Abstract
The ability to make choices and carry out appropriate actions is critical for individual survival and well-being. Choice behaviors, from hard-wired to experience-dependent, have been observed across the animal kingdom. Although differential engagement of sensory neuronal pathways is a known mechanism, neurobiological substrates in the brain that underlie choice making downstream of sensory perception are not well understood. Here, we report a behavioral paradigm in zebrafish in which a half-light/half-dark visual image evokes an innate choice behavior, light avoidance. Neuronal activity mapping using the immediate early gene c-fos reveals the engagement of distinct brain regions, including the medial zone of the dorsal telencephalic region (Dm) and the dorsal nucleus of the ventral telencephalic area (Vd), the teleost anatomical homologs of the mammalian amygdala and striatum, respectively. In animals that were subjected to the identical sensory stimulus but displayed little or no avoidance, strikingly, the Dm and Vd were not engaged, despite similar levels of activation in the brain nuclei involved in visual processing. Based on these findings and previous connectivity data, we propose a neural circuitry model in which the Dm serves as a brain center, the activity of which predicates this choice behavior in zebrafish.
Keywords: emotion, fear, anxiety, decision-making
Choosing among alternative behaviors, whether they be regulated by external stimuli, internal drives, or cognitive processes, is a fundamental ability that all living animals possess (1–7). How brains make choices has been studied in disciplines ranging from psychology/cognition to neuroethology/behavior (8–10) using, for example, awake behaving monkeys that perform specific tasks or invertebrate animals that choose between swimming or crawling. Despite the significant advancements that these studies have brought to our understanding of behavioral choices or decision making, each system has limitations. The complex brains of primates make it difficult to delineate neural circuitry underlying behavior, whereas the nervous systems of invertebrates are significantly different from those of humans, making it difficult to extrapolate mechanistic findings from these systems directly to mammals. Therefore, animals with intermediate complexity and similarity may provide an interface that could bridge the currently available model systems and help us to understand the molecular and cellular mechanisms underlying behavioral choices and decision making.
Utilization of distinct sensory neuronal subtypes and “gating” out sensory input by presynaptic inhibition are mechanisms at the sensory end to elicit choice behaviors when animals face alternative sensory cues (1, 11, 12). When faced with identical sensory stimuli, the invertebrate medicinal leech employs “group discriminator” neurons to “decide” between crawling and swimming (7). In more complex animals, it is less clear what brain regions might dictate choice making. Here, we report a study of an innate light/dark (L/D) choice behavior in zebrafish, a vertebrate genetic model organism with a brain similar to that of mammals but with significantly less complexity (13, 14). Our findings show that the medial zone of the dorsal telencephalic region (Dm), the teleost anatomical homolog of the mammalian amygdala (14–17), and the dorsal nucleus of the ventral telencephalic area (Vd), the zebrafish anatomical homolog of the mammalian striatum (18), are differentially activated between animals that display light avoidance and those that do not, whereas the brain nuclei involved in visual processing are similarly activated among these animals. Because Vd is likely downstream of Dm (15), we suggest that Dm serves as an internal center, the activity of which discriminates the outcome of this choice behavior.
Results and Discussion
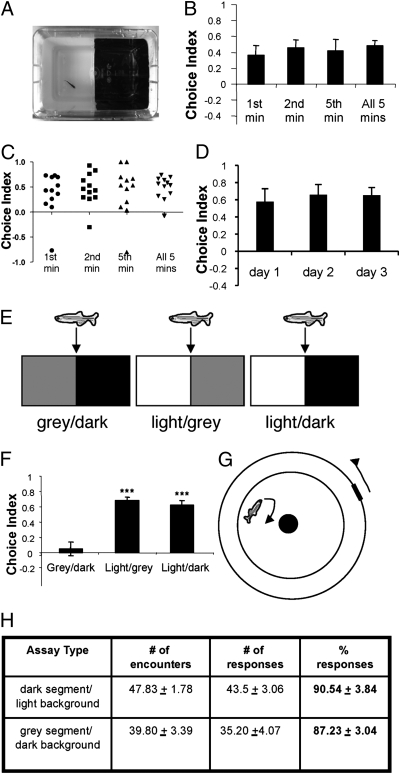
Phototaxis, which is a rapid and short-lived (usually reaching completion within a minute) swim toward or away from a light source, has been reported in larval zebrafish (1, 19, 20). L/D choice behavior is similarly light-driven yet distinct in duration (observable within minutes to tens of minutes), and it has been reported in adult zebrafish (13, 21–23). After being individually introduced into a L/D choice chamber (light intensity readings of 300 and 160 Lux, respectively, on each side) (Fig. 1A), zebrafish explore randomly but exhibit a bias by turning away at the border to avoid the more illuminated space (Movie S1). This was measured by a choice index [(% time in dark − % time in light)/100] (Fig. 1B). When the data were presented as a scatter plot (Fig. 1C), a distribution of the choice index was observed, with most animals displaying a positive value, meaning less time spent on the light side. When zebrafish were tested repeatedly in the same chamber (three trials with a 24-h interval between each trial), we did not observe significant habituation or sensitization of the choice behavior (Fig. 1D, n = 13; P = 0.64), which was consistent with what has been reported recently (24).
