Abstract
Improved protein-based vaccines should facilitate the goal of effective vaccines against HIV and other pathogens. With respect to T cells, the efficiency of immunization, or “immunogenicity,” is improved by targeting vaccine proteins to maturing dendritic cells (DCs) within mAbs to DC receptors. Here, we compared the capacity of Langerin/CD207, DEC205/CD205, and Clec9A receptors, each expressed on the CD8+ DC subset in mice, to bring about immunization of microbial-specific T cells from the polyclonal repertoire, using HIV gag-p24 protein as an antigen. α-Langerin mAb targeted splenic CD8+ DCs selectively in vivo, whereas α-DEC205 and α-Clec9A mAbs targeted additional cell types. When the mAb heavy chains were engineered to express gag-p24, the α-Langerin, α-DEC205, and α-Clec9A fusion mAbs given along with a maturation stimulus induced comparable levels of gag-specific T helper 1 (Th1) and CD8+ T cells in BALB/c × C57BL/6 F1 mice. These immune T cells were more numerous than targeting the CD8− DC subset with α-DCIR2-gag-p24. In an in vivo assay in which gag-primed T cells were used to report the early stages of T-cell responses, α-Langerin, α-DEC205, and α-Clec9A also mediated cross-presentation to primed CD8+ T cells if, in parallel to antigen uptake, the DCs were stimulated with α-CD40. α-Langerin, α-DEC205, and α-Clec9A targeting greatly enhanced T-cell immunization relative to nonbinding control mAb or nontargeted HIV gag-p24 protein. Therefore, when the appropriate subset of DCs is targeted with a vaccine protein, several different receptors expressed by that subset are able to initiate combined Th1 and CD8+ immunity.
Keywords: antigen presentation, C-type lectins, cross-priming
A major goal in the development of effective vaccines against pathogens such as HIV and malaria is the induction of durable and protective T-cell immunity. Attenuated viral vectors are being emphasized widely as a vaccine platform to elicit T-cell immunity in humans (1). Attenuated vectors have potential limitations with respect to immunogenicity and repeated use, however (2). Protein vaccines could provide a stand-alone or complementary platform (e.g., to viral vectors), with relative ease of production and ability to be repeatedly injected. Proteins are poorly immunogenic for T cells, however, even when administered repeatedly in high doses.
Recent progress in immunobiology provides the potential to overcome this obstacle. The immunogenicity of proteins can be greatly enhanced by improving the delivery of protein to dendritic cells (DCs). To do this, one approach is to introduce the protein into mAbs that efficiently and specifically target to DC receptors in situ, within lymphoid tissues, and then to coadminister the fusion antibody with an appropriate agonist for DC maturation [reviewed in (3, 4)]. Delivery of vaccine proteins within mAbs increases the efficiency of antigen presentation on MHC class I and II molecules ∼100-fold and allows protein vaccines to induce T helper 1 (Th1) type CD4+ T cells and CD8+ T cells (5–8).
The DC system is composed of different subsets with distinct cell surface markers and biological functions (9–11). Three of the major DC subsets in mouse secondary lymphoid organs are as follows: (i) CD11chighCD8− DCs, (ii) CD11chighCD8+ DCs, and (iii) CD11cintCD45RA+ Gr1+ plasmacytoid DCs (PDCs) (12). For example, when CD8− DCs take up antigen, they more efficiently form MHC II complexes (13) and elicit T cells producing IFN-γ and IL-4 (14, 15). By contrast, CD8+ DCs are the major producer of IL-12 on activation (16, 17) and promote Th1 responses. These DCs also are specialized to capture dying cells (18, 19) and to cross-present antigens on MHC class I (20–24). The ability of CD8+ DCs to initiate combined Th1 and cytotoxic responses in vivo has made them a most logical target for vaccine development, including protein vaccines.
