Abstract
The vertebrate planar cell polarity (PCP) pathway shares molecular components with the β-catenin–mediated canonical Wnt pathway but acts through membrane complexes containing Vang or Frizzled to orient neighboring cells coordinately. The molecular interactions underlying the action of Vang in PCP signaling and specification, however, are yet to be delineated. Here, we report the identification of Rack1 as an interacting protein of a vertebrate Vang protein, Vangl2. We demonstrate that Rack1 is required in zebrafish for PCP-regulated processes, including oriented cell division, cellular polarization, and convergent extension during gastrulation. We further show that the knockdown of Rack1 affects membrane localization of Vangl2 and that the Vangl2-interacting domain of Rack1 has a dominant-negative effect on Vangl2 localization and gastrulation. Moreover, Rack1 antagonizes canonical Wnt signaling. Together, our data suggest that Rack1 regulates the localization of an essential PCP protein and acts as a molecular switch to promote PCP signaling.
Planar cell polarization (PCP) refers to cellular processes that establish coordinated polarity of neighboring cells in the 2D plane of a cell sheet. One such process is convergent extension (CE), in which cellular polarization along one planar axis drives cells to converge along the same axis and results in concomitant extension of the tissue along a perpendicular axis (1). PCP is also required for many other cellular processes, such as oriented cell division (2–4) and the precise orientation of sensory hair cells in the vertebrate inner ear (5) and in the zebrafish lateral line (6).
The study of PCP in many tissues across organisms identified key players and common membrane-bound PCP complexes for PCP regulation. PCP signaling requires an evolutionarily conserved “core” group of proteins to establish a tissue-wide polarity. Two essential core PCP components are the membrane receptor Frizzled (Fz) and the membrane protein Van gogh (Vang) or Vang-like proteins (7). Fz receptors can activate a cascade of downstream events leading to the stabilization of β-catenin and transcriptional regulation of target genes for the canonical Wnt signaling transduction upon binding of Wnt morphogens (8). During PCP signaling, however, the membrane distribution of Fz receptors is polarized along the planar polarity axis of the tissue. Cytoplasmic proteins, such as dishevelled (dsh), are associated with Fz to direct polarized cytoskeleton changes (9–11). The recruitment of Dsh to the membrane by Fz also operates as a switch to PCP signaling from canonical Wnt signaling (8). Furthermore, several members that are linked to vertebrate Dishevelled (Dvl), including Diversin (12) and the primary cilia (13), can suppress canonical Wnt signaling while promoting PCP-regulated processes. The essential Vang protein also shows polarized membrane distribution along the planar polarity axis of the tissue (7). Data from Drosophila indicates that interaction between Vang protein and Fz, in conjunction with Flamingo, propagates the polarity signal across the tissue (7), and that Vang can act with downstream effectors intracellularly for morphological polarization (11, 14).
The Vang-like 2 (Vangl2) protein (15) is a vertebrate Vang protein and essential for all of the known PCP processes in vertebrates. A recent study found that it is selectively sorted into COPII vesicles by Sec24b for transport from the endoplasmic reticulum to the Golgi (16). Vangl2 has also been reported to interact with Rac1 to regulate adherens junctions during PCP signaling in vertebrates (17). The molecular networks that underlie its membrane targeting and its action in vertebrate PCP signaling, however, remain largely unknown. To further explore vertebrate PCP signaling concerning the central player Vangl2, we used the C-terminal cytoplasmic domain of Vangl2 as the bait to screen a cDNA library from embryonic day 15 mouse cochlear epithelia. We identified Rack1, receptor for activated C kinase 1 (18), as a Vangl2-interacting protein. We confirmed that Rack1 physically interacts with Vangl2. We further showed that, like Vangl2, Rack1 is required for multiple PCP processes in zebrafish, including CE during gastrulation, oriented cell division, and cellular polarization. Moreover, the interaction of Rack1 with Vangl2 is required for Vangl2 localization and PCP signaling. Knocking down Rack1 disrupts membrane localization of Vangl2, and the Vangl2-interacting domain of Rack1 prevents Vangl2 localization in a dominant-negative manner. Finally, we demonstrated that Rack1 inhibits canonical Wnt signaling both in vivo and in vitro. Together, our data identified Rack1 as an essential component for Vangl2 membrane targeting and revealed an additional molecular component required for PCP signaling while modulating canonical Wnt signaling.
Results
Rack1 Interacts with PCP Protein Vangl2.