Fig. 1.
Adult zebrafish display an innate light avoidance behavior. (A) Photograph of the L/D choice chamber. (B) Choice indices in the choice chamber during first, second, fifth, and all 5 min analyzed (a choice index of 1.0 represents 100% time spent on the dark side, and an index of −1.0 represents 100% time spent on the light side) (n = 12 for each group). (C) Scatter plot of B, showing the distribution of choice indices in the tested animals. (D) Choice indices of three trials with a 24-h interval between each trial. The means for choice indices are day 1 (0.58 ± 0.15), day 2 (0.66 ± 0.12), and day 3 (0.65 ± 0.09) (n = 13; P = 0.64, ANOVA followed by the Bonferroni post hoc test). (E) Schemes of different choice chambers. (F) Choice indices in different choice chambers (L/D, n = 26; light/gray, n = 23; gray/dark, n = 24; F = 28.55, ***P < 0.001 compared with gray/dark, Tukey's test). (G) Scheme of a visual acuity chamber for testing the ability of zebrafish to discriminate between gray and dark. (H) Comparable visual discrimination of gray segment over dark background (n = 6) vs. dark segment over light background (n = 5), as indicated by comparable % responses (F = 0.36, P = 0.56, Tukey's test).
This choice behavior was observed in several different genetic backgrounds in both male and female zebrafish and was unaffected by whether the animal was initially introduced into the light side or the dark side or whether it had been raised in dim or bright lighting conditions (Fig. S1 A–D). Moreover, either individual- or group-raised animals exhibited similar light avoidance behavior (Fig. S1E). Interestingly, using an L/D choice chamber designed proportionally to the size of larval zebrafish under the same experimental conditions, we found that 1- and 2-wk-old larvae preferred the light environment instead of avoiding it (Fig. S1F). These results indicate that a choice reversal occurs during the development and maturation of zebrafish, with adult zebrafish generally exhibiting a light avoidance behavior. The neural basis of this choice reversal is currently not known and will be the subject of future investigations.
To characterize the sensitivity of the adult choice behavior to lighting conditions further, we introduced adult zebrafish into gray/dark or light/gray chambers (Fig. 1E, the light intensity readings of the light, gray, and dark areas are 300, 200, and 160 Lux, respectively). In the light/gray chamber, zebrafish spent significantly more time on the gray side, similar to their preference in the L/D chamber (Fig. 1F, center bar compared with right bar). In contrast, in the gray/dark chamber, they displayed no preference and spent about equal time on either side (Fig. 1F, left bar). Such lack of preference in the gray/dark chamber is not attributable to a lack of visual discrimination, because zebrafish could visually discriminate gray from dark in a visual acuity test (Fig. 1 G and H). We next asked whether increased light intensity would enhance the avoidance behavior. Increasing the ambient light from 300 to 1,000 Lux did not further enhance the avoidance behavior (Fig. S1G). Taken together, these results suggest that adult zebrafish avoid a lit environment when the light intensity is above a certain threshold (at least above 200 Lux).