To date, the uptake receptors expressed by CD8+ DCs include several molecules with external C-type lectin domains (25), such as DEC205/CD205, Langerin/CD207, and Clec9A (26–30). There is little information comparing the immunogenicity of proteins targeted to the same subset of DCs using different α-receptor mAbs, however. Previously, we have compared the presentation of ovalbumin (OVA) to T-cell receptor (TCR) transgenic T cells using α-DEC205 and α-Langerin mAbs for CD8+ DCs and α-DCIR2 mAb for CD8− DCs (31). Here, we have assessed the capacity of different receptors on the CD8+ DC subset to bring about the priming of scarce microbial-specific T cells in the polyclonal repertoire, using the HIV protein gag-p24 as an antigen, and compared these lectins with gag targeted to the DCIR2 lectin on splenic CD8− DCs. We show that α-Langerin targets the CD8+ DC subset selectively but that α-Langerin, α-DEC205, and α-Clec9A mAb are each effective in inducing gag-specific Th1 and CD8+ T-cell responses. α-DCIR2 targeting was less effective at priming such responses from a polyclonal T-cell repertoire, however. Our findings indicate that uptake of proteins through different receptors on CD8+ DCs has similar potential for inducing or priming Th1 and CD8+ immunity.
Results
α-Langerin Selectively Targets CD8+ DCs in Spleen, Whereas α-DEC205 and α-Clec9A Label CD8+ DCs and Some Other Leukocytes.
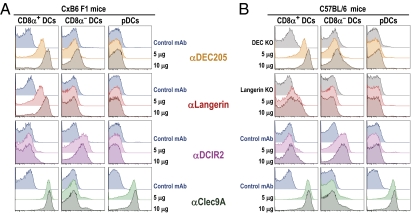
To assess specific targeting of α-receptor mAbs to DCs in vivo, 10 or 5 μg of Alexa 647-labeled α-Langerin, α-DEC205, α-Clec9A, α-DCIR2, or control mAb was injected i.p. into C57BL/6 or C×B6 F1 mice. The percentage of splenic DCs containing Alexa 647 3 h after administration was subsequently examined by FACS (Fig. 1, using the gating strategy in Fig. S1). In both strains of mice, the injected α-DEC205 mAb labeled most of the CD8+ DCs, but no labeling was observed in DEC205 B6 KO mice. In addition to CD8+ DCs, α-DEC205 bound to other leukocyte populations (Fig. S2), which corresponds to the observed expression of DEC205 in these cells (32), although the labeling of CD8+ DCs (mean fluorescence intensity) was threefold higher on CD8+ DCs relative to granulocytes and greater than threefold relative to other types of leukocytes (Fig. 1 and Fig. S2). α-Clec9A targeted to CD8+ DCs and PDCs but not to other leukocytes (Fig. 1 and Fig. S2). In contrast to α-DEC205 or α-Clec9A, α-Langerin targeted only to CD8+ DCs in the spleens of C×B6 F1 mice (Fig. 1A) and not to PDCs or other cells, including B cells, T cells, natural killer (NK) cells, monocytes, macrophages, and granulocytes (Fig. S2 A and B), consistent with prior data that Langerin is a specific marker for CD8+ DCs (27). Only 25% of CD8+ DCs in B6 mice were targeted with α-Langerin mAb (Fig. 1B), however, which is consistent with data showing that Langerin is only expressed at low levels in CD8+ DCs in B6 mice (33, 34). Labeling of CD8+ DCs was abrogated in Langerin B6 KO mice. In both B6 and C×B6 F1 strains, α-DCIR2 selectively stained CD8− DCs and nontargeted control mAb did not label any leukocyte (Fig. 1 and Fig. S2). These results indicate that i.p. injection of α-Langerin mAbs targets CD8+ DCs selectively in vivo, and in C×B6 F1 mice, it labels this subset to a magnitude comparable to that of α-DEC205 mAbs and α-Clec9A.
Fig. 1.
α-Langerin, α-DEC205, and α-Clec9A are captured by CD8+ DCs in vivo, whereas α-DCIR2 is taken up by CD8− DCs. C×B6 F1 mice (A) or C57BL/6 mice (B) were inoculated i.p. with 5 or 10 μg of each Alexa 647-labeled α-Langerin, α-DEC205, α-Clec9A, and α-DCIR2 mAb. GL117 mAb labeled with Alexa 647 (blue histograms) was used as a control mAb. Data are plotted with the higher dose of GL117 because we did not see differences between inoculation of 5 or 10 μg. When available, we also added KO mice in the C57BL/6 background as an additional control (DEC205 KO and Langerin KO mice, gray histograms). Uptake of labeled mAb by splenocytes was evaluated 3 h after inoculation by multicolor flow cytometry (Fig. S1) in CD11chighCD8α+ DCs (Fig. S1, population C), CD8α− DCs (Fig. S1, population B), and PDCA-1+ PDCs (Fig. S1, population D). One experiment representative of two to three with similar results is shown.
