Abstract
The regional community concept embraces the idea that species interactions across large areas shape both the geographic/ecological distributions and the local abundances of populations. Within this framework, I analyzed the distribution and abundance of 79 species of land birds across 142 ca. 10-ha census plots from standardized breeding bird censuses in deciduous and mixed forests of eastern North America. To characterize the regional ecological space, plots were ordinated on the basis of species abundances. Within the regional community defined by these synthetic axes, the distribution and abundance of individual species did not appear to be shaped by competition or to reflect the adaptations of individuals: (i) local abundance and population extent across the ordination axes were unrelated, (ii) pairwise correlation coefficients of species abundances were centered on 0, (iii) average species distribution and abundance were independent of the number of close relatives, and (iv) distribution and abundance exhibited no evolutionary (phylogenetic) conservatism. To explain these seemingly random patterns, I speculate that species are approximately evenly matched competitors over much of the region and that their distributions and relative abundances are determined by the labile coevolutionary outcomes of interactions with specialized pathogens. Thus, despite the appearance that random processes determine patterns in the distribution and abundance of populations in the regional community, it is plausible that species-specific deterministic interactions are responsible. Although competition is a dominant force in ecological communities, variation in the distribution and abundance of individual species might instead reflect the outcome of interactions with specialized antagonists, including pathogens.
Keywords: niche, population distribution, community assembly, macroecology, variance in abundance
Biological communities comprise large numbers of potentially interacting species. Although ecologists broadly embrace this concept of community (1, 2), they are not agreed on the integrity of communities as ecological entities or the scale on which communities can be perceived (3). Interactions between populations in local assemblages are evident through natural (4, 5) and experimental (2, 6) manipulations, but dispersal of individuals across space also connects and integrates assemblage dynamics within the larger region. The local presence and relative abundance of species integrate processes of evolutionary diversification and ecological interaction operating at large spatial and temporal scales (7). Accordingly, ecological communities make sense only as regional entities. Nonetheless, factors that influence the numbers and distributions of species within the regional community are poorly understood.
The distribution and abundance of a species have two components (8). The individual component reflects adaptations to use substrates and exploit resources within the individual's activity space, which is also influenced by its tolerance of physical conditions (1, 9, 10). The population component reflects the changing balance of births and deaths over space as they are affected by regional conditions and interacting populations (11). The individual (primarily within-habitat) component tends to be evolutionarily conservative—for example, most warblers glean insects from foliage and most woodpeckers scale bark and excavate wood to find their prey. In contrast, the population component is highly labile (8, 12), being influenced by small changes in the balance of births and deaths.
In this essay, I argue that although resources limit populations, making competition a potent force, the distribution and abundance of a particular species are also influenced by coevolved interactions with specialized antagonists, plausibly pathogens. The idiosyncratic and evolutionarily dynamic nature of host–pathogen interactions creates the appearance of randomness in community organization, which has largely defeated attempts by ecologists to relate distribution and abundance to species characteristics (13, 14). I explore this paradox further here: a) I begin by describing a regional community of forest birds in eastern North America, for which population densities of individual species are available for a sample of local forest plots. b) I use an ordination based on species abundances to place the plots in a multidimensional space reflecting axes of variation that are meaningful to bird populations, i.e., taking a bird's-eye view of the ecological space. Both species richness and total density on the plots are strongly related to plot positions on the ordination axes, suggesting regulation of overall community attributes. c) Within the regional community, population density and distribution vary independently among species and, lacking phylogenetic signal, these traits appear to be evolutionarily labile. d) This evidence of species-level idiosyncrasy is reinforced by the absence of negative pairwise correlations among local species abundances, which could be expected from competitive interactions among ecologically similar species. e) Finally, lacking evidence for strong competitive structuring, I speculate that distribution and abundance of species within the regional community reflect evolutionarily labile interactions between populations and pathogens or other antagonists.
Results and Discussion
Regional Community.
Although the concept of the regional community is straightforward, working with its structure and dynamics is challenging because the spatial scale is large, regions are not integral ecological entities (their boundaries are poorly defined), and species distributions are complex and only partially overlapping. How can one comprehend the regional community? Consider that populations are the core entities of ecological communities and that their interactions extend over their spatial extent. It follows that regional distributions of populations should provide insight into the organization of the regional community. With this in mind, I characterize attributes of the distributions of forest birds in eastern North America. Analysis of these distributions suggests that the component populations of the regional community are individually and independently dynamic over periods that are short relative to the time for species formation and extinction. Moreover, I show that ecological and geographic distributions are independent of local abundance, both of which vary without regard to the number of species in an evolutionary clade and hence the number of ecologically similar species that are likely to be close competitors (15). Owing to the complexity of interactions influencing each species, the internal structure of the regional community appears deceptively random. Indeed, species give the impression of being relatively evenly matched as competitors over much of the regional community landscape, with their distributions and abundances appearing to reflect stochastic processes (16).