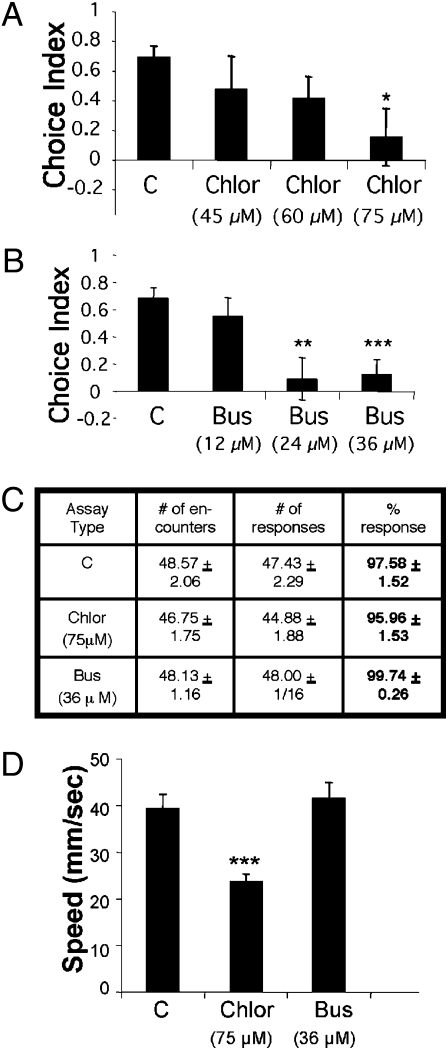
In rodents, avoidance of a brightly lit environment is sensitive to anxiolytic compounds (25). Similarly, the light avoidance behavior in zebrafish was attenuated by two common anxiolytics (Fig. 2 A and B), chlordiazepoxide and buspirone, which enhance GABAergic (26) and monoaminergic (27) signaling, respectively, in mammals and have effects in zebrafish at both behavioral and tissue-binding levels (28, 29) (Fig. S2, chlordiazepoxide had a Ki of 143 nM; see SI Materials and Methods). Because the drugs were administered directly in the tank water and needed to cross multiple barriers (including the absorption and blood–brain barriers) to reach the site of action, a higher concentration than that used during the in vitro binding experiment was necessary to achieve an in vivo effect. Using the visual acuity assay, we found that neither compound affected visual capability at the highest drug concentrations used in our experiments (Fig. 2C). Although buspirone had no effect on locomotor activity, the highest concentration of chlordiazepoxide (75 μM) did reduce locomotor speed (Fig. 2D). Such effects could not account for the reduced avoidance behavior, however, because zebrafish swimming at such a reduced speed could still easily reach either side of the choice chamber. Thus, the light avoidance behavior in adult zebrafish employs GABAergic and monoaminergic substrates, which are likely required in specialized functional brain areas rather than in sensory or motor neurons.
Fig. 2.
The light avoidance behavior is attenuated by commonly used anxiolytics. (A) Chlordiazepoxide reduces the light avoidance behavior in a dose-dependent manner. C (control), n = 11; Chlor: 45 μM, n = 11; 60 μM n = 11; 75 μM n = 12; F = 1.65, *P < 0.05 compared with control, t test. (B) Buspirone reduces the light avoidance behavior in a dose-dependent manner. C, n = 11; Bus: 12 μM, n = 11; 24 μM = 12; 36 μM = 12; F = 5.75, **P < 0.01 compared with control, ***P < 0.001 compared with control, t test. (C) Effect of chlordiazepoxide and buspirone on visual acuity at the highest concentration tested. C, n = 7; Chlor: 75 μM, n = 8, P = 0.47 compared with control; Bus: 36 μM = 8; P = 0.16 compared with control, t test. (D) Effect of chlordiazepoxide and buspirone on swim speed at the highest concentration tested (n = 8 for each group; F = 12.56, ***P < 0.001 compared with control, t test). Mean ± SEM are shown.