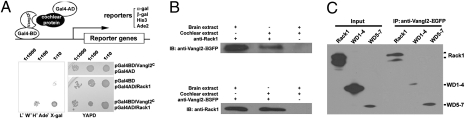
The murine Vangl2 protein contains putatively an N-terminal cytoplasmic region, four transmembrane domains, and a 283-aa C-terminal cytoplasmic tail (15). To identify candidate proteins that interact with Vangl2 for vertebrate PCP signaling, we fused the C-terminal cytoplasmic tail of Vangl2 in frame with the Gal4 DNA binding domain (Fig. 1A) and constructed a mouse embryonic cochlear cDNA library cloned into a Gal4 DNA activation domain expression vector (Fig. 1A). The plasmid pGal4-BD/Vangl2C alone with the empty Gal4-AD vector does not activate reporter expression (Fig. 1A). We cotransfected pGal4-BD/Vangl2C and the cDNA library DNA into yeast reporter cells and screened for colonies that grew on histidine- and adenine-deficient plates and turned blue under the conditions for α-galactosidase (α-gal) and β-gal assays (Fig. 1A). One of the verified positive clones encodes WD40 repeats 3–7 of mouse Rack1 (Fig. 1A) (18).
Fig. 1.
Rack1 interacts with Vangl2. (A) A diagram depicting the use of Vangl2c as the bait to screen a cochlear cDNA library to identify cytoplasmic Vangl2-interacting proteins. Yeast cells were serially diluted and replicated on rich media YAPD (yeast media containing yeast extract, peptone, dextrose plus adenine) plates and minimal select plates. (B) Vangl2EGFP and Rack1 can be coimmunoprecipitated with embryonic day 16.5 mouse embryo brain and cochlear extracts prepared from transgenic mice expressing Vangl2-GFP.(C) Rack1WD5–7 was efficiently pulled down by Vangl2, and there are two bands for HA-Rack1. The rabbit polyclonal antibody against Rack1 also recognizes two bands for the endogenous Rack1 (C), whereas the monoclonal antibody recognizes one band (B).
To confirm the interaction between Rack1 and Vangl2, we performed coimmunoprecipitation of Vangl2 and Rack1. Rack1 antibody can bring down Vangl2-GFP in mouse brain and cochlear extracts isolated from mice expressing Vangl2-GFP fusion protein (Fig. 1B) that shows asymmetric membrane enrichment identical to the endogenous Vangl2 in these mice (19). Conversely, the antibody against GFP can specifically pull down Rack1 from the extracts (Fig. 1B).
Rack1 is a highly conserved protein, whose zebrafish, mouse, and human orthologs share >95% identity. It contains seven tandem WD40 motifs. We further performed experiments to determine the domain in Rack1 that mediates its interaction with Vangl2 (Fig. 1C and Fig. S1). Rack1 WD40 repeats 1–2 (Rack1WD1–2), Rack1WD2–4, or Rack1WD5–7 was cloned into Gal4-AD and transfected alone or with Gal4-BD/Vangl2C into yeast cells. Rack1WD1–2 or Rack1WD2–4 is not sufficient to mediate a detectable interaction with Vangl2C in the yeast two-hybrid assay, whereas Rack1WD5–7 is toxic to yeast cells (Fig. S1). We also coexpressed Rack1WD1–4 or Rack1WD5–7 domains with Vangl2-GFP and performed coimmunoprecipitation. In comparison with the intact Rack1, Rack1WD1–4 protein was much less efficiently immunoprecipitated with Vangl2 (Fig. 1C). On the contrary, Rack1WD5–7 was efficiently coimmunoprecipitated with Vangl2 (Fig. 1C). It is noted that the transfection rate of HEK293 cells with Rack1WD5–7 was very low. The cultures were scaled up significantly for coimmunoprecipitation.
Rack1 Is Required for Gastrulation.
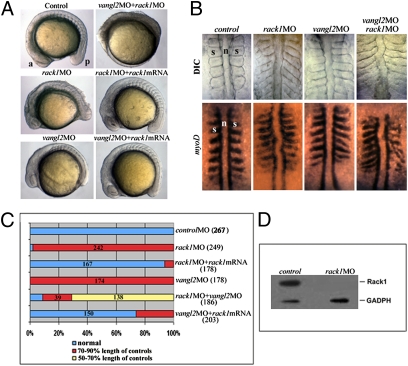
Vangl2 is essential for all known PCP processes. To test whether its interacting protein Rack1 also has a role in PCP signaling, we investigated the effect of knocking down Rack1 in zebrafish. Zebrafish rack1 mRNA is expressed ubiquitously from fertilization through midsomitogenesis (Fig. S2). We designed a translation-blocking morpholino oligonucleotide (MO) against rack1 (Fig. 2). Approximately 97% of the embryos injected with the rack1 MO exhibit a shortened anterior–posterior body axis, an undulating notochord accompanied by widened or irregular somites (Fig. 2 A–C). These phenotypes are commonly associated with gastrulation defects resulting from impaired PCP signaling, such as those caused by vangl2 MO (Fig. 2 A–C) (20). Furthermore, coinjection of suboptimal levels of rack1 and vangl2 MOs demonstrated a synergistic genetic interaction, where gastrulation defects observed in double morphants were more severe than those in embryos injected with either single morpholino (Fig. 2 A–C).
Fig. 2.