α-Langerin-, α-DEC205-, and α-Clec9A-gag-p24 Inoculated in the Presence of α-CD40 and Induces Comparable Th1 and CD8+ T-Cell Responses in C×B6 F1 Mice.
To examine the capacity of different receptors to immunize scarce microbial-specific T cells in the polyclonal repertoire, as contrasted with a prior study on presentation to OVA-specific TCR transgenic T cells using α-Langerin, α-DEC205, and α-DCIR2 (31), we genetically engineered mAbs against Langerin (L31) (33), DCIR2 (33D1) (35), DEC205 (NLDC145) (36, 37), and a nonreactive control Ig (GL117) (38) to express HIV gag-p24. We also cloned and engineered an antibody against the more recently described receptor Clec9A to express HIV gag-p24 (10B4) (28). In each case, the fusion H chain had a molecular mass of ∼75 kDa (Fig. S3A, Left), as opposed to ∼50 kDa for unmodified mouse IgG1, and it also reacted with α-mouse Ig and α-p24 mAbs by Western blotting (Fig. S3A, Right). In contrast to control Ig-gag-p24, these fusion mAbs bound to CHO cells expressing the respective receptor (Fig. S3B, red histograms) but not to untransfected CHO (Fig. S3B, blue histograms, CHO neo). Thus, each mAb to four endocytic receptors can be successfully engineered to express HIV gag-p24 as an antigen.
To compare T-cell responses induced by HIV gag-p24 targeted within different α-receptor antibodies, we immunized C×B6 F1 mice with 5 μg of α-Langerin-, α-DEC205-, α-Clec9A-, α-DCIR2-, and control Ig-gag-p24 mAbs along with 25 μg of α-CD40 mAb and 50 μg of polyriboinosinic:polyribocytidylic acid (poly IC) as a maturation stimulus. C×B6 F1 mice were chosen for this experiment for several reasons. First, broad CD4+ T-cell responses directed to at least three different defined CD4+ T-cell peptide mimetopes are achieved by combining H-2d and H-2b haplotypes in F1 mice (6). Second, the CD8+ T-cell response is directed to a defined gag 197–205 peptide presented only on H-2d (39) but not on H-2b. Finally, only in C×B6 F1 but not in C57BL/6 mice were all CD8+ DCs similarly targeted with α-Langerin, α-DEC205, and α-Clec9A mAbs (Fig. 1).
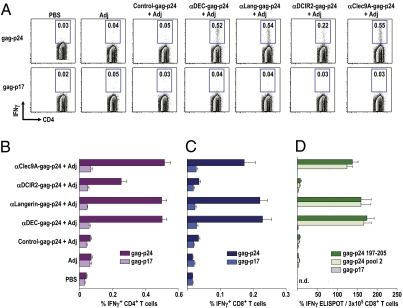
Two weeks after immunization, antigen-specific responses were evaluated by IFN-γ production in response to HIV gag-p24 peptides by multicolor flow cytometry. α-Langerin-, α-DEC205-, and α-Clec9A-gag-p24 induced significantly stronger gag-specific, IFN-γ–producing, CD4+ T-cell responses compared with α-DCIR2-gag-p24 (Fig. 2 A and B; P < 0.01, data not shown) with frequencies of >0.5% of IFN-γ–producing CD3+CD4+ T cells after a single i.p. injection. No CD4+ T-cell immunity was observed following a single immunization with control Ig-gag-p24 and when fusion antibodies were given in the absence of an adjuvant, as shown previously (6). When we performed similar experiments in C57BL/6 mice, α-DEC205-gag-p24 led to higher CD4+ T-cell responses than α-Langerin-gag-p24 (Fig. S4 A and B), possibly because Langerin was less expressed on CD8+ DCs in C57BL/6 mice (33, 34). Fewer responding CD4+ T cells were seen with one dose of α-DCIR2-gag-p24 or nontargeted HIV gag-p24 protein given along with α-CD40 and poly IC.