Eastern North American Forest Birds.
The pertinent “region” in this analysis corresponds to the extent of broad-leaved and mixed forest in eastern North America (17), within which few natural barriers impede the movement of birds and the distributions of their populations. The 142 local assemblages included 79 species of birds that are typical of forested habitats in the region (SI Text S1). Bird distributions reflect variations in physical conditions of the environment, including climate, but also variations in habitat structure, including openness of the forest canopy, shrub layer density, presence of standing water, and the mix of broad-leaved and needle-leaved trees (18). I realized this multidimensional nature of the regional community using a Bray–Curtis (BC) ordination (19) of the forest census sites based on the distributions of the bird species among them (SI Text S2). Ordination extracts synthetic axes of plot and species distribution while preserving pairwise dissimilarity between plots as distance in the ordination space. Except for the first BC axis, which represents a north–south gradient, the remaining ordination axes were only modestly related to climate variables (SI Text S3).
Summary Attributes of Local Assemblages.
Two assemblage-level (i.e., local census) characteristics—the number of species of birds and their summed population densities at each census site—were strongly correlated with the position of the site within the ordinated regional community space. As one would expect from sampling effects, the logarithm of the number of species was related to the logarithm of the total number of bird territories per site with a slope of 0.34 ± 0.03 se, explaining 27% of the variance. Additional statistical association with BC axes 4 and 6 increased the explained variance in the number of species to 73%. Likewise, 78% of the variance in the overall density of territories (pairs per hectare) was explained by the BC ordination axes, with large contributions (P < 0.0001) from BC axes 1, 2, 3, 6, and 7, and a contribution of <2% from the log-transformed number of species (P = 0.002). Similar analyses relating log-transformed species richness and population density to climate variables explained only 49% and 22% of the variance, respectively (SI Text S4), emphasizing that bird species sort themselves more by habitat structure than by physical (climate) conditions of the environment. The strong relationship between total numbers of birds and the ordination axes additionally implies regulation of total population density independently of the composition of the community. Density dependence in local populations is further indicated by the generally lower variance in population size over years compared with the mean (SI Text S5).
Local Density and Regional Distribution of Populations.
Although the species richness and average total density of the bird assemblages varied systematically over the ordination space, the average local density of any particular bird species was independent of the breadth of its distribution on any ordination axis. Distributional breadth can be characterized by the SD of a species’ occurrence in local counts over each of the nine ordination axes. Surprisingly, neither the total number of individual territories of each species reported over the 142 census sites (F18,60 = 1.55, P = 0.10) nor the average density of each species in occupied censuses (F18,60 = 1.33, P = 0.20) was related to the mean position or the breadth of the species distribution on nine ordination axes.
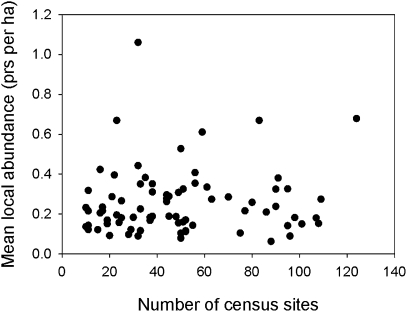
The local abundance of each species is presumably related to its local resource base. If local abundance of resources were positively related across localities, one would expect locally abundant species to be widely distributed (20), as often appears in regional analyses at coarse scales (21). However, the average density of each species within local sites and the number of sites occupied were unrelated among species of forest birds in eastern North America (Fig. 1), further emphasizing the apparent independence of populations from the distribution of particular resources. Moreover, neither distribution (P = 0.33) nor local abundance (P = 0.67) varied significantly among 20 ecologically defined groups (SI Text S7).
Fig. 1.
Mean local abundance is unrelated to number of census sites occupied (F1,77 = 0.6, P = 0.45).
Evolutionary Lability of Population Attributes.
