, once passed, allows cells to respond appropriately to activity-dependent cues. In this context, it is worth noting that hair cell maturation is suspended at an equivalent level not only in IHCs and OHCs but in a progressive wave along the cochlea, starting with the basal cells, which develop a few days before those in the apex (25). This suggests that the developmental checkpoint for basal cells is likely to occur at late embryonic stages.
miR-96 Can Act as a Master Switch for IHC and OHC Functional Differentiation.
miRNAs can regulate various aspects of cell function and may act as stoichiometric inhibitors of mRNA translation (41). Although they can repress hundreds of proteins, this repression appears to be relatively mild (42). Although mi-RNAs are thought to act by both repression of translation and decreasing the levels of target mRNA, the regulation of target RNA levels is the predominant mechanism in mammals (11). These broad functions of miRNAs cannot explain the highly coordinated phenotype that we observe in hair cells with the diminuendo mutation. So, does miR-96 regulate hair cell development by repressing genes that impede hair cell maturation? Furthermore, how many genes must miR-96 regulate to achieve such a coherent response? It has been suggested that miRNAs can act as complex molecular switches, with some promoting and others inhibiting cell differentiation (43). miR-9 has been proposed as a master switch in the CNS because it targets a group of 11 RNAs implicated in adaptation to alcohol (44). miR-96 directly or indirectly affects the expression of a large number of downstream genes implicated in cochlear function, development, and survival (23). In diminuendo mice, the mutation affecting miR-96 leads to the down-regulation of Tmc1 (Fig. S5), which is a transmembrane protein required for the expression of adult-like biophysical characteristics in hair cells (8), and Ptprq (23), which is a component of the hair bundle stereocilia involved in their development (45). Furthermore, in the organ of Corti with mutant miR-96, Gfi1, which is a transcription factor critical for hair cell differentiation and survival from just before or at around birth (46), is down-regulated (23). The absence of Gfi1 causes the loss of hair cells from the base to the apex of the cochlea (46), which is consistent with our findings in diminuendo mutant mice, in which basal hair cell development is likely to be halted earlier than in apical hair cells. The similarity in the morphological and physiological abnormalities caused by these downstream genes to those observed in diminuendo mice further supports a role for miR-96 as a master switch for the functional development of the mammalian cochlea. Although the direct targets of miR-96 are not clear, it could exert its role by regulating the expression of specific gene transcripts required for hair cell development as well as down-regulating genes that, although necessary for embryonic stages, block maturation after birth. The latter could explain how miR-96 is able to up-regulate important developmental genes, such as Tmc1, Ptprq, and Gfi1, indirectly. miRNAs have been shown to be important for defining and maintaining the identity of different cell types (47). In the olfactory system, miRNAs are crucial for the differentiation of progenitor cells into mature olfactory neurons, but they are not crucial in initial cell fate specification or during adult stages (48). It is likely that miR-96 fulfils a similar role in the auditory system.
We propose that miR-96 is a late embryonic/early postnatal upstream regulator of mRNA translation that ensures controlled and highly coordinated differential development of mammalian cochlear IHCs and OHCs. This occurs through a sustainable progression of physiological and structural changes and includes a pivotal role for miR-96 in the coordinated regulation of the different elements required for neuronal plasticity and sensory maturation. Understanding the mechanism by which miR-96 is able to orchestrate such a complex and integrated expression of genes required for cochlear function, could provide us with clues to help develop therapies to ameliorate the effects associated with nonsyndromic progressive hearing loss.
Materials and Methods
Electrophysiology.
Electrophysiological recordings were made from IHCs and OHCs of diminuendo mutant mice. A detailed description of voltage and current recordings and real-time ΔCm is available in SI Materials and Methods. Statistical comparisons of means were made by a Student's two-tailed t test or one-way ANOVA. Unless otherwise specified, mean values are quoted ± SEM, where P < 0.05 indicates statistical significance.
ABR Recordings.
ABRs were used to assess hearing threshold of diminuendo mice (details are provided in SI Materials and Methods).
Immunostaining.
Cochleae from diminuendo mice were fixed with 4% (weight/vol) paraformaldehyde (PFA) for 2 h. The primary antibody directed against neurofilaments was detected with Alexa Fluor 488-conjugated antibodies. The tissue was then imaged using a confocal system (details are provided in SI Materials and Methods).
Transmission Electron Microscopy.
Cochleae were fixed for 2 h with 2.5% (vol/vol) glutaraldehyde. Radial and horizontal ultrathin sections were examined in a transmission electron microscope (details are provided in SI Materials and Methods).
SEM.
Inner ears from diminuendo mice were fixed for 3 h with 2.5% (vol/vol) glutaraldehyde. The samples were examined using a scanning electron microscope (details are provided in SI Materials and Methods).
Quantification of Hair Cell Length.
