Abstract
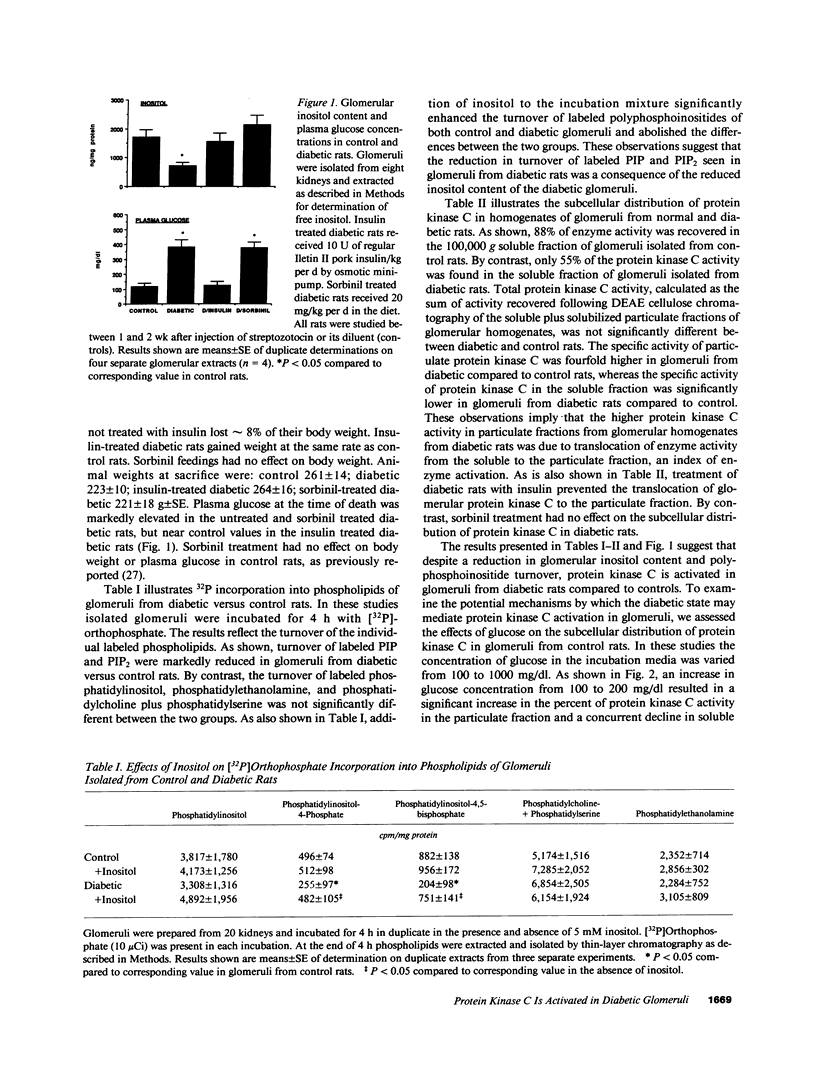
Glomerular inositol content and the turnover of polyphosphoinositides was reduced by 58% in 1-2 wk streptozotocin diabetic rats. Addition of inositol to the incubation medium increased polyphosphoinositide turnover in glomeruli from diabetic rats to control values. Despite the reduction in inositol content and polyphosphoinositide turnover, protein kinase C was activated in glomeruli from diabetic rats, as assessed by an increase in the percentage of enzyme activity associated with the particulate cell fraction. Total protein kinase C activity was not different between glomeruli from control and diabetic rats. Treatment of diabetic rats with insulin to achieve near euglycemia prevented the increase in particulate protein kinase C. Moreover, incubation of glomeruli from control rats with glucose (100-1,000 mg/dl) resulted in a progressive increase in labeled diacylglycerol production and in the percentage of protein kinase C activity which was associated with the particulate fraction. These results support a role for hyperglycemia per se in the enhanced state of activation of protein kinase C seen in glomeruli from diabetic rats. Glucose did not appear to increase diacylglycerol by stimulating inositol phospholipid hydrolysis in glomeruli. Other pathways for diacylglycerol production, including de novo synthesis and phospholipase C mediated hydrolysis of phosphatidylcholine or phosphatidyl-inositol-glycan are not excluded.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Berridge M. J. Growth factors, oncogenes and inositol lipids. Cancer Surv. 1986;5(2):413–430. [PubMed] [Google Scholar]
- Berti-Mattera L. N., Peterson R. G., Eichberg J. Insulin reverses enhanced incorporation of 32P into polyphosphoinositides in peripheral nerve of the streptozotocin diabetic rat. J Neurochem. 1986 Dec;47(6):1932–1935. doi: 10.1111/j.1471-4159.1986.tb13110.x. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer-Mears A., Ku L., Cohen M. P. Glomerular polyol accumulation in diabetes and its prevention by oral sorbinil. Diabetes. 1984 Jun;33(6):604–607. doi: 10.2337/diab.33.6.604. [DOI] [PubMed] [Google Scholar]
- Beyer-Mears A. The polyol pathway, sorbinil, and renal dysfunction. Metabolism. 1986 Apr;35(4 Suppl 1):46–54. doi: 10.1016/0026-0495(86)90187-3. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Hutson N. J. Introduction: evidence for the role of the polyol pathway in the pathophysiology of diabetic complications. Metabolism. 1986 Apr;35(4 Suppl 1):1–3. doi: 10.1016/0026-0495(86)90178-2. [DOI] [PubMed] [Google Scholar]
- Cohen M. P. Aldose reductase, glomerular metabolism, and diabetic nephropathy. Metabolism. 1986 Apr;35(4 Suppl 1):55–59. doi: 10.1016/0026-0495(86)90188-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Klepser H. Binding of an aldose reductase inhibitor to renal glomeruli. Biochem Biophys Res Commun. 1985 Jun 14;129(2):530–535. doi: 10.1016/0006-291x(85)90184-6. [DOI] [PubMed] [Google Scholar]