None of the variables used to describe the occupation of community space by individual species exhibited phylogenetic conservatism. Each variable was subjected to a nested analysis of variance with respect to the taxonomic levels superfamily, family, genus, and species (the latter representing the residual or error variance) (SI Text S5). In not a single case did the means or SDs of the ordination axis scores have significant variance components at any level above species within genus, for which the species-level variance was 75.6–100% (means) and 70.2–100% (SDs) of the total, respectively. This was also true of the number of sites occupied (82.1%), the total abundance of each species over the entire region (99.4%), and the average density at occupied census sites (91.7%). Thus, the distributions of species within the regional community appear evolutionarily labile within the time duration of individual species, emphasizing the dynamic nature of the regional community (12–14, 22). In contrast, the principal components scores of species based on morphological measurements exhibited significant variation above the species-within-genus level (42–95%), as one would expect of evolutionarily conservative traits (SI Text S5).
Competition Within the Regional Community.
Bird populations are limited largely by resources (23). Accordingly, competition—particularly between evolutionarily closely related species—should impact the abundances and local population densities of individual species. Seeking evidence of competitive effects, I calculated pairwise Spearman rank correlation coefficients (rS) between the average densities of all 79 species over the 142 census sites (SI Text S6). If species interactions strongly influenced species population sizes, one could expect the distribution of correlation coefficients to be skewed toward negative values, particularly among species with similar resource requirements. Alternatively, both positive and negative correlations could result from similarities and differences in habitat preferences of bird species across census sites, even in the absence of direct competitive interactions.
Over all 79 species (3,081 pairwise comparisons), rS was centered close to 0 (0.034 ± 0.232 sd) (Table 1). Randomly shuffling local densities among species within sites or over the entire data matrix resulted in mean rS close to 0, with SDs of 0.082 and 0.086, respectively. Among fully randomized data, 95% of observations would lie between −0.167 and 0.167 (24). Of the observed correlations, 27% were more positive and 19% were more negative than these boundaries. Thus, birds are not distributed randomly with respect to each other over the regional community, but the slight predominance of positive correlations suggests that associations between species more likely derive from similar and dissimilar habitat preferences than from pairwise competitive interactions. The distributions of rS values within smaller groups of species having similar resource requirements [warblers (Parulidae), vireos (Vireonidae)], woodpeckers (Picidae), and flycatchers (Tyrannidae)] were also centered on 0 (−0.002–0.088) and had SDs similar to the complete sample (0.17–0.24) (Table 1). A more telling result is that the 140 pairwise comparisons between warblers and woodpeckers, which have little in common with respect to resource requirements, nonetheless exhibit a similar distribution of rank correlations (rS = −0.010 ± 0.215). Because the correlations within and between taxonomic groups of species do not differ from the correlations in the whole set, pairwise species competition does not appear to influence distribution and abundance.
Table 1.
Pairwise Spearman's rank correlation statistics between the densities over 142 census sites for the complete species set and several subsets of species
| Species | N | Average | SD | |
| All species | 79 | 3,081 | 0.0334 | 0.232 |
| Row shuffled (randomized) | 79 | 3,081 | 0.0244 | 0.082 |
| Fully shuffled (randomized) | 79 | 3,081 | −0.0014 | 0.086 |
| Warblers | 20 | 190 | 0.0654 | 0.201 |
| Vireos | 5 | 10 | −0.002 | 0.241 |
| Woodpeckers | 7 | 42 | 0.088 | 0.213 |
| Flycatchers | 5 | 10 | 0.006 | 0.174 |
| Woodpeckers × warblers | 7 × 20 | 140 | −0.010 | 0.215 |
N refers to the number of pairwise comparisons among species used to calculate the observed average correlation and the SD of the distribution of correlations.
Independence of Population Attributes and Clade Size.
If species interactions influenced the abundance of species locally, one might expect species in larger evolutionary clades to divide resources more finely, leading to reduced abundance locally and/or globally. I assigned species in the analysis into 20 monophyletic taxonomic/ecological groups, within which body size, foraging behavior, diet, and other ecological variables are uniform compared with their distributions among all of the species (SI Text S7). The largest groups were warblers (22 species), finches (10 species), woodpeckers (7 species), and vireos, thrushes, and flycatchers (5 species each); 7 of the groups were represented by single species. Unexpectedly, neither the number of sites occupied by each species (F19,59 = 1.15, P = 0.33) nor the mean density of each species in occupied sites (F19,59 = 0.82, P = 0.67) varied significantly among groups.