Fig. 2.
α-Langerin, α-DEC205, and α-Clec9A induce comparable Th1 and CD8+ T-cell responses in C×B6 F1 mice. (A) C×B6 F1 mice were immunized with 5 μg of α-receptor fusion mAbs in the presence of 50 μg of poly IC and 25 μg of α-CD40 [adjuvant (Adj)]. Fourteen days later, splenocytes were restimulated in vitro with HIV gag-p24 or HIV gag-p17 (negative control) peptide mix in the presence of Brefeldin A for 6 h. Intracellular staining was performed to detect IFN-γ in CD3+CD4+ T cells. (B) As in A, but % of IFN-γ+ CD4+ T cells is shown as mean ± SD of four to six experiments with two to three mice per group. (C) As in A, but this time, the % of IFN-γ+ CD8+ T cells is shown as mean ± SD of four to six experiments with two to three mice per group. (D) As in A, but after 14 d, purified splenic CD8+ T cells were restimulated with CD11c+ bead-purified DCs and CD8 nanomer (gag-p24 197-205), p24 reactive pool 2, or p17 nonreactive peptide mix. IFN-γ production was evaluated by ELISPOT. Data are representative of three to four similar experiments with three mice pooled in each experiment. n.d., not determined.
When we measured CD8+ T-cell responses in C×B6 F1 mice by intracellular cytokine staining, we found that a single vaccination of α-Langerin-, α-DEC205-, and α-Clec9A-gag-p24 mAb plus α-CD40/poly IC induced comparable significant numbers of IFN-γ–producing CD8+ T cells, whereas α-DCIR2-gag-p24 did not cross-prime (Fig. 2C; P < 0.01, data not shown). An enzyme-linked immunospot (ELISPOT) assay revealed that the CD8+ T-cell response was directed to a peptide in p24 pool 2, which contained a previously defined gag 197–205 peptide sequence presented on H-2Kd (39) (Fig. 2D). We conclude that a single immunization of HIV gag-p24 targeted within 5 μg of α-Langerin, α-DEC205, and α-Clec9A fusion mAb induced comparable Th1 and CD8+ T-cell responses, which were greater relative to DCIR2 targeting.
HIV gag-p24 Targeted in α-Langerin, α-DEC205, and α-Clec9A mAbs Requires an Adjuvant for in Vivo Cross-Presentation to Primed gag-Specific CD8+ T Cells.
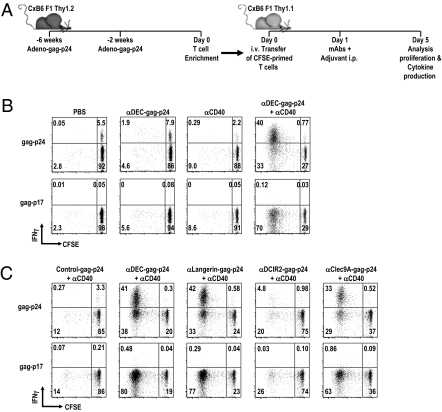
To assess the early proliferative response to HIV gag protein following targeting to Langerin, DEC205, and Clec9A in vivo, we used polyclonal memory T cells from C×B6 F1 mice that had been immunized twice with adenovirus expressing HIV gag (Fig. 3A). As shown previously (6), adenovirus-gag induced high frequencies of gag-specific CD8+ T cells (∼5%) but low frequencies of gag-specific CD4+ T cells (∼0.3%). In initial experiments, gag-specific splenic T cells were enriched from C×B6 F1 Thy 1.2+ mice, labeled with carboxylfluorescein succinimidlylester (CFSE), and adoptively transferred into C×B6 F1 Thy 1.1+ recipients (Fig. 3A). One day later, the mice were injected with either PBS, α-DEC205-gag-p24 mAb alone, α-CD40 alone, or a combination of α-DEC205-gag-p24 mAb plus α-CD40 (Fig. 3 A and B), where prior data had shown that α-CD40 allows DC-targeted antigens to elicit immunity (38). Cross-presentation was evaluated in the spleen 4 d later by T-cell proliferation and IFN-γ production of gag p24-specific CD8+ T cells in a CFSE dilution and intracellular cytokine assay (Fig. 3 A and B). The gag-specific CD8+ T cells proliferated and produced IFN-γ on restimulation with gag-p24 peptide mix only when mice were coinoculated with α-DEC-gag-p24 plus α-CD40, whereas no proliferative or IFN-γ responses were induced to either α-DEC-gag-p24 alone, α-CD40 alone, or medium. Thus, DEC205 mediated efficient cross-presentation of HIV gag-p24 in vivo but only when α-CD40 was given along with the fusion mAb. We also tested CD40−/− mice to show that CD40 expression on recipient but not donor cells was essential for DEC-mediated cross-presentation of HIV gag in vivo (Fig. S5A). In addition, α-DEC-gag-p24 plus α-CD40 induced responses by adenovirus-gag–primed CD8+ T cells but not by adenovirus-OVA–specific CD8+ T cells, indicating that proliferation and IFN-γ production were gag-specific (Fig. S5B). These in vivo findings complement prior studies showing that targeting to DEC205 enhanced cross-presentation of HIV gag to antigen-primed human and mouse CD8+ T cells in vitro (7, 8).