- Cohen P., Broekman M. J., Verkley A., Lisman J. W., Derksen A. Quantification of human platelet inositides and the influence of ionic environment on their incorporation of orthophosphate-32P. J Clin Invest. 1971 Apr;50(4):762–772. doi: 10.1172/JCI106547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., Briggs R., DeRubertis F. R. Calcium-dependent action of osmolality on adenosine 3',5'-monophosphate accumulation in rat renal inner medulla: evidence for a relationship to calcium-responsive arachidonate release and prostaglandin synthesis. J Clin Invest. 1980 Feb;65(2):529–542. doi: 10.1172/JCI109697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., Caines M. A., DeRubertis F. R. Sequential alterations in glomerular prostaglandin and thromboxane synthesis in diabetic rats: relationship to the hyperfiltration of early diabetes. Metabolism. 1987 Jan;36(1):95–103. doi: 10.1016/0026-0495(87)90070-9. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Patterson M. C., DeRubertis F. R. Role for protein kinase C in A23187 induced glomerular arachidonate release and PGE2 production. Biochem Biophys Res Commun. 1987 Dec 16;149(2):658–664. doi: 10.1016/0006-291x(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Patterson M. C., DeRubertis F. R. Role for protein kinase C in the modulation of glomerular PGE2 production by angiotensin II. Biochem Biophys Res Commun. 1988 May 16;152(3):1481–1489. doi: 10.1016/s0006-291x(88)80453-4. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Patterson M. C., DeRubertis F. R. Role of enhanced arachidonate availability through phospholipase A2 pathway in mediation of increased prostaglandin synthesis by glomeruli from diabetic rats. Diabetes. 1988 Apr;37(4):429–435. doi: 10.2337/diab.37.4.429. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation and reactive oxygen formation by bile acids. J Clin Invest. 1987 Feb;79(2):532–541. doi: 10.1172/JCI112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethy J. M., Callaert-Deveen B., Janssens M., Lenaers A. Determination of sorbitol and galactitol at the nanogram level in biological samples by high-performance liquid chromatography. Anal Biochem. 1984 Nov 15;143(1):119–124. doi: 10.1016/0003-2697(84)90565-7. [DOI] [PubMed] [Google Scholar]
- Donnelly T. E., Jr, Birt D. F., Sittler R., Anderson C. L., Choe M., Julius A. Dietary fat regulation of the association of protein kinase C activity with epidermal cell membranes. Carcinogenesis. 1987 Dec;8(12):1867–1870. doi: 10.1093/carcin/8.12.1867. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Activity of endogenous phospholipase C and phospholipase A2 in glucose stimulated pancreatic islets. Biochem Biophys Res Commun. 1984 May 16;120(3):820–827. doi: 10.1016/s0006-291x(84)80180-1. [DOI] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Glucose-induced phospholipid-dependent protein phosphorylation in neonatal rat islets. Arch Biochem Biophys. 1986 Aug 1;248(2):562–569. doi: 10.1016/0003-9861(86)90509-6. [DOI] [PubMed] [Google Scholar]
- Esmatjes E., Fernandez M. R., Halperin I., Camps J., Gaya J., Arroyo V., Rivera F., Figuerola D. Renal hemodynamic abnormalities in patients with short term insulin-dependent diabetes mellitus: role of renal prostaglandins. J Clin Endocrinol Metab. 1985 Jun;60(6):1231–1236. doi: 10.1210/jcem-60-6-1231. [DOI] [PubMed] [Google Scholar]
- Farese R. V., DiMarco P. E., Barnes D. E., Sabir M. A., Larson R. E., Davis J. S., Morrison A. D. Rapid glucose-dependent increases in phosphatidic acid and phosphoinositides in rat pancreatic islets. Endocrinology. 1986 Apr;118(4):1498–1503. doi: 10.1210/endo-118-4-1498. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Farese R. V., Jr, Sabir M. A., Larson R. E., Trudeau W. L., 3rd, Barnes D. The mechanism of action of insulin on phospholipid metabolism in rat adipose tissue. Requirement for protein synthesis and a carbohydrate source, and relationship to activation of pyruvate dehydrogenase. Diabetes. 1984 Jul;33(7):648–655. doi: 10.2337/diab.33.7.648. [DOI] [PubMed] [Google Scholar]