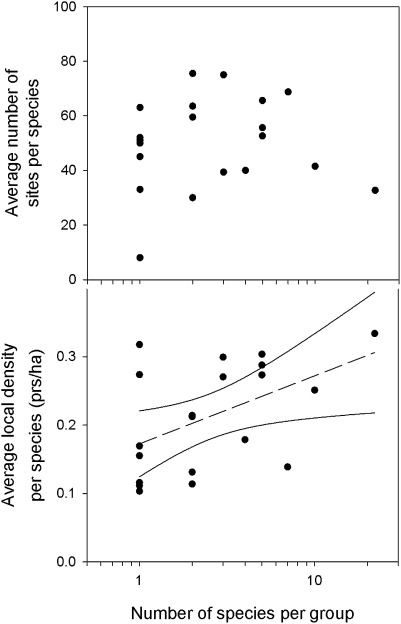
These population traits also were not negatively related to the number of species per group, as could have been expected on the basis of competition and evolutionary conservatism of resource use; local density was weakly related to the number of species but the relationship was positive (Fig. 2). The independence of local density per species and the number of species per group could reflect a relationship between group size and resource abundance, but data are not available to test this hypothesis. Alternatively, larger groups might use a broader spectrum of the available local resources. To the extent that morphology reflects variation in foraging substrates and movements, as well as variation in prey types (25), larger groups might exhibit greater variation in morphology (26). In fact, however, the relationship between the SDs of species positions on the eight morphological principal components and the logarithm of the number of species per group was insignificant (P > 0.05, R2 < 0.2) for all but principal component (PC) 5 (1.6% of the total morphological variance), which represented the trait combination tarsus length × bill width/bill depth (F1,11 = 8.0, P = 0.017, R2 = 0.42). Thus, the morphological space occupied by each of the clades bore little relationship to clade size.
Fig. 2.
Distribution and local density of species as a function of the number of species in 20 taxonomically and ecologically defined groups of birds. Number of sites per species is independent of number of species per group (F1,18 = 0.1, P = 0.75); local density per species increased with the size of the ecological group (F1,18 = 5.8, P = 0.027, R2 = 0.24; slope 0.099 ± 0.041 SE pairs per hectare per log10-transformed number of species).
Conclusions
Analyses of the structure of the regional community led to the following conclusions:
i) Variation in local species richness and number of individuals is strongly related to ordination scores based on the distribution of species among sites, suggesting that local attributes of the environment constrain the combined abundance of local bird populations. Over the range of environments considered in this analysis, climate variables predict combined abundances in local assemblages less well than ordination axes based on species distributions, emphasizing the contribution of locally variable forest structure to habitat quality for bird populations.
ii) Local abundance and regional distribution of populations are largely uncoupled (Fig. 1). Although several analyses have shown positive correlations between range size and local abundance, most of these cases have involved ecologically varied regions and coarse spatial scales (21, 27, 28), potentially confounding local abundance, distribution, and environmental heterogeneity.
iii) Local abundance and geographic distribution are unrelated to number of presumed close competitors (i.e., close relatives) (Fig. 2), as shown also with respect to regional distribution, habitat distribution, and local abundance in South American passerine birds (29) and with respect to the abundance of tree species on Barro Colorado Island, Panama (8). Accordingly, competition cannot account for variation in the distribution and abundance of species within the regional community.
iv) Evidence of pairwise competitive effects among populations across the region is lacking. Correlation coefficients are centered close to 0 (Table 1), and significantly positive and negative values would appear to represent similar or different habitat preferences among species rather than the outcome of species interactions.
v) The relative abundance of species over the whole region corresponds closely to the expectation of a homogeneous random birth–death process (SI Text S8). Accordingly, one could not reject a neutral model of population dynamics within the region (16). To the extent that the population of a species is a cohesive entity, the relevant dynamics are regional rather than local, for which the summed population over the whole region is the appropriate unit of comparison.
vi) Local abundance and ecological distribution exhibit little indication of phylogenetic conservatism (8, 12), suggesting that population characteristics are labile over time spans within the durations of individual species (30, 31).
Ecologists have traditionally conceptualized local biological communities in terms of strong interactions between ecologically specialized species, with deterministic outcomes (e.g., refs. 15 and 32–34). The absence of several expected patterns in the distribution and abundance of species and clades within the regional community challenges this concept. The picture of the regional community that emerges from the analysis of forest birds of eastern North America seems closer to the zero-sum ecological drift model of Hubbell (16), without strong ecological distinction between species across environmental gradients or evolutionary conservatism in their regional distributions. Of course, the pattern at the community level, particularly regarding species abundance distributions and species–area relationships, often provides little insight into the underlying community processes (35).
Rationalizing the Regional Community.
How can we reconcile the contrasting viewpoints of ecological determinism and ecological indifference in the distribution and abundance of species (36)? The combined densities of local populations appear to be constrained by the availability of resources. For example, in island compared with continental avifaunas, lower species richness is compensated by higher individual population densities (4, 5, 37). Decades of experimentation in ecology, including “natural experiments” involving ecological release on islands, have further demonstrated the strength of interspecific competition in limiting populations (38–40). In the present analysis, we see this resource dependence in the strong correlation between the summed densities of breeding birds and the positions of census sites on the ordination axes, reflecting both physical conditions of the environment and structure of the habitat.