Cochleae were fixed in 4% (weight/vol) PFA. The primary antibody directed against Myosin7a was detected with Alexa Fluor 488-conjugated antibodies and viewed using a confocal microscope (details are provided in SI Materials and Methods).
Real-Time PCR.
Semiquantitative real-time PCR was carried out on cDNA made from RNA that had been extracted from the organ of Corti of P4 homozygote and WT littermates (details are provided in SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank G. P. Richardson for his critical feedback on an earlier version of the manuscript. We thank S. Pearson for assistance with ABR recordings. This work was supported by Royal National Institute for Deaf People (RNID) Grant G41 (to W.M.), Wellcome Trust Grant 088719 and a VIP award (to W.M.), Wellcome Trust Grant 077189 (to K.P.S.), and Deafness Research UK (to W.M.). D.N.F. is supported by the Henry Smith Charity. V.Z. was supported by the Physiological Society (International Junior Research Grant) and the University of Sheffield (devolved funds). W.M. is a Royal Society University Research Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016646108/-/DCSupplemental.
References
- 1.Glowatzki E, Grant L, Fuchs PA. Hair cell afferent synapses. Curr Opin Neurobiol. 2008;18:389–395. doi: 10.1016/j.conb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews G, Fuchs PA. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liberman MC, et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 4.Guinan JJ., Jr. Physiology of olivocochlear efferents. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer; 1996. pp. 435–502. [Google Scholar]
- 5.Dror AA, Avraham KB. Hearing loss: Mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. doi: 10.1146/annurev-genet-102108-134135. [DOI] [PubMed] [Google Scholar]
- 6.Rüsch A, et al. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21:9792–9800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcotti W, Erven A, Johnson SL, Steel KP, Kros CJ. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J Physiol. 2006;574:677–698. doi: 10.1113/jphysiol.2005.095661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidrych P, et al. Otoferlin interacts with myosin VI: Implications for maintenance of the basolateral synaptic structure of the inner hair cell. Hum Mol Genet. 2009;18:2779–2790. doi: 10.1093/hmg/ddp213. [DOI] [PubMed] [Google Scholar]
- 10.Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells—Beyond the transducer. J Membr Biol. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- 11.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 13.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 14.Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 15.Mencía A, et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41:609–613. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- 16.Friedman LM, et al. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci USA. 2009;106:7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282:25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 18.Pierce ML, et al. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev. 2008;10:106–113. doi: 10.1111/j.1525-142X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacheli R, et al. Expression patterns of miR-96, miR-182 and miR-183 in the development inner ear. Gene Expr Patterns. 2009;9:364–370. doi: 10.1016/j.gep.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Wienholds E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Kloosterman W, Fekete DM. MicroRNA-183 family members regulate sensorineural fates in the inner ear. J Neurosci. 2010;30:3254–3263. doi: 10.1523/JNEUROSCI.4948-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis MA, et al. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009;41:614–618. doi: 10.1038/ng.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitosis. Acta Otolaryngol Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- 25.Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548:383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol. 1999;520:653–660. doi: 10.1111/j.1469-7793.1999.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcotti W, Géléoc GSG, Lennan GWT, Kros CJ. Developmental expression of an inwardly rectifying potassium conductance in inner and outer hair cells along the mouse cochlea. Pflugers Arch. 1999;439:113–122. doi: 10.1007/s004249900157. [DOI] [PubMed] [Google Scholar]
- 28.Marcotti W, Johnson SL, Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol. 2004;560:691–708. doi: 10.1113/jphysiol.2004.072868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson SL, Franz C, Knipper M, Marcotti W. Functional maturation of the exocytotic machinery at gerbil hair cell ribbon synapses. J Physiol. 2009;587:1715–1726. doi: 10.1113/jphysiol.2009.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson SL, et al. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nat Neurosci. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci. 1982;2:942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pujol R, Lavigne-Rebillard M, Lenoir M. Development of sensory and neural structures in the mammalian cochlea. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer; 1998. pp. 146–192. [Google Scholar]
- 34.Cotanche DA, Kaiser CL. Hair cell fate decisions in cochlear development and regeneration. Hear Res. 2010;266:18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- 36.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SL, Adelman JP, Marcotti W. Disruption of spontaneous action potential activity in inner hair cells of SK2 knockout mice prevents the normal development of exocytotic machinery. J Physiol. 2007;583:631–646. doi: 10.1113/jphysiol.2007.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 39.Beurg M, et al. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci. 2008;28:1798–1803. doi: 10.1523/JNEUROSCI.4653-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10:837–841. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selbach M, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Pietrzykowski AZ, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodyear RJ, et al. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci. 2003;23:9208–9219. doi: 10.1523/JNEUROSCI.23-27-09208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallis D, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- 47.Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 48.Choi PS, et al. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca2+ on Ca2+-activated K+ currents in mature mouse inner hair cells. J Physiol. 2004;557:613–633. doi: 10.1113/jphysiol.2003.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kubisch C, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.