Rack1 is required for zebrafish gastrulation. (A–C) Both rack1 and vangl2 morphants exhibited a shortened anterior–posterior axis as well as widened somites as shown by lateral (A) and dorsal (B) views of live embryos (B Upper) and embryos hybridized with myoD1 in situ probes (B Lower). The images in A and B are representative of each group of ∼200 embryos (C). Note that cellular shedding at the dorsal side was observed in rack1 or rack1 and vangl2 morphants but not vangl2 morphants. (D) Although Rack1 protein is abundant in control embryos of six-somite stage, it is barely detectable in rack1 morphant embryos of the same stage. a, anterior; p, posterior; n, notochord; s, somite.
To determine the efficacy and specificity of the rack1 MO, we analyzed Rack1 protein levels and attempted to rescue the rack1 morphant phenotype by coinjecting rack1 mRNA. The level of Rack1 protein was decreased to barely detectable levels in rack1 morphant embryos (Fig. 2D), and coinjection of full-length mouse rack1 mRNA with rack1 MO rescued the gastrulation defects observed in rack1 morphants (Fig. 2 A and C). Interestingly, rack1 mRNA injection also rescued the gastrulation defects seen in vangl2 morphants (Fig. 2 A and C). In contrast, overexpression of vangl2 mRNA failed to rescue the rack1 morphant phenotype.
Rack1 Regulates Cellular Polarization, Oriented Cell Division, and CE During Zebrafish Gastrulation.
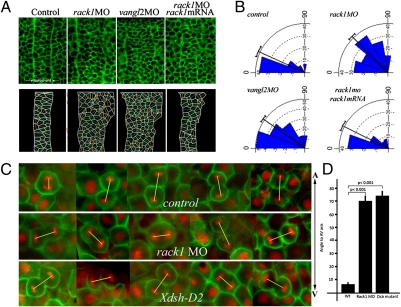
We further determined whether the gastrulation defects in Rack1 morphants are associated with defects in PCP-regulated processes. We analyzed cellular morphology in the notochord during gastrulation. The cells are polarized along the mediolateral axis of the embryo with their long axis oriented along the mediolateral axis, and this oriented cellular polarization is disrupted in PCP mutants (20). We calculated and plotted the geometric long axis of irregular polygons and quantified the angles formed between the mediolateral axis and the long axis of the cell. The long axes of notochord cells at the tail bud stage are oriented closely to the mediolateral axis in control morpholino-injected embryos (Fig. 3 A and B). In both rack1 and vangl2 morphants, the notochord was widened and had an undulating appearance, and most of cells were no longer oriented along the mediolateral axis (Fig. 3 A and B). Injection of rack1 mRNA almost completely rescued the morphological defects caused by rack1 MO (Fig. 3 A and B).
Fig. 3.
Cellular polarization and oriented cell division are disrupted in rack1 morphants. (A and B) Cellular morphology in the notochord at the bud stage was visualized with fluorescein-labeled BODIPY ceramide (A Upper) and diagramed (A Lower). The angles formed between the long axis of each cell and the mediolateral axis of each embryo were calculated and plotted with the Oriana 3 program (B). (C and D) Embryos injected with control or rack1 MOs, or RNAs encoding Xdsh-D2, Histone 2B-RFP, and membrane GFP were imaged at the shield stage from the dorsal side to visualize the orientation of mitotic divisions in dorsal epiblast cells (C). The angles formed between the direction of each mitotic spindle and the animal–vegetal axis of each embryo were quantified (D). The yellow lines depict the orientation of mitotic spindles. A-V, animal–vegetal axis.
The PCP pathway is also essential for oriented cell division, a driving force for axis elongation, in the dorsal epiblast during zebrafish gastrulation (2). We investigated whether deregulation of oriented cell division in rack1 morphants may also contribute to the gastrulation defects observed. Embryos at the one- or two-cell stage were injected with control or rack1 MOs or RNA encoding Xdsh-D2, a dominant-negative form of Dvl lacking the PDZ domain that strongly inhibits PCP but maintains its activity in canonical Wnt signaling (2, 9), along with RNAs encoding Histone 2B-RFP and membrane GFP. Cell divisions throughout the depth of the dorsal epiblast were recorded at the shield to 75% epiboly stage from the dorsal side, and the orientation of cell division was analyzed by measuring the angle formed between the projection of the axis of mitotic spindles to the plane of the epiblast and the animal–vegetal axis. As previously reported, the mitotic spindles were oriented mostly along the animal–vegetal axis in control morphants (Fig. 3 C and D). In rack1 morphants, the trend of oriented cell division in dorsal epiblast was lost (Fig. 3 C and D), similar to what was observed in the embryos that express the dominant-negative form of Dvl (Fig. 3 C and D) as reported previously (2). The alteration in the orientation of cell division in the dorsal epiblasts in rack1 morphants or Xdsh-D2–injected embryos is statistically significant compared with that of controls (Fig. 3D).