At the species level, however, the regional community of forest birds in eastern North America shows little evidence of structuring by competition; abundance and distribution appear unrelated to ecological adaptations of species in that these population parameters do not exhibit evolutionary conservatism. Negative correlations between the local population densities of potentially competing species are not evident in pairwise comparisons. The similar distributions of correlation coefficients between pairs of ecologically related and ecologically distant species suggest that significant negative and positive correlations reflect the outcome of different or similar habitat selection and range distribution with respect to major climate and habitat-structure gradients. Although individuals of the same and different species “compete” for resources, in the sense that resource use reduces availability (33), interspecific competitors might be so closely matched, and their effects spread so diffusely through the regional community, that competition between species is a weak force in establishing species abundances and setting boundaries to species distributions.
What is the regional community? How is the regional community organized? What factors influence the structure of the regional community? To the degree that the dispersal of individuals integrates a population over space and time, populations should be considered as the basic units of ecological communities. To the extent that geographic or habitat boundaries circumscribe the distributions of a set of species, as in the case of the forest birds of eastern North America, natural boundaries can define a regional community within which ecologically similar species interact and influence each others’ distribution and abundance. The regional community is not a discrete entity, but might be conveniently bounded in such a way that it functions as a relatively self-contained unit and can be comprehended as an integrated whole.
Proposal for the Regional Community.
I suggest that the apparent indeterminism of population properties within the regional community follows from three underlying principles: (i) Species are approximately equivalent in competitive ability over large portions of the region; (ii) small differences in the demography of a population can cause large changes in abundance and distribution; and (iii) population processes are influenced by highly species-specific influences in the environment, for which specialized pathogens are potential candidates, leading to idiosyncratic outcomes.
Proposition (i).
Species are approximately equally competent competitors, at least within the relatively homogeneous forests of eastern North America. Most ecological groups contain some species that are distributed throughout most of the region, indicating that a particular ecological type can persist in most of the census sites that contribute to the present analysis. Moreover, all of the major ecological types of species occur at all of the sites, and individual species have persisted over hundreds of thousands or millions of years, likely within their present habitat types. The fact that closely related species can exhibit any combination of low to high local abundance and narrow to broad ecological distribution within the region suggests that the properties of populations within the regional community do not reflect conservative adaptations associated with particular foraging substrate types, foraging movements, or prey items, i.e., the traditional concept of the ecological niche of an individual (34, 41, 42).
Proposition (ii).
Small differences in demography translate into large variation in distribution and abundance. When species are approximately equal competitors across large expanses of ecological space, small changes in population productivity can produce large changes in the geographic and ecological extent of a population. Along a single dimension of ecological space, such a dynamic might lead to frequent extinction and the long-term coexistence of relatively few species (32). However, in regional communities of higher dimension—forest birds of eastern North America appear to recognize multiple environment/habitat dimensions—many more species are able to stay in the game, so to speak (43). Nevertheless, the extent of each population might be labile within the regional community, at least on the timescale of species existences, judging from the concentration of variance in distribution and abundance at the species-within-genus taxonomic level (12, 22). Accordingly, one would not expect to find a close correlation between species ecology and population parameters.
Proposition (iii).
The idiosyncratic nature of species abundances and distributions gives the appearance of randomness and lack of structure within the regional community, particularly in ecologically homogeneous regions. Nonetheless, these apparently stochastic manifestations likely reflect multiple, independent, but deterministic forces shaping the distributions of each species. Species can partition the environment in ways that promote diversity, but only in an environment having high dimensionality (43, 44).
I argue here that one of the ways species can differ is with respect to their interactions with specialized pathogens. Specialized pathogens create largely independent ecological axes and can make the dimensionality of the environment comparable to the number of host species. Host–pathogen interactions, by their nature, are well matched to the apparently independent, labile determination of host abundance and distribution (8). Pathogens exhibit rapid coevolutionary dynamics capable of uncoupling population properties from ecological adaptations; they normally evolve toward relatively benign effects on host populations, although novel host or pathogen mutations, or changes in the environment, can upset this balance. Because pathogen transmission often is directly related to host density (45–48), small host population sizes might be self-correcting owing to poor pathogen transmission, thereby preventing extinction of narrowly distributed populations and stabilizing community diversity.