- Fox J. A., Soliz N. M., Saltiel A. R. Purification of a phosphatidylinositol-glycan-specific phospholipase C from liver plasma membranes: a possible target of insulin action. Proc Natl Acad Sci U S A. 1987 May;84(9):2663–2667. doi: 10.1073/pnas.84.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi S., Merola L. O., Kinoshita J. H. Altering the course of cataracts in diabetic rats. Invest Ophthalmol Vis Sci. 1980 Mar;19(3):313–315. [PubMed] [Google Scholar]
- Gabbay K. H. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973 Apr 19;288(16):831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N., Tomlinson D. R. Myo-inositol and sorbitol metabolism in relation to peripheral nerve function in experimental diabetes in the rat: the effect of aldose reductase inhibition. Diabetologia. 1983 Oct;25(4):365–371. doi: 10.1007/BF00253203. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Transport of myo-inositol into endoneurial preparations of sciatic nerve from normal and streptozotocin-diabetic rats. Biochem J. 1983 Mar 15;210(3):775–781. doi: 10.1042/bj2100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb S., Simmons D. A., Kern E. F. Amelioration of glomerular hyperfiltration in acute experimental diabetes mellitus by dietary myo-inositol supplementation and aldose reductase inhibition. Trans Assoc Am Physicians. 1986;99:67–72. [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve. Implications for (Na,K)-ATPase regulation and diabetic complications. Diabetes. 1986 Feb;35(2):242–245. doi: 10.2337/diab.35.2.242. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Hise M. K., Mehta P. S. Characterization and localization of calcium/phospholipid-dependent protein kinase-C during diabetic renal growth. Endocrinology. 1988 Sep;123(3):1553–1558. doi: 10.1210/endo-123-3-1553. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Thomas T. P., Chik C. L., Anderson W. B., Klein D. C. Protein kinase C: subcellular redistribution by increased Ca2+ influx. Evidence that Ca2+-dependent subcellular redistribution of protein kinase C is involved in potentiation of beta-adrenergic stimulation of pineal cAMP and cGMP by K+ and A23187. J Biol Chem. 1988 Jul 5;263(19):9292–9297. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol-4,5-bisphosphate phosphodiesterase and phosphomonoesterase activities of rat brain. Some properties and possible control mechanisms. Biochem J. 1984 Feb 15;218(1):177–185. doi: 10.1042/bj2180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. K., Steven K., Blaehr H., Christiansen J. S., Parving H. H. Effects of indomethacin on glomerular hemodynamics in experimental diabetes. Kidney Int. 1986 Feb;29(2):490–495. doi: 10.1038/ki.1986.26. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum M. A., Chaudhari A. Effect of experimental diabetes on glomerular filtration rate and glomerular prostanoid production in the rat. Miner Electrolyte Metab. 1986;12(5-6):352–355. [PubMed] [Google Scholar]
- Kolesnick R. N., Paley A. E. 1,2-Diacylglycerols and phorbol esters stimulate phosphatidylcholine metabolism in GH3 pituitary cells. Evidence for separate mechanisms of action. J Biol Chem. 1987 Jul 5;262(19):9204–9210. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Patel P. Y. The effects of insulin, glucose and diabetes on prostaglandin production by rat kidney glomeruli and cultured glomerular mesangial cells. Prostaglandins Leukot Med. 1983 Aug;11(4):431–442. doi: 10.1016/0262-1746(83)90097-5. [DOI] [PubMed] [Google Scholar]