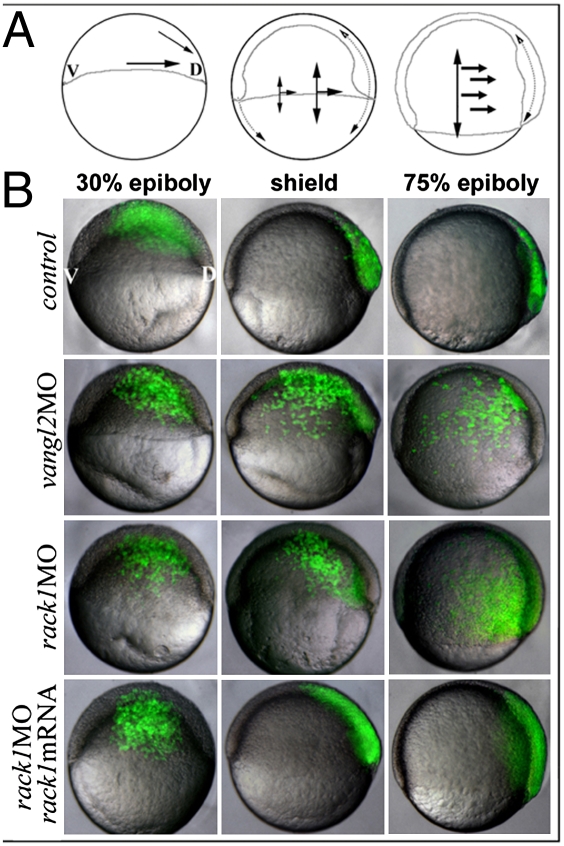
Additionally, from 30% to 75% epiboly, cells undergo ventral to dorsal convergence concomitant with anterior–posterior axis extension under the influence of the PCP pathway (Fig. 4A) (21). We used a previously published protocol (13) to visualize cell movements during gastrulation. A single marginal blastomere of morpholino-injected embryos was injected at the 32-cell stage with fluorescein dextran (10,000 Mr). Injected embryos were individually separated, and cell populations were monitored beginning at 30% epiboly. Only embryos with fluorescent clones located within the dorsal region of embryos at the onset of gastrulation were included for imaging. Images shown are representative of 30 embryos containing dorsal fluorescent clones of each experimental type. In 30 of 30 (30/30) control morphants, labeled cells underwent normal convergence, congregating at the dorsal side of the embryos (Fig. 4B). Consistent with previous studies demonstrating a role for Vangl2 in CE movements during gastrulation, labeled cells of vangl2 morphants (29/30) failed to converge properly and were dispersed across the dorsal and the lateral sides of the gastrula (Fig. 4B). Similar results were observed in rack1 morphant embryos (26/30) (Fig. 4B), suggesting a defect in CE during gastrulation. The CE defect observed in rack1 morphant embryos was rescued by coinjection of mouse rack1 mRNA (Fig. 4B). Of the 30 embryos coinjected with rack1 mRNA, 24 embryos were normal.
Fig. 4.
Rack1 is required for CE during gastrulation. (A) The diagrams illustrate the directions of cellular movement during zebrafish gastrulation. (B) Images of live embryos injected with vangl2 MO, rack1 MO, or rack1 MO plus mouse rack1 mRNA and subsequently labeled single blastomere cell with fluorescein dextran at the 32-cell stage. V, ventral; D, dorsal.
Rack1 Is Required for Membrane Localization of Vangl2.
The synergistic effect of vangl2 MO and rack1 MO in multiple PCP processes, the rescue of the vangl2 morphant gastrulation phenotype by rack1 mRNA, and the physical interaction between Rack1 and Vangl2 imply that Rack1 forms a complex with Vangl2 and acts in the same genetic pathway with Vangl2 for gastrulation. A prediction is that overexpression of the protein domain in Rack1 that interacts with Vangl2 is likely to have a dominant-negative effect on gastrulation. We injected mRNA encoding Rack1WD1–4 or Rack1WD5–7 in zebrafish embryos at the one- or two-cell stage. We found that Rack1WD1–4 did not cause discernable gastrulation defects when overexpressed in zebrafish, whereas overexpression of Rack1WD5–7 led to strong gastrulation defects, including a shortened body axis, an undulating notochord, and widened and asymmetric somites (Fig. S3 A–C).