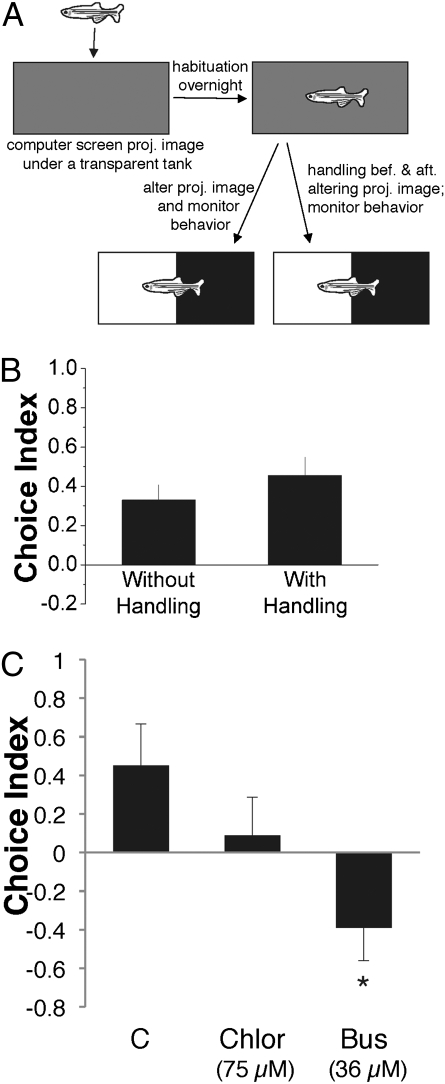
The behavioral paradigm presented in Fig. 1 involved netting zebrafish into a different chamber, a process expected to engage multiple sensory modalities, including visual and mechanosensory as well as stress-modulating pathways, such as the catecholaminergic pathway. These complex polymodal stimuli are difficult to standardize, and therefore are likely to elicit variable patterns of neuronal activity. To obtain consistent neural activity data, we tested whether a unimodal sensory stimulus, (i.e., the L/D visual image) is able to evoke the avoidance behavior. Individual zebrafish were allowed to habituate overnight to a transparent testing chamber atop a computer monitor projecting a brown-colored image that matched the behavioral testing environment. The next day, a computer-generated L/D visual image was remotely projected underneath the tank (Fig. 3A). Animals exposed to such a visual image showed light avoidance behavior (Fig. 3B and Movie S2), which was slightly less intense but, nevertheless, comparable to that of animals that were subjected to both the visual image and handling (netting) (P = 0.13). These data suggest that the L/D visual stimulus is sufficient to evoke the avoidance behavior. We next determined whether chlordiazepoxide and buspirone affected this choice behavior evoked solely by the L/D visual stimulus. Whereas chlordiazepoxide did not significantly reduce light avoidance in this setting, strikingly, buspirone-treated animals reversed their choice by showing a preference for the light side (Fig. 3C). Thus, monoaminergic neural substrates are likely to play a central role in regulating the L/D choice.
Fig. 3.
The L/D visual sensory stimulus is sufficient to trigger the avoidance behavior that is reversible by buspirone. (A) Scheme of the experimental design that allows the sensory stimulus of a single modality (visual) to be presented and compared with the effect of visual stimulus plus handling. (B) L/D visual stimulus alone is capable of evoking an avoidance behavior similar to that evoked by L/D + H. Mean ± SEM values are shown (n = 19 for each group; P = 0.13, t test). (C) Effect of chlordiazepoxide and buspirone on the choice behavior evoked solely by the L/D visual sensory stimulus. Mean ± SEM values are shown (n = 8; *P < 0.05, comparing buspirone-treated with control, ANOVA followed by Dunnett's post hoc test).
With a unimodal visual sensory stimulus-evoked behavior, we sought to determine the underlying neural correlates through analysis of behaviorally driven expression of c-fos, one of the best-characterized molecular markers for neural activity (30–32). Because c-fos–assisted neuronal activity mapping has not been previously carried out in adult zebrafish, we first assessed the basal expression level of c-fos in animals that were habituated to their environment (either their natural housing system or the behavioral testing room) for at least 24 h. Little c-fos expression was detected in the brain, suggesting low basal expression (Fig. S3A). We next determined the inducibility of c-fos in animals subjected to vigorous handling stress (continuous shaking for 30 min in the confinement of a net). Robust induction of c-fos was observed in many brain regions, including the hypothalamus (Hy; Fig. S3B), consistent with its known role in teleost stress responses (33). Therefore, c-fos in situ hybridization is a suitable method for evaluating stimulus-induced neuronal activity in most, if not all, neurons of the adult zebrafish brain.
During the L/D choice behavioral testing, we noted that a small percentage of animals (∼10%), which were siblings of the rest of the animals tested, displayed little avoidance yet had normal visual sensory capacity as assessed by the visual acuity test (Fig. S4A and Movie S2). These nonresponders also did not show significant avoidance behavior when exposed to more intense light (Fig. S4B) or given more time to respond (Fig. S4C). The presence of light-avoidant vs. non–light-avoidant zebrafish thus presented an opportunity to compare the underlying neuronal activity and identify potential neural correlates for the preference behavior. Zebrafish were subjected to the L/D visual stimulus, and their choice behavior was quantified by the choice index. Analyses of c-fos expression were carried out in animals that exhibited high avoidance (with choice indices of 0.2 and higher) as well as in animals that displayed little avoidance (with choice indices of 0.02 and lower).