Plausible alternative explanations would have to predict the high lability and independence of range size and local abundance, the lack of correlation between clade size and species distributions, and the absence of skew toward negative correlations in pairwise comparisons of species abundances. Models of species coexistence based on strong habitat selection and narrow ecological ranges of competitive dominance depend on ecological specialization and are not favorable toward population lability, even in an environment that has changed in response to glacial climate cycles. Models of species formation based on vicariant or peripatric division of populations might create the appearance of lability in population density and range (49), but there is little evidence for lineage splitting within the regional community considered here (SI Text S9). Moreover, few barriers impede the dispersal of forest species, and range expansion and contraction quickly obliterate distribution asymmetries following allopatric or peripatric species formation (14, 30, 50).
The apparent approximate competitive equivalence of species over large parts of the regional community and the stochastic-appearing distribution of overall species abundances within the region are suggestive of neutral or nearly neutral population dynamics, as envisioned by Hubbell (16). By suggesting that species tend to be broadly equivalent competitors over much of the regional community, I basically support the fundamental assumption of ecological equivalence, upon which neutral theory is based, at least within an ecologically homogeneous region. I differ, however, with respect to the mechanisms that determine population abundance and distribution. In neutral systems, population size depends on the history of stochastic variation in births and deaths. In contrast, I envision deterministic influences of specialized pathogens on host population size and distribution. One can imagine that the appearance of randomness in the regional community arises from specific coevolutionary outcomes that reflect a history of random mutation in both parties that influences pathogen virulence and host resistance, each engendering strong selection.
Neutral models have very slow dynamics where large populations are concerned (51, 52). Extinction times, measured in generations, are on the order of the population size. With the land area of eastern North America (from the western Mississippi River border states to the east) at ∼300 × 106 ha, species densities averaging 0.089 ± 0.091 pairs/ha in forested habitats over the entire region, and an average generation time of 3 y (representing maturity at 1 y of age and 50% annual adult survival rate), stochastic extinction times would be on the order of tens of millions of years and >100 million years for the more abundant species (53). Because the life spans of bird species appear to be less than this by one or two orders of magnitude (53–55), bird abundance and distribution cannot be the outcome of purely random processes. Deterministic influences must prevail, but these influences are sufficiently independent and variable for each population, as would be the case for specialized pathogens, that they give the appearance of randomness.
A high degree of specificity in the distribution and abundance of individual species also could be achieved by differential responses to generalized ecological factors in an environment with high dimensionality, perhaps on the order of the number of species (43, 44). Depending on trade-offs in the responses of each species to these factors, resource partitioning with respect to a sufficient number of these factors could allow many species to coexist within a regional community. What are these factors and what phenotypic trade-offs constrain individual species to respond to these factors in a unique manner? Ordinations of species abundances across communities in this study revealed a limited number (seven to nine) of significant axes of variation (i.e., shared among species) within the regional community. Perhaps, combined with within-habitat opportunities for resource and structural niche partitioning, seven axes are sufficient to explain coexistence within the regional community. This mechanism does not, however, explain the lack of phylogenetic signal in distribution and abundance or the absence of clear indications of between-species competition in these population attributes.
Pathogens hold promise as environmental factors that can promote coexistence among multiple species of hosts by limiting host populations. Host specificity of pathogens generates a high dimensionality among limiting environmental factors; selection for pathogen resistance can create the kinds of trade-offs needed to explain unique population responses of host species; density-dependent transmission tends to stabilize host–parasite systems; and parasite–host coevolution decouples host distributions from the host phylogenetic relationship. Time will tell whether these speculations are useful. However, they suggest that pathogens, and probably other specialized microbes with beneficial effects on fitness, might hold a key to understanding the organization and maintenance of diversity in regional communities.
Materials and Methods
I analyzed the abundances of 79 species of bird that are typical of forested habitats distributed across 142 local assemblages selected from Breeding Bird Censuses (SI Text S1). Individual census plots harbored 12–51 of the 79 species (26.4 ± 7.70 SD), and individual species were distributed over 8–124 of the 142 census plots (47.4 ± 29.4 SD). Of the total cells in the 142 sites × 79 species matrix (11,218 cells), 3,746 (33.4%) were occupied. Details of the parameters used in the Bray–Curtis ordination of these sites are described in SI Text S2. Mean scores and their SDs on each ordination axis were calculated for both census sites and species presences. Statistical analyses of variance and covariance were carried out in SAS procedure GLM. Evolutionary conservatism was determined by nested analysis of variance (SAS procedures NESTED and MIXED) on the basis of superfamily, family, and genus taxonomy described in SI Text S5. Rank correlations between abundances of pairs of species across censuses were calculated in SAS procedure CORR (SI Text S6). To determine the relationship between population distribution and clade size, species were assigned to 1 of 20 monophyletic ecological groups (SI Text S7).