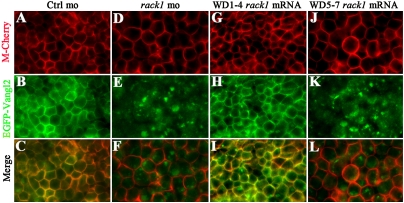
The dominant-negative effect of Vangl2-interacting Rack1WD5–7 domain on gastrulation supports that the interaction between Rack1 and Vangl2 underlies the role of Rack1 in PCP signaling. The highly conserved Rack1 is believed to function as a scaffold protein on which protein partners can dock and form signaling complexes in a cell context–dependent manner (22, 23). In particular, Rack1 is implicated in cell adhesion, spreading, and vesicle trafficking and targeting of membrane receptors (18, 24–29). To further explore the mechanism underlying the role for Rack1 in PCP signaling, we first verified the association of EGFP-Vangl2 and Rack1 in zebrafish extracts (Fig. S4). We then examined the localization of Vangl2 or Rack1 in zebrafish embryos injected with rack1 or vangl2 MOs, respectively (Fig. 5 and Fig. S5). Injections of mRNA encoding Rack1 protein in vangl2 morphants revealed a general localization within the cytoplasm of Rack1 in control and vangl2 morphants (Fig. S5). Conversely, we injected mRNA encoding EGFP-Vangl2 protein and membrane Cherry (M-Cherry) protein with rack1 MO into one-cell embryos and visualized EGFP-Vangl2 localization in live gastrulas (Fig. 5 A–F). As expected, EGFP-Vangl2 is exclusively localized to cell membrane in embryos injected with control morpholino (Fig. 5 A–C). Interestingly, coinjection of rack1 MOs abolished cell membrane localization of EGFP-Vangl2, and EGFP-Vangl2 appears as granules within the cells (Fig. 5 D–F). We also tested whether the Vangl2-interacting domain of Rack1, Rack1WD5–7, has a dominant-negative effect on Vangl2 localization (Fig. 5 G–L). The injection of mRNA encoding Rack1WD1–4 had no effect on EGFP-Vangl2 localization (Fig. 5 G–I). In contrast, the injection of mRNA encoding the Vangl2-interacting Rack1WD5–7 domain led to the removal of cell membrane localization of EGFP-Vang2 protein (Fig. 5 J–L). These data indicate that Rack1 is required for Vangl2 membrane localization and that the interaction between Rack1 and Vangl2 is essential for Vangl2 cell membrane targeting.
Fig. 5.
Rack1 is required for membrane targeting of Vangl2. Injections of mRNA encoding EGFP-Vangl2 in control gastrulating (A–C) or rack1 morphant embryos (D–F). Coinjection of mRNA encoding Rack1 WD40 repeats 1–4 and EGFP-Vangl2 (G–I) or Rack1 WD40 repeats 5–7 and EGFP-Vangl2 (J–L). Views are of enveloping layer cells of a shield-stage embryo. RNA encoding membrane Cherry protein (M-Cherry) was included in all of the experiments to outline the cell membrane.
Rack1 Inhibits Canonical Wnt Signaling.
In addition to gastrulation defects characteristic of defective CE, we consistently observed body axis duplication in ∼5% and ∼15% of rack1 morphant and rack1 and vangl2 double-morphant embryos, respectively (Fig. S6 A and B). Such a phenotype is commonly associated with elevated canonical Wnt signaling (30). The PCP signaling pathway shares several components with the canonical Wnt signaling pathway, and recent studies have revealed a recurring theme in which canonical Wnt signaling is inhibited at multiple steps during PCP signaling to ensure the specificity of downstream activity (13, 31). The formation of double body axes in rack1 morphant embryos implied that Rack1 might act as a molecular switch to promote PCP signaling while inhibiting canonical Wnt signaling. To explore this possibility (Fig. S6), we first examined the expression of chordin (chd), a known target of canonical Wnt signaling (32). We also compared and tested the synergistic effect of rack1 MO and wnt5b MO, a morpholino known to increase canonical Wnt activity (33–36). The expression of chd was expanded along the dorsal axis of wnt5b morphants as expected (Fig. S6 C–E). The expression of chd in rack1 morphants was also expanded, comparable to what was observed in wnt5b morphants (Fig. S6 C–E). In addition, ectopic expression of chd was observed in a small but significant number of rack1 morphants (Fig. S6 F and L), consistent with the formation of a duplicate axis observed. Coinjection of suboptimal doses of rack1 and wnt5b MOs resulted in a similar frequency of increased or ectopic chd expression (Fig. S6 G, H, and L), suggesting that Rack1 acts similarly to Wnt5b and also inhibits canonical Wnt activity.
To further confirm that formation of duplicate axes observed in rack1 morphants was caused by increased canonical Wnt activity, we next used a transgenic zebrafish line containing a dominant-negative form of the canonical Wnt effecter tcf3a under the inducible control of a heat-shock promoter [Tg(hsp70l:tcf3-GFP)w26/+; Δtcf] (37). Injection of rack1 MO, vangl2 MO, or suboptimal doses of rack1 and vangl2 MOs into non-heat-shocked Δtcf embryos resulted in frequencies of ectopic head formation that were similar to ectopic chd expression observed in rack1 or wnt5b morphants (Fig. S6 I and L). Heat-shocking double morphants before the onset of gastrulation (∼50% epiboly) eliminated the presence of ectopic heads in nearly all embryos examined (Fig. S6 J and L), suggesting that duplicate axes observed in rack1 morphants require TCF.