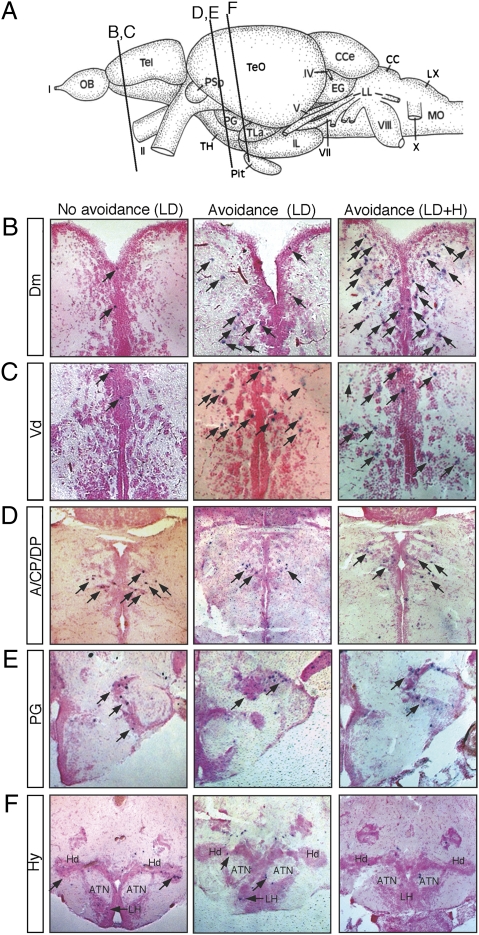
The results of c-fos expression are shown in Fig. 4, and the anatomical regions shown in Fig. 4 are schematized in Fig. S5. In animals that exhibited high avoidance of the light area, c-fos expression was detected in the telencephalic Dm and Vd (Fig. 4 B and C, Center), the zebrafish anatomical homologs of the mammalian amygdala and striatum, respectively (14, 18). In the diencephalon, c-fos expression was detected in the dorsal thalamus [anterior nucleus (A), dorsal posterior thalamic nucleus (DP), and central posterior thalamic nucleus (CP)] (Fig. 4D, Center), the preglomerular nucleus (PG) (Fig. 4E, Center), and the Hy (Fig. 4F, Center). The A, CP, DP, and PG are visually activated nuclei in the teleost brain (34). Although our analyses were focused on the forebrain, c-fos expression was also detected in the optic tectum (TeO) periventricular gray zone (PGZ) and the cerebellum of the middle hind-brain region (Fig. S6). In animals that exhibited low or no avoidance of the light area, although comparable c-fos expression was detected in the visually related brain nuclei and Hy (Fig. 4 D–F, Left compared with Center), little c-fos was detected in the Dm and Vd (Fig. 4 B and C, Left compared with Center).
Fig. 4.
Mapping of c-fos neuronal activity. In all images, c-fos–positive cells are shown in purple (arrows) and brain sections are counterstained with the nuclear fast red. (Left) Brain sections from an animal that was stimulated with the L/D visual stimulus and displayed little avoidance behavior (choice index: −0.04). (Center) Brain sections from an animal that was stimulated with the L/D visual stimulus and displayed avoidance behavior (choice index: 0.40). (Right) Brain sections from animals that were stimulated with the L/D + H and displayed avoidance behavior (choice index: 0.92). (A) Schematic showing the section positions. (B) Dm. (C) Vd. (D) Dorsal thalamus (A, CP, and DP). (E) Preglomerular complex (PG). (F) Hy [anterior tuberal nucleus (ATN), dorsal zone of the periventricular hypothalamus (Hd), and lateral hypothalamic nucleus (LH)].
We also analyzed neuronal activity in animals that were exposed to the L/D visual stimulus and handling (L/D + H) (Fig. 4, Right). In these animals, in addition to the regions mentioned above, c-fos expression was detected in other regions of the telencephalon, such as the supracommissural nucleus of ventral telencephalic region (Vs); postcommissural nucleus of ventral telencephalic region (Vp); lateral zone of the dorsal telencephalic region (Dl); posterior zone of the dorsal telencephalic region (Dp); and central zone of the dorsal telencephalic region (Dc); and diencephalon, including the dorsal habenula nucleus (Had), ventrolateral thalamic nucleus (VL), and periventricular nucleus of posterior tuberculum (TPp) (Fig. S7), highlighting the importance of having a well-defined behavioral paradigm devoid of extraneous stimuli for neuronal activity mapping. We also noted that c-fos expression in the Dm and Vd appeared broader in these animals than in the animals exposed to only L/D (Fig. 4 B and C, Right compared with Left and Center). Such broader engagement of brain structures in animals exposed to L/D + H is consistent with the pharmacological data (Figs. 2A and 3C) showing that chlordiazepoxide attenuated the choice behavior only in the animals that were exposed to L/D + H.