Fig. 3.
α-Langerin-, α-DEC205-, and α-Clec9A–targeted HIV gag-p24 requires α-CD40 for in vivo cross-presentation by DCs to primed gag-specific CD8+ T cells. (A) Schematic for the design of the adoptive transfer experiment (Materials and Methods). (B) Thy 1.1 mice transferred 1 d before with CFSE-labeled Thy 1.2+ adenovirus-primed T cells were challenged with 25 μg of α-CD40 alone, 5 μg of α-DEC-gag-p24 alone, medium, or a combination of 5 μg of α-DEC-gag-p24 and 25 μg of α-CD40. Four days after challenge, IFN-γ production and proliferation of transferred Thy 1.2+ CD3+CD8+ T cells were assessed in a 6-h in vitro restimulation assay. (C) As in B, but adoptively transferred, gag-primed, enriched T cells were challenged with 25 μg of α-CD40 in combination with 5 μg of α-DEC205-, α-Langerin-, α-DCIR2-, α-Clec9A-, or control Ig-gag-p24 mAbs. In all cases, one experiment of two with similar results is shown.
With these results, we proceeded to compare the capacity of several DC uptake receptors to mediate cross-presentation of HIV gag in vivo. Specifically, C×B6 F1 Thy 1.1+ mice were injected with 5 μg of α-Langerin-, α-DEC205-, α-Clec9A-, α-DCIR2-, and control Ig-gag-p24 fusion mAb along with α-CD40 1 d after inoculating CFSE-labeled gag-specific enriched T cells. When we analyzed the proliferation and IFN-γ secretion by gag-primed CD8+ T cells 4 d later in splenocytes restimulated for 6 h in the presence or absence of reactive gag-peptide mix, we found that α-Langerin-, α-DEC205-, and α-Clec9A-gag-p24 induced proliferation and IFN-γ production by gag-specific CD8+ T cells but that α-DCIR2- or control Ig-gag-p24 did not (Fig. 3C). In contrast, all antireceptor mAbs, including α-DCIR2-gag p24, were able to expand adenovirus-gag–primed gag-specific CD4+ T cells (Fig. S5C). Similar results were found when 1 vs. 5 μg of receptor mAbs was given along with α-CD40. We conclude from these experiments that α-Langerin, α-DEC205, and α-Clec9A mAbs are comparable in their capacity to mediate cross-presentation of HIV gag in vivo by DCs and that cross-presentation of antigen to polyclonal primed T cells only occurs if the DCs undergo maturation in parallel to antigen uptake.
Enhanced gag-Specific CD4+ T-Cell Immunity Following Prime/Boost Immunization Using Poly IC or Poly ICLC as the Only Adjuvant.