We also injected rack1 MO into a transgenic zebrafish line containing GFP under the control of a TOPFlash reporter [Tg(TOP:GFP)w25/+] (38) and obtained consistent data supporting that Rack1 is capable of inhibiting canonical Wnt target genes in vivo. Injection of rack1 MO into Tg(TOP:GFP)w25/+ embryos resulted in an increase in GFP protein accumulation relative to controls (Fig. S6M). These in vivo results were further supported by TOPFlash reporter assays performed in cell culture, where control or Rack1-expressing plasmid was cotransfected into cells together with a luciferase reporter plasmid driven by canonical Wnt activity and a normalizing plasmid expressing β-gal. Exposure of transfected cells to Wnt3a media led to drastically increased levels of luciferase expression when transfected with control plasmid (Fig. S6N). Transfection of Rack1-expressing plasmid, however, significantly reduced activation of the canonical Wnt reporter (Fig. S6N). Together, our results suggest that Rack1 directly inhibits the canonical Wnt pathway.
Discussion
Studies of vertebrate homologs of the Drosophila PCP genes have revealed a conserved genetic pathway that regulates PCP in several developmental processes, including CE during gastrulation and neurulation, oriented cell division, hair-cell orientation in the zebrafish lateral line and the mouse inner ear, and hair-follicle alignment in mouse epidermis (5, 39). The vertebrate PCP pathway appears to use a conserved mechanism of polarized sorting of membrane-associated complexes to propagate the polarity signal and to recruit intracellular effectors for morphological polarization.
The vertebrate Vang protein, Vangl2, is predicted to consist of four transmembrane domains and is localized to the membrane (19, 40). Its membrane localization via a Sec24b-dependent transport from the endoplasmic reticulum to the Golgi appears to be essential for its role in PCP signaling (16). However, the molecular network that directs the membrane targeting and its action in PCP signaling is not delineated. In this study, we identified Rack1 as a Vangl2-interacting protein in a yeast two-hybrid screen by using the C-terminal cytoplasmic domain of Vangl2 (Fig. 1). We verified the interaction between Vangl2 and Rack1 by coimmunoprecipitation and determined the region in Rack1 that mediates its interaction with Vangl2 (Fig. 1). We demonstrated that Rack1 is required for notochord cell polarization, oriented cell division, and CE during gastrulation and for neurulation in zebrafish (Figs. 2–4). We further showed that the interaction between Rack1 and Vangl2 is essential for gastrulation (Fig. S3). Together, these data identify Rack1 as a previously uncharacterized component of the vertebrate PCP signaling pathway.
Rack1 is a highly conserved protein and is predicted to have seven WD40 repeats folding into a β-propeller structure (18). It is believed to serve as a scaffold signaling protein and act in multiple cellular processes in complex with different proteins. It has been implicated in FAK complexes to regulate cell spreading and cell polarity in migrating cells (29), in targeting a tumor suppressor, p63, for proteasomal degradation (41), in a nuclear BML1 complex as integral components of the mammalian circadian clock (42), and in regulating cell membrane targeting of several receptors (24, 27, 28). We found that Rack1 is required for membrane localization of Vangl2 (Fig. 5). Moreover, we found that the Vangl2-interacting domain of Rack1 has a dominant-negative effect on membrane targeting of Vangl2, parallel to its dominant-negative effect on gastrulation (Fig. 5 and Fig. S3). These data suggest that the interaction between Rack1 and Vangl2 and the membrane targeting of Vangl2 underlie the requirement for Rack1 in PCP signaling.
Rack1 was originally identified on the basis of its ability to bind to the activated form of PKC (18). Subsequent studies of Rack1 suggest that its ability for differential PKC binding in a cell context–dependent manner may underlie its various functions in diverse cellular processes. Indeed, Rack1 acts with PKC in both membrane targeting of receptors (24) and in the regulatory circadian feedback loop (42). It is possible that the role for Rack1 in membrane targeting of Vangl2 is also mediated by its association with PKC. Atypical PKCs (aPKCs) are known regulators cell polarity and adhesion. Morpholino injections of the aPKCζ ortholog in Xenopus or zebrafish lead to CE defects (43) or deregulated cell division orientation in the eye (44), respectively. We found that an antibody against aPKCζ can also coimmunoprecipitate Rack1 and Vangl2 from mouse brain and inner ear tissues (Fig. S7). It is possible that the interaction between Rack1 and aPKCζ regulates the process of Vangl2 membrane targeting. However, it cannot currently be excluded whether this interaction between Rack1 and aPKCζ is involved in other events in PCP signaling or other cellular processes independent of PCP signaling. In particular, cell shedding at the dorsal side was observed in of the rack1 or rack1 and vangl2 morphants (Fig. 2), suggesting a defect in cell adhesion. Because Rack1-interacting protein aPKC can regulate the balance between microtubule and actin cytoskeleton to influence adherens junctions (45) and Rack1 can interact with integrins (46, 47), it is possible that Rack1 plays a direct role in cell adhesion and migration in zebrafish independent of PCP signaling. Alternatively, Rack1 mediates cell adhesion regulation during PCP signaling (48), in addition to its role in Vangl2 targeting. The interaction between Rack1 and aPKCζ (Fig. S7) also implicated a potential molecular role for Rack1 in regulating PCP gene Dvl (43). In Xenopus, aPKCζ interacts with and regulates plasma membrane localization of Dvl to activate JNK for CE. Because aPKCζ interacts with Rack1 (Fig. S7), it is possible that Rack1 provides a molecular scaffold for aPKCζ to regulate the plasma membrane localization of Dvl.