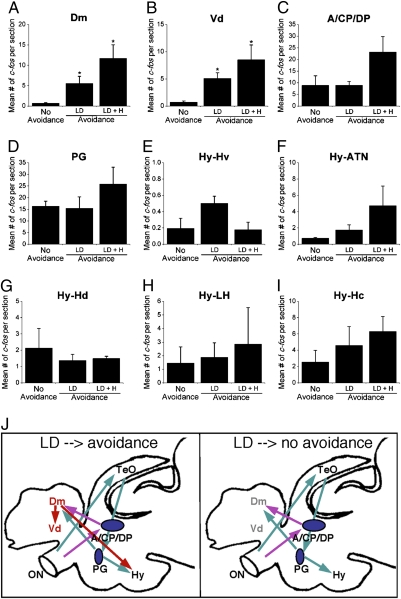
The qualitative observation of preferential activation of the Dm and Vd in the animals that displayed an avoidance behavior is intriguing. We therefore quantified the number of c-fos–expressing cells in various regions. These quantitative analyses showed that animals exhibiting the avoidance behavior displayed significantly more c-fos–expressing cells in the Dm and Vd regions than those exhibiting no avoidance (Fig. 5 A and B), whereas no significant correlation was found in other regions of the brain analyzed, including the A, CP, DP, and PG, which are visually activated nuclei in the brain, as well as in various subregions of the Hy (Fig. 5 C–I). These results suggest that there is a circuitry within the brain that determines whether the avoidance behavior will be performed or not, regardless of sensory activation. The differential activity of Dm and Vd in animals with or without avoidance behavior suggests that these regions are likely to be part of such circuitry.
Fig. 5.
Quantification of c-fos expression reveals differential activation of the Dm and Vd in high light-avoidant vs. low light-avoidant animals. Mean number of c-fos cells in the DM (A); Vd (B); A, CP, and DP (C); PG (D); ventral hypothalamus (E, Hy-Hv); anterior tuberal nucleus of hypothalamus (F, Hy-ATN); dorsal zone of periventricular hypothalamus (G, Hy-Hd); lateral hypothalamic nucleus (H, Hy-LH); and caudal hypothalamus (I, Hy-Hc). Mean ± SEM are shown (n = 3 for each group; *P < 0.05 compared with no avoidance, t test). (J) Scheme of a core neural circuitry underlying the L/D choice behavior. Details are presented in the text.
Our neural activity mapping has revealed the activation of discrete brain structures. This, together with previous tracing studies of the visual pathway and Dm connectivity (15, 34), allows us to propose a circuitry model underlying the L/D choice behavior (Fig. 5J). The L/D visual stimulus is transmitted through the retina and optic nerve to activate neurons in the PGZ of the TeO. Tectal neurons, in turn, project to and activate cells in the PG. Subsequently, the PG projects to and activates neurons in the Dm. In addition to this pathway, a second visual pathway exists involving direct sensory activation of neurons in the thalamus (A, CP, and DP), because the dendrites of such neurons have previously been shown to reach into the retinal terminal fields (35). Thalamic neurons also send projections to and activate the Dm. Analyses of efferent connections from the Dm reveal their projections to the dorsal subdivisions of the Vd, which controls motor output, and to the Hy, which regulates physiological output (15).
Neurobiological mechanisms underlying choice behaviors are a fascinating subject for investigation across multiple disciplines, including neuroscience, psychology, and ethology (8–10). Both our daily life observations (e.g., fight or flight reactions in the presence of a threatening stimulus) and scientific studies (e.g., swimming or crawling in the medicinal leech facing identical sensory stimuli) (7) point to the existence of a brain circuitry that can dictate behavioral choices in the face of identical sensory input potentially to maximize survival and well-being. Here, we show that adult zebrafish display a simple L/D choice behavior to avoid the more illuminated space. It is conceivable that such choice is guided by two opposing instincts: One is to explore the entire space, and the other is to avoid a potentially “dangerous” environment. Using this paradigm, we have shown differential engagement of the Dm and Vd in the light-avoidant and non–light-avoidant animals. Given that the Vd is likely downstream of the Dm, it is plausible that the Dm plays a critical role as a “choice center” in this behavior. Interestingly, lesions of the Dm in the goldfish abrogate a conditioned avoidance behavior (36). Imaging studies in humans uncover a key role for the amygdala, the mammalian counterpart of the Dm, in decision making that comes from value-related predictions (37). Together, these findings suggest a potentially evolutionarily conserved role of the Dm/amygdala in mediating behavioral choices.