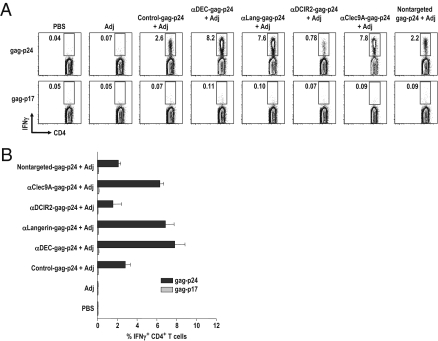
To test the efficacy of α-receptor antibodies to induce antigen-specific CD4+ T cells in a prime-boost regimen, we injected C57BL/6 mice twice i.p. over a 4-wk period with 5 μg of α-Langerin-, α-DEC205-, α-Clec9A-, α-DCIR2, or control Ig-gag-p24 (which contain ∼1.5 μg of gag-p24 protein) or with 5 μg of nontargeted HIV gag-p24 protein along with 50 μg of poly IC or poly ICLC as an adjuvant. The latter is poly IC formulated together with poly l-lysine and carboxymethylcellulose. Poly IC and poly ICLC are synthetic dsRNAs that are proving to be superior adjuvants for T-cell immune responses (40, 41). Because both poly IC and poly ICLC were comparable in the response induced after prime-boost, they were used interchangeably. Previously, we reported that two immunizations with α-DEC205-gag-p24 and 50 μg of poly IC induced higher frequencies of cytokine-producing CD4+ T cells than a single immunization of α-DEC205-gag-p24 plus 50 μg of poly IC or two doses of α-DEC205-gag-p24 plus 10 μg of poly IC (42). Two weeks after the boost, the frequency of gag p24-specific CD4+ T cells producing IFN-γ, IL-2, or TNF-α was greatest with two doses of α-Langerin, α-DEC205, and α-Clec9A and poly IC or poly ICLC (Fig. 4 A and B and Fig. S6 A and B). In contrast, fewer IFN-γ–producing CD4+ T cells were seen with two doses of α-DCIR2- or control Ig-gag-p24 or with soluble HIV gag-p24 protein plus poly IC or poly ICLC. We did not find significant differences between α-Langerin-, α-DEC205-, and α-Clec9A-gag-p24; however, targeting any of these lectins showed significant differences from either control Ig-, α-DCIR2-, or nontargeted gag-p24 protein (P < 0.01, data not shown). When we tested a lower concentration of protein vaccine (i.e., 0.5 μg of fusion mAb or nontargeted p24 protein given with poly IC or poly ICLC), we identified IFN-γ–producing CD4+ T cells only in mice immunized with two doses of α-DEC205-gag-p24 mAb and poly IC or poly ICLC (Fig. S6C). We conclude that targeting vaccine proteins with 5 μg of α-Langerin, α-DEC205, or α-Clec9A mAb is more effective at initiating IFN-γ–producing CD4+ T-cell immunity than using nontargeted gag-p24 protein or α-DCIR2- and control Ig-gag-p24.
Fig. 4.
Enhanced gag-specific immunity by prime/boost immunization. (A) C57BL/6 mice were primed and boosted 4 wk apart with 5 μg of α-receptor mAbs or nontargeted gag-p24 protein in the presence of 50 μg of poly IC or poly ICLC as an adjuvant (Adj). IFN-γ production was evaluated by intracellular cytokine staining 2 wk after boost in the CD3+CD4+ gated cells in response to reactive HIV gag-p24 or nonreactive HIV gag-p17 peptide mix. (B) As in A, but mean ± SD from three to five experiments with three to four mice per group are shown.
Discussion
The development of protein-based vaccines will benefit from identifying principles that lead to cross-presentation of antigens on MHC I molecules and cross-priming of CD8+ T cells. Prior studies primarily examining the responses of OVA-specific T cells in C57BL/6 mice, often with TCR transgenic T cells as reporters, have shown that the CD8+ subset of DCs is particularly effective at cross-presentation. In this study, we addressed the logical need to compare different surface receptors on CD8+ DCs for their capacity to improve antigen uptake and presentation in vivo, particularly cross-presentation and cross-priming.