- Kreutter D., Caldwell A. B., Morin M. J. Dissociation of protein kinase C activation from phorbol ester-induced maturation of HL-60 leukemia cells. J Biol Chem. 1985 May 25;260(10):5979–5984. [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moel D. I., Safirstein R. L., McEvoy R. C., Hsueh W. Effect of aspirin on experimental diabetic nephropathy. J Lab Clin Med. 1987 Sep;110(3):300–307. [PubMed] [Google Scholar]
- Mogensen C. E., Andersen M. J. Increased kidney size and glomerular filtration rate in untreated juvenile diabetes: normalization by insulin-treatment. Diabetologia. 1975 Jun;11(3):221–224. doi: 10.1007/BF00422325. [DOI] [PubMed] [Google Scholar]
- Nagao S., Seishima M., Mori S., Nozawa Y. Increased protein kinase C activity in fibroblast membranes from psoriatic patients. J Invest Dermatol. 1988 Mar;90(3):406–408. doi: 10.1111/1523-1747.ep12456509. [DOI] [PubMed] [Google Scholar]
- Patel D. G. Rate of insulin infusion with a minipump required to maintain a normoglycemia in diabetic rats. Proc Soc Exp Biol Med. 1983 Jan;172(1):74–78. doi: 10.3181/00379727-172-41529. [DOI] [PubMed] [Google Scholar]
- Peter-Riesch B., Fathi M., Schlegel W., Wollheim C. B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988 Apr;81(4):1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Bauer C. Pertussis toxin abolishes angiotensin II-induced phosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Biochem J. 1986 May 15;236(1):289–294. doi: 10.1042/bj2360289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAABO E., TERKILDSEN T. C. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12(4):402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- Ramsdell J. S., Pettit G. R., Tashjian A. H., Jr Three activators of protein kinase C, bryostatins, dioleins, and phorbol esters, show differing specificities of action on GH4 pituitary cells. J Biol Chem. 1986 Dec 25;261(36):17073–17080. [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Schambelan M., Blake S., Sraer J., Bens M., Nivez M. P., Wahbe F. Increased prostaglandin production by glomeruli isolated from rats with streptozotocin-induced diabetes mellitus. J Clin Invest. 1985 Feb;75(2):404–412. doi: 10.1172/JCI111714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrama L. H., Berti-Mattera L. N., Eichberg J. Altered protein phosphorylation in sciatic nerve from rats with streptozocin-induced diabetes. Diabetes. 1987 Nov;36(11):1254–1260. doi: 10.2337/diab.36.11.1254. [DOI] [PubMed] [Google Scholar]
- Seyer-Hansen K. Renal hypertrophy in experimental diabetes mellitus. Kidney Int. 1983 Apr;23(4):643–646. doi: 10.1038/ki.1983.71. [DOI] [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Florholmen J., Turk J., McDaniel M. L. Studies of the Ca2+ requirements for glucose- and carbachol-induced augmentation of inositol trisphosphate and inositol tetrakisphosphate accumulation in digitonin-permeabilized islets. Evidence for a glucose recognition site in insulin secretion. J Biol Chem. 1988 Mar 15;263(8):3565–3575. [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Huang F. L., Nakabayashi H., Huang K. P. Tissue distribution and developmental expression of protein kinase C isozymes. J Biol Chem. 1988 Jul 15;263(20):9868–9873. [PubMed] [Google Scholar]