Supplementary Material
Acknowledgments
James Lowe provided the Breeding Bird Census data; Jonathan Chase, Walter Jetz, and Jason Knouft provided insightful discussion and helpful comments on the manuscript; W. Jetz prepared Fig. S1. Dr. Martin Wikelski and the Max Planck Institute for Ornithology in Radolfzell, Germany, are thanked for their hospitality. Support for this work was provided by the Alexander von Humboldt Foundation and the Curators of the University of Missouri.
Footnotes
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018642108/-/DCSupplemental.
References
- 1.Chase JM, Leibold MA. Ecological Niches. Linking Classical and Contemporary Approaches. Chicago: Univ of Chicago Press; 2003. [Google Scholar]
- 2.Morin P. Community Ecology. Oxford: Blackwell; 1999. [Google Scholar]
- 3.Ricklefs RE. Disintegration of the ecological community. Am Nat. 2008;172:741–750. doi: 10.1086/593002. [DOI] [PubMed] [Google Scholar]
- 4.Cox GW, Ricklefs RE. Species diversity, ecological release, and community structuring in Caribbean land bird faunas. Oikos. 1977;29:60–66. [Google Scholar]
- 5.Wright SJ. Density compensation in island avifaunas. Oecologia. 1980;45:385–389. doi: 10.1007/BF00540211. [DOI] [PubMed] [Google Scholar]
- 6.Pfister CA. Estimating competition coefficients from census data: A test with field manipulations of tidepool fishes. Am Nat. 1995;146:271–291. [Google Scholar]
- 7.Ricklefs RE, Schluter D. Species diversity: Regional and historical influences. In: Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities. Historical and Geographical Perspectives. Chicago: Univ of Chicago Press; 1993. pp. 350–363. [Google Scholar]
- 8.Ricklefs RE. Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proc Natl Acad Sci USA. 2010;107:1265–1272. doi: 10.1073/pnas.0913626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchinson GE. Concluding remarks. Cold Spring Harb Symp Quant Biol. 1957;22:415–427. [Google Scholar]
- 10.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 11.Holt RD. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc Natl Acad Sci USA. 2009;106(Suppl 2):19659–19665. doi: 10.1073/pnas.0905137106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaston KJ. Species-range size distributions: Products of speciation, extinction and transformation. Philos Trans R Soc Lond B Biol Sci. 1998;353:219–230. [Google Scholar]
- 13.McGill BJ. Exploring predictions of abundance from body mass using hierarchical comparative approaches. Am Nat. 2008;172:88–101. doi: 10.1086/588044. [DOI] [PubMed] [Google Scholar]
- 14.Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 15.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- 16.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 17.Braun EL. Deciduous Forests of Eastern North America. New York: Hafner; 1950. [Google Scholar]
- 18.James FC. Ordinations of habitat relationships among breeding birds. Wilson Bull. 1971;83:215–236. [Google Scholar]
- 19.Legendre P, Legendre L. Numerical Ecology. 2nd Ed. Amsterdam: Elsevier; 1998. [Google Scholar]
- 20.Brown JH. On the relationship between the abundance and distribution of species. Am Nat. 1984;124:255–279. [Google Scholar]
- 21.Gaston KJ, Blackburn TM. Pattern and Process in Macroecology. Oxford: Blackwell; 2000. [Google Scholar]
- 22.Scheuerlein A, Ricklefs RE. Prevalence of blood parasites in European passeriform birds. Proc Biol Sci. 2004;271:1363–1370. doi: 10.1098/rspb.2004.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton I. Population Limitation in Birds. London: Academic; 1998. [Google Scholar]
- 24.Sokal RR, Rohlf FJ. Biometry. New York: Freeman; 1995. [Google Scholar]
- 25.Miles DB, Ricklefs RE. The correlation between ecology and morphology in deciduous forest passerine birds. Ecology. 1984;65:1629–1640. [Google Scholar]
- 26.Ricklefs RE. Cladogenesis and morphological diversification in passerine birds. Nature. 2004;430:338–341. doi: 10.1038/nature02700. [DOI] [PubMed] [Google Scholar]
- 27.Brown JH. Macroecology. Chicago: Univ of Chicago Press; 1995. [Google Scholar]
- 28.Bock CE, Ricklefs RE. Range size and local abundance of some North American songbirds: A positive correlation. Am Nat. 1983;122:295–299. [Google Scholar]