We have observed a choice reversal between larval and adult zebrafish, such that adults avoid light, whereas the juveniles prefer light. Furthermore, the light avoidance behavior in adult zebrafish can be reversed to light preference by treatment with buspirone, a common anxiolytic that targets monoaminergic (27) signaling. Therefore, it would be of great interest to determine in the future whether this developmentally regulated choice reversal is attributable to age-dependent plasticity in monoaminergic neurons.
Three possible scenarios might explain the failure to display an avoidance behavior in some animals that are exposed to the same L/D visual sensory stimulus. First, although the visually related brain regions (e.g., A, CP, DP, PG) appear to be well-activated in these animals, such activation fails to recruit Dm because of insufficient presynaptic input from these sensory-processing brain nuclei to the Dm. Second, these animals may have an intrinsic defect in Dm excitability despite normal input from the A, CP, DP, and PG. Finally, these animals may have insufficient neuromodulatory tone, such as the noradrenergic drive (38), to facilitate Dm recruitment. It is also of great interest to investigate further whether the L/D choice behavior exhibits plasticity in a given animal, a question that we were not able to address in this study because animals had to be killed after a single behavioral readout to perform c-fos labeling. Together, the simplicity of this unimodal visual sensory stimulus-evoked L/D choice behavior in a genetically amenable vertebrate affords a great opportunity to explore further how the Dm becomes differentially activated between light-avoidant and non–light-avoidant fish and how such activation may dictate the animal's choice to avoid the light.
Materials and Methods
Experimental Animals.
Adult zebrafish (AB, EK, and Wik) were maintained and bred following standard procedures (39).
Behavioral Assays.
Animals tested in a given experiment were siblings group-housed (8–12 per housing unit) in identical tanks and tested in identical experimental settings. Light avoidance assays were carried out in either a behavioral chamber (taped half dark and half light) or a transparent chamber with a computer-projected L/D visual image from the bottom. Locomotor activity was assessed by video recording and analyzed by a Dynamic Image Analysis System (Soltec) or by Ethovision (Noldus) and Excel (Microsoft). Visual acuity assay was carried out as previously described (40).
Pharmacological Study.
Buspirone hydrochloride and chlordiazepoxide hydrochloride were purchased from Sigma–Aldrich. Zebrafish were treated for 1 h, followed by behavioral analyses as described above.
Statistical Analysis.
The Student's t test or one-way ANOVA, followed by post hoc tests (whenever appropriate, either Bonferroni, Dunnett's, or Tukey's test), were used. Data are considered significantly different when P < 0.05.
Analysis of c-fos.
The c-fos gene sequence information (National Center for Biotechnology Information Gene ID code 394198) was used to prepare the in situ probe. For c-fos induction, 30 min after initial exposure to either L/D or L/D + H, zebrafish were killed and their brains were processed for in situ hybridization and counterstained with nuclear fast red. A time frame of 30 min was chosen, based on many previous studies showing the highest expression of c-fos mRNA 30 min after behavioral induction of neuronal activities [reviewed in (41) and references therein]. Images were taken with a Zeiss compound microscope. For the quantitative analysis of c-fos–positive cells, total numbers of c-fos–positive cells were counted in all sections containing the brain regions of interest. The interpretation of neuroanatomy follows the adult zebrafish brain atlas (42). Data were presented as the average number of c-fos–positive cells per 30-μm section.
Supplementary Material
Acknowledgments
We thank E. Hurlock, B. Lu, and T. Mueller for helpful comments on the manuscript and Michael Munchua for excellent fish husbandry. B.Y.B.L. thanks K. Krishnan for support. This work was supported by the Byers Award, Sandler Award, Packard Foundation, and National Institutes of Health Grant AA016021 (to S.G.) and by a Sigma Delta Epsilon Fellowship. Funding was supported by Grant T42CCT610417 (to G.G.G.) from the National Institute for Occupational and Environmental Health (NIOSH)/Centers for Disease Control and Prevention (CDC) to the Southwest Center for Occupational and Environmental Health (SWCOEH), a NIOSH Education and Research Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018275108/-/DCSupplemental.