OVA has been a valuable tool for research because of its high sensitivity [e.g., responses are observed with just 1 pM SIINFEKL peptide in vitro (43)]. Here, we focused on the more representative protein, HIV gag-p24, where it is known that targeting to DEC205+ DCs leads to strong Th1 immunity in mice (8, 42). HIV gag is likely to be an important antigen for eliciting T-cell immunity with an HIV vaccine [e.g., when this protein is the only HIV antigen in a vaccine, significant protection is induced in the simian immunodeficiency virus rhesus macaque model of disease (44)]. Using HIV gag-p24 targeted within mAbs to different C-type lectins on CD8+ DCs, we found that targeting of any one of three receptors—Langerin/CD207, DEC205/CD205, and Clec9A/DNGR1—led to similar levels of priming of CD4+ and CD8+ T cells. Targeting of DCIR2 on another CD8− DC subset failed to cross-prime and induced weaker CD4+ IFN-γ–secreting T-cell responses. Consistent with previous reports (5, 29, 42), we used an adjuvant, α-CD40 plus poly IC, to elicit T-cell immunity with these α-receptor mAbs. Our findings are at this time limited to the protocol we used in a large number of animals and experiments [i.e., we used one dose (5 μg) of fusion mAb and one type of adjuvant, and we evaluated the acute response to priming and boosting by cytokine-producing T cells]. Further research is needed to assess the capacity of different DC uptake receptors to induce memory T-cell responses as well as acute and memory B-cell responses. Nonetheless, our results indicate that several receptors allow CD8+ DCs to exert their capacity to bring about combined CD8+ and CD4+ T-cell immunity.
When we used sensitive FACS analyses to compare the targeting of each of the mAbs to the major types of leukocytes in spleen, we found that α-DEC205 was picked up in large amounts by CD8+ DCs but also in lower amounts by T cells, granulocytes, NK cells, and B cells. For α-Clec9A, uptake was observed in both CD8+ DCs and PDCs. Only α-Langerin was fully selective for targeting CD8+ DCs in spleen. These mAb targeting results replicate prior data on the expression of these lectins by DCs (27–29, 32, 34). DEC205 and Langerin are also expressed by skin dermal DCs and Langerhans cells (45). Because immunization of CD8+ T cells was similar when gag-p24 was delivered in three mAbs to distinct lectins on the CD8+ DC subset, our findings imply that the subset of DCs and not the receptor-capturing antigen is a dominant force in bringing about the measured T-cell priming following antigen uptake and that the CD8+ subset and not other DCs or other leukocytes that were targeted by α-DEC205 acts as the dominant cell for cross-priming in vivo when α-CD40 or poly ICLC is used as an adjuvant.
We also noted that the targeting of each lectin on CD8+ DCs led to Th1 type CD4+ T-cell immunity, whereas targeting via DCIR2 on CD8− DCs was less efficient at inducing IFN-γ. At this time, it is not feasible to target other lectins selectively on CD8− DCs. Nevertheless, our data lead to the conclusion that the induction of combined Th1 and CD8+ T-cell immunity to a protein is primarily determined by the subset of DCs rather than the particular receptor for antigen uptake, with additional input from the adjuvant used.
Materials and Methods
Mice.
We purchased C57BL/6J (B6) and BALB/CJ mice from the The Jackson Laboratory and BALB/c × C57BL/6 (C×B6) F1 Thy 1.2+ mice from Harlan. CD40−/− BALB/c mice were from The Jackson Laboratory. C×B6 F1 Thy 1.1+ mice were obtained by breeding B6 Thy 1.1+ male mice (The Jackson Laboratory) with BALB/c Thy 1.1+ female mice. DEC205 KO mice were obtained from M. Nussenzweig (The Rockefeller University, New York). Langerin diphtheria toxin receptor (DTR) was kindly provided by one of the authors (B.E.C.), and mice were bred in-house as homozygotes [Langerin DTR+/+ mice do not express Langerin, becoming Langerin KO mice (46)]. Mice were maintained under specific pathogen-free conditions and used at 6–8 wk of age in accordance with The Rockefeller University Animal Care and Use Committee guidelines.
Reagents.
All fluorochrome-labeled antibodies are listed in Table S1. mAbs to Langerin (L31) (33), DCIR2 (33D1) (35), DEC205 (NLDC145) (36, 37), Clec9A (10B4) (28), control Ig (GL117) (38), and CD40 (IC10) were produced from hybridoma supernatants; purified on protein G (Pierce); and, when necessary, labeled with Alexa 647 (Invitrogen) per the manufacturer's instructions. Anti-CD11c beads (N418), anti-CD8 beads (Ly-2), and anti-CD19 beads were from Miltenyi Biotec. Other reagents were Live/Dead Fixable Aqua or Violet vitality dye (Invitrogen) and DAPI (Sigma).