- 29.Ricklefs RE. Speciation, extinction, and diversity. In: Butlin R, Bridle J, Schluter D, editors. Speciation and Patterns of Diversity. Cambridge, UK: Cambridge Univ Press; 2009. pp. 257–277. [Google Scholar]
- 30.Webb TJ, Gaston KJ. Geographic range size and evolutionary age in birds. Proc Biol Sci. 2000;267:1843–1850. doi: 10.1098/rspb.2000.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb TJ, Gaston KJ. On the heritability of geographic range sizes. Am Nat. 2003;161:553–566. doi: 10.1086/368296. [DOI] [PubMed] [Google Scholar]
- 32.MacArthur RH, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am Nat. 1967;101:377–385. [Google Scholar]
- 33.Tilman D. Resource Competition and Community Structure. Princeton: Princeton Univ Press; 1982. [PubMed] [Google Scholar]
- 34.Cody ML. Competition and the Structure of Bird Communities. Princeton: Princeton Univ Press; 1974. [PubMed] [Google Scholar]
- 35.Chave J. Neutral theory and community ecology. Ecol Lett. 2004;7:241–253. [Google Scholar]
- 36.Holt RD. Emergent neutrality. Trends Ecol Evol. 2006;21:531–533. doi: 10.1016/j.tree.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 37.MacArthur RH. Geographical Ecology. Patterns in the Distribution of Species. New York: Harper & Row; 1972. [Google Scholar]
- 38.Connell JH. On the prevalence and relative importance of interspecific competition: Evidence from field experiments. Am Nat. 1983;122:661–696. [Google Scholar]
- 39.Gurevitch J, Morrow LL, Wallance A, Walsh JS. A meta-analysis of competition in field experiments. Am Nat. 1992;140:539–572. [Google Scholar]
- 40.Schoener TW. Field experiments on interspecific competition. Am Nat. 1983;122:240–285. [Google Scholar]
- 41.Pianka ER. The structure of lizard communities. Annu Rev Ecol Syst. 1973;4:53–74. [Google Scholar]
- 42.Vitt LJ, Sartorius SS, Avila-Pires TCS, Esposito MC, Miles DB. Niche segregation among sympatric Amazonian teiid lizards. Oecologia. 2000;122:410–420. doi: 10.1007/s004420050047. [DOI] [PubMed] [Google Scholar]
- 43.Clark JS. Individuals and the variation needed for high species diversity in forest trees. Science. 2010;327:1129–1132. doi: 10.1126/science.1183506. [DOI] [PubMed] [Google Scholar]
- 44.Clark JS, et al. High-dimensional coexistence based on individual variation: A synthesis of evidence. Ecol Monogr. 2010;80:569–608. [Google Scholar]
- 45.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 46.Arneberg P. Host population density and body mass as determinants of species richness in parasite communities: Comparative analyses of directly transmitted nematodes of mammals. Ecography. 2002;25:88–94. [Google Scholar]
- 47.Begon M, et al. Transmission dynamics of a zoonotic pathogen within and between wildlife host species. Proc Biol Sci. 1999;266:1939–1945. doi: 10.1098/rspb.1999.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. 2001;292:1109–1112. doi: 10.1126/science.1059026. [DOI] [PubMed] [Google Scholar]
- 49.Barraclough TG, Vogler AP. Detecting the geographical pattern of speciation from species-level phylogenies. Am Nat. 2000;155:419–434. doi: 10.1086/303332. [DOI] [PubMed] [Google Scholar]
- 50.Lovette IJ, Hochachka WM. Simultaneous effects of phylogenetic niche conservatism and competition on avian community structure. Ecology. 2006;87(Suppl 7):S14–S28. doi: 10.1890/0012-9658(2006)87[14:seopnc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Leigh EG., Jr The average lifetime of a population in a varying environment. J Theor Biol. 1981;90:213–239. doi: 10.1016/0022-5193(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 52.Ricklefs RE. A comment on Hubbell's zero-sum ecological drift model. Oikos. 2003;100:185–192. [Google Scholar]
- 53.Ricklefs RE. The unified neutral theory of biodiversity: Do the numbers add up? Ecology. 2006;87:1424–1431. doi: 10.1890/0012-9658(2006)87[1424:tuntob]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Rabosky DL. Heritability of extinction rates links diversification patterns in molecular phylogenies and fossils. Syst Biol. 2009;58:629–640. doi: 10.1093/sysbio/syp069. [DOI] [PubMed] [Google Scholar]
- 55.Weir JT, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1574–1576. doi: 10.1126/science.1135590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.