References
- 1.Burgess HA, Schoch H, Granato M. Distinct retinal pathways drive spatial orientation behaviors in zebrafish navigation. Curr Biol. 2010;20:381–386. doi: 10.1016/j.cub.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 3.Tang S, Guo A. Choice behavior of Drosophila facing contradictory visual cues. Science. 2001;294:1543–1547. doi: 10.1126/science.1058237. [DOI] [PubMed] [Google Scholar]
- 4.Lau B, Bretaud S, Huang Y, Lin E, Guo S. Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav. 2006;5:497–505. doi: 10.1111/j.1601-183X.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 5.Bretaud S, et al. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 6.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggman KL, Abarbanel HD, Kristan WBJ., Jr Optical imaging of neuronal populations during decision-making. Science. 2005;307:896–901. doi: 10.1126/science.1103736. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: Neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- 9.Kristan WB. Neuronal decision-making circuits. Curr Biol. 2008;18:R928–R932. doi: 10.1016/j.cub.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 10.Grodzinski U, Clayton NS. Problems faced by food-caching corvids and the evolution of cognitive solutions. Philos Trans R Soc Lond B Biol Sci. 2010;365:977–987. doi: 10.1098/rstb.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 12.Gaudry Q, Kristan WBJ., Jr Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nat Neurosci. 2009;12:1450–1457. doi: 10.1038/nn.2400. [DOI] [PubMed] [Google Scholar]
- 13.Guo S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 14.Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- 15.Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- 16.Northcutt RG. Forebrain evolution in bony fishes. Brain Res Bull. 2008;75:191–205. doi: 10.1016/j.brainresbull.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 17.Braford MRJ., Jr Comparative aspects of forebrain organization in the ray-finned fishes: Touchstones or not? Brain Behav Evol. 1995;46:259–274. doi: 10.1159/000113278. [DOI] [PubMed] [Google Scholar]
- 18.Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res Bull. 2002;57:385–387. doi: 10.1016/s0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 19.Brockerhoff SE, et al. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orger MB, Baier H. Channeling of red and green cone inputs to the zebrafish optomotor response. Vis Neurosci. 2005;22:275–281. doi: 10.1017/S0952523805223039. [DOI] [PubMed] [Google Scholar]
- 21.Serra EL, Medalha CC, Mattioli R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res. 1999;32:1551–1553. doi: 10.1590/s0100-879x1999001200016. [DOI] [PubMed] [Google Scholar]
- 22.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 23.Maximino C, Marques de Brito T, Dias CA, Gouveia AJ., Jr Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protoc. 2010;5:209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- 24.Maximino C, et al. Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res. 2010;210:1–7. doi: 10.1016/j.bbr.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 26.Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- 27.Cervo L, Munoz C, Bertaglia A, Samanin R. Alnespirone and buspirone have anxiolytic-like effects in a conflict procedure in rats by stimulating 5-HT(1A) receptors. Behav Pharmacol. 2000;11:153–160. doi: 10.1097/00008877-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Renier C, et al. Genomic and functional conservation of sedative-hypnotic targets in the zebrafish. Pharmacogenet Genomics. 2007;17:237–253. doi: 10.1097/FPC.0b013e3280119d62. [DOI] [PubMed] [Google Scholar]
- 29.Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 32.Guzowski JF, et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Wendelaar Bonga SE. The stress response in fish. Physiol Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- 34.Wullimann MF. The central nervous system. In: Evans DH, editor. The Physiology of Fishes. New York: CRC Press LLC; 1997. pp. 245–281. [Google Scholar]
- 35.Schellart NAM. The visual pathways and central non-tectal processing. In: Douglas RH, Djamgoz MBA, editors. The Visual System of Fish. London: Chapman & Hall; 1990. [Google Scholar]
- 36.Portavella M, Torres B, Salas C. Avoidance response in goldfish: Emotional and temporal involvement of medial and lateral telencephalic pallium. J Neurosci. 2004;24:2335–2342. doi: 10.1523/JNEUROSCI.4930-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: Opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish, Brachydanio rerio. 3rd Ed. Eugene, OR: Univ of Oregon Press; 1995. [Google Scholar]
- 40.Li L, Dowling JE. A dominant form of inherited retinal degeneration caused by a non-photoreceptor cell-specific mutation. Proc Natl Acad Sci USA. 1997;94:11645–11650. doi: 10.1073/pnas.94.21.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovács KJ. Measurement of immediate-early gene activation—c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 42.Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain. Basel: Birkhauser; 1996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.