It is worth noting that a small but statistically significant percentage of rack1 morphants and rack1 and vangl2 double morphants have duplicated body axes, which typically result from increased canonical Wnt activity (30). We verified an inhibitory role for Rack1 on canonical Wnt signaling not only by examining the expression of canonical Wnt reporter genes in zebrafish and in cultured cells but also by rescuing the ectopic axis phenotype through the inhibition of canonical Wnt effectors (Fig. S6). The regulation of canonical Wnt signaling by a molecule involved in targeting of an essential PCP membrane protein has been reported previously. VhaPRR is found to be specifically associated with PCP protein Fz for its membrane targeting in Drosophila wing (49). Interestingly, VhaPRR also acts as a modulator for canonical Wnt signaling in the wing tissue (49). In summary, our current study identified a previously uncharacterized component of the vertebrate PCP signaling pathway that promotes PCP signaling while attenuating canonical Wnt signaling. Future studies aimed at exploring the mechanisms underlying the role of Rack1 in vertebrate PCP signaling and canonical Wnt signaling will delineate further the molecular compositions and events of these two essential biological pathways.
Methods
Zebrafish Strains and Husbandry.
Zebrafish were maintained in an Emory University facility under standardized conditions in compliance with Institutional Animal Care and Use Committee regulations. Wild-type and transgenic zebrafish embryos were maintained at 28.5 °C and staged as previously described (50).
Coimmunoprecipitation, Western Blot Analysis, and Immunohistochemistry.
Coimmunoprecipitation assays of Rack1 and Vangl2-GFP were performed with brain and/or cochlear extracts prepared from embryonic day 16.5 mouse embryos expressing Vangl2-GFP fusion protein. Vangl2-GFP shows asymmetric subcellular localization, identical to that of the endogenous Vangl2 protein.
The following antibodies were used: anti-Rack1 (BD Biosciences, Santa Cruz Biotechnology, and Transduction Laboratories), anti-GFP (Chemicon and Santa Cruz Biotechnology), anti-HA (Cell Signaling and Santa Cruz Biotechnology), anti-GADPH (Proteus BioSciences), and anti–α-tubulin (Sigma).
MOs and RNA Injections.
The translation-blocking MO against rack1 was as follows: 5′-CCC TTA CTG TCA TCT GCT CGG TCAT- 3′. The vangl2 MO has been previously described (51). For single-morpholino injections, 2 ng of MO was injected into embryos at the one- or two-cell stage. For double-morpholino injections, ∼1 ng of each MO was injected. The control morpholino sequence used was 5′-CCT CTT ACC TCA GTT ACA ATT TATA-3′. All of the MOs were synthesized by Gene Tools.
For RNA injection, ∼100–200 pg of mouse rack1 or vangl2 mRNA was injected into embryos at the one- or two-cell stage together with rack1 or vangl2 MO for rescue experiments. For overexpression of Rack1 WD40 motifs, 500–2,000 pg of mRNA was used.
Monitoring Gastrulation Cellular Movements, Mitotic Spindle Orientation, and Notochord Cellular Morphology.
CE movements during gastrulation were analyzed as previously described (13). Fluorescein dextran (10,000 Mr) was injected into a single blastomere of a 32-cell morphant embryo. Ten embryos with fluorescent clones located within the dorsal region of embryos at the onset of gastrulation for each experimental group were identified for live embryo imaging. The experiments were repeated for three times. Images shown are representative of 30 embryos containing dorsal fluorescent clones of each experimental group. Live images were taken at 30% epiboly, shield, and 75% epiboly stages of the same embryos.
Mitotic spindle orientation in dorsal epiblast cells during early gastrulation was recorded and analyzed as described (2). To assay notochord cell morphology, embryos were raised in 10 μM BODIPY ceramide (Invitrogen) as previously described (20).
Lef/Tcf Canonical Wnt Reporter Assays.
TOPFlash luciferase reporter construct and a pSV-β-gal expression vector were cotransfected with the plasmid expressing Rack1 or with the control plasmid into HEK293 cells by using FuGENE 6. Transfected cells were treated with Wnt3a conditioned medium (isolated from Wnt3A-expressing cell line CRL-2647; ATCC) or control medium (isolated from L2648; CRL-2648 ATCC) and harvested for standard luciferase assays, all of which were normalized with β-gal activity. The difference between samples of control plasmid and Rack1-expressing plasmid was subjected to two-tailed unpaired type-2 Student's t test.
Supplementary Material
Acknowledgments
We thank Dr. Pablo Lopez Bergami, Dr. Ze'ev Ronai, Xing Dai, Harish Joshi, Herwig Baier, Auta Keeter, Dr. Scott Fraser, Devon Jensen, and Dr. Randy Schekman for various research reagents, including the reporter and normalization plasmids for canonical Wnt signaling, Rack1 plasmids, the zebrafish canonical Wnt reporter line, the Histone 2B-RFP and the dominant-negative Xdsh-D2 plasmids, and various Vangl2 plasmids. This research was supported by March of Dimes research Grant FY08-490 and National Institutes of Health research Grants RO1 DC007423 and DC005213 (to P.C.). R.E. was supported by National Institutes of Health Grant RO1 DC00701 (to A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013170108/-/DCSupplemental.
References
- 1.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 2.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 3.Ségalen M, et al. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell. 2010;19:740–752. doi: 10.1016/j.devcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quesada-Hernández E, et al. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr Biol. 2010;20:1966–1972. doi: 10.1016/j.cub.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Jones C, Chen P. Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol. 2008;85:197–224. doi: 10.1016/S0070-2153(08)00808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Schier H, Hudspeth AJ. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc Natl Acad Sci USA. 2006;103:18615–18620. doi: 10.1073/pnas.0608536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- 11.Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh K, Jenny A, Mlodzik M, Sokol SY. Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci. 2009;122:3791–3798. doi: 10.1242/jcs.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdes JM, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, et al. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics. 2008;180:219–228. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 16.Merte J, et al. Sec24b selectively sorts vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2010;12:41–46. doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindqvist M, et al. Vang-like protein 2 and Rac1 interact to regulate adherens junctions. J Cell Sci. 2010;123:472–483. doi: 10.1242/jcs.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ron D, et al. Cloning of an intracellular receptor for protein kinase C: A homolog of the β subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian D, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jessen JR, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–R228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: A dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 23.Sklan EH, Podoly E, Soreq H. RACK1 has the nerve to act: Structure meets function in the nervous system. Prog Neurobiol. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Parent A, Laroche G, Hamelin E, Parent JL. RACK1 regulates the cell surface expression of the G protein-coupled receptor for thromboxane A2. Traffic. 2008;9:394–407. doi: 10.1111/j.1600-0854.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 25.López-Bergami P, et al. RACK1 mediates activation of JNK by protein kinase C. Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ai E, Poole DS, Skop AR. RACK-1 directs dynactin-dependent RAB-11 endosomal recycling during mitosis in Caenorhabditis elegans. Mol Biol Cell. 2009;20:1629–1638. doi: 10.1091/mbc.E08-09-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikebuchi Y, et al. Receptor for activated C-kinase 1 regulates the cellular localization and function of ABCB4. Hepatol Res. 2009;39:1091–1107. doi: 10.1111/j.1872-034X.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohgaki R, Fukura N, Matsushita M, Mitsui K, Kanazawa H. Cell surface levels of organellar Na+/H+ exchanger isoform 6 are regulated by interaction with RACK1. J Biol Chem. 2008;283:4417–4429. doi: 10.1074/jbc.M705146200. [DOI] [PubMed] [Google Scholar]
- 29.Serrels B, et al. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–1092. doi: 10.1016/j.cub.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Sokol S, Christian JL, Moon RT, Melton DA. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz-Romond T, et al. The ankyrin repeat protein Diversin recruits Casein kinase Iε to the β-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled β-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–3911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- 33.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westfall TA, et al. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topol L, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres MA, et al. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis JL, et al. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- 38.Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/β-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- 39.Mlodzik M. Planar cell polarization: Do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 40.Montcouquiol M, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Peart MJ, Prives C. Stxbp4 regulates DeltaNp63 stability by suppression of RACK1-dependent degradation. Mol Cell Biol. 2009;29:3953–3963. doi: 10.1128/MCB.00449-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robles MS, Boyault C, Knutti D, Padmanabhan K, Weitz CJ. Identification of RACK1 and protein kinase Cα as integral components of the mammalian circadian clock. Science. 2010;327:463–466. doi: 10.1126/science.1180067. [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui S, Otten C, Rohr S, Abdelilah-Seyfried S, Link BA. Analysis of aPKCλ and aPKCζ reveals multiple and redundant functions during vertebrate retinogenesis. Mol Cell Neurosci. 2007;34:431–444. doi: 10.1016/j.mcn.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–738. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vomastek T, et al. RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell motility. Mol Cell Biol. 2007;27:8296–8305. doi: 10.1128/MCB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buensuceso CS, Woodside D, Huff JL, Plopper GE, O'Toole TE. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J Cell Sci. 2001;114:1691–1698. doi: 10.1242/jcs.114.9.1691. [DOI] [PubMed] [Google Scholar]
- 48.Solnica-Krezel L. Gastrulation in zebrafish—all just about adhesion? Curr Opin Genet Dev. 2006;16:433–441. doi: 10.1016/j.gde.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Hermle T, Saltukoglu D, Grünewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–1276. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 50.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 51.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.