Peptides.
Overlapping (staggered by four amino acids) 15-mer peptides spanning the entire HIV gag-p17 or -p24 sequence were synthesized by H. Zebroski in the Proteomics Resource Center of The Rockefeller University. HIV gag-p17 or -p24 peptides were resuspended at 3 mg/mL per peptide in DMSO and added to immune assays at a rate of 1 μg/mL.
Fusion HIV gag-p24 mAbs.
DNA for HIV gag-p24 (aa 133–363 derived from HIV isolate BH10) was cloned in frame into the COOH terminus of the heavy chains of α-mouse-DEC205, -Langerin, -DCIR2, -Clec9A, and -control Ig, as previously described (6). Fusion mAbs against DEC205, Langerin, DCIR2, and control IgG were expressed by transient transfection (calcium phosphate) in 293T cells in serum-free DMEM supplemented with Nutridoma SP (Roche Applied Science). Fusion mAbs for α-Clec9A-gag-p24 were produced by transient transfection in Freestyle 293F cells (Invitrogen) using the Freestyle 293 Expression System (Invitrogen). The mAbs were purified on protein G columns (GE Healthcare Bio-Sciences Corp.) and characterized by SDS/PAGE and Western blotting using α-mouse IgG1-HRP (Southern Biotech) or HRP-α-gag-p24 (ImmunoDiagnostics). mAb binding was verified on CHO cells stably transfected with the respective receptor by FACS using phycoerythrin-conjugated goat α-mouse IgG (Jackson ImmunoResearch).
Soluble gag-p24 Protein.
Soluble FLAG gag-p24 protein (8) was expressed by CHO cells and purified from culture supernatant using anti-FLAG M1 Affinity Gel (Sigma–Aldrich) following the manufacturer's instructions.
Targeting of Alexa 647-Labeled mAbs.
C57BL/6 or C×B6 F1 mice were injected i.p. with 10 or 5 μg of α-receptor or control mAbs labeled with Alexa 647 for 3 h.
Immunizations.
Mice were injected i.p. once with fusion mAbs or HIV gag-p24 protein with a stimulus for DC maturation, which was 50 μg of poly IC (InVivoGen) together with 25 μg of IC10 agonistic α-CD40 mAb (47). In other experiments, we used a prime boost regimen in which α-CD40 was omitted and 50 μg of poly IC (Thermo Scientific) or poly ICLC (Oncovir, Inc.) was the adjuvant for both prime and boost, which were given 1 mo apart. To assess cross-presentation of HIV gag-p24 in vivo, mice were immunized twice i.m. with 107 pfu of adenovirus expressing HIV gag-p24 or OVA.
Intracellular Cytokine Staining.
Bulk splenocytes were either restimulated with the entire reactive HIV gag-p24 or nonreactive HIV gag-p17 15-mer peptide mix (peptides at 1 μg/mL) in the presence of 2 μg/mL costimulatory α-CD28 (clone 37.51; American Type Culture Collection) for 6 h at 37 °C, adding Brefeldin A (10 μg/mL; Sigma–Aldrich) for the last 5 h to allow accumulation of intracellular cytokines. Cells were washed, incubated for 10 min at 4 °C with 2.4G2 mAb to block Fcγ receptors, and stained with mAbs against surface molecules for 20 min at 4 °C. Cells were then fixed, permeabilized (BD Pharmingen), and stained with mAbs against cytokines. A total of 1–3 × 105 live-CD3+ cells were acquired on a Becton Dickinson (BD) LSR II flow cytometer, and data were analyzed with FlowJo Software (Tree Star, Inc.).
ELISPOT.
ELISPOT Multiscreen-HA MAHA 54510 (Millipore) plates were coated with 10 μg of purified α-mouse IFN-γ mAb (clone R4-6A2; BD Biosciences) overnight at 4 °C and subsequently blocked by incubation with 1% PBS-BSA for at least 1 h at 37 °C. A total of 3 × 105 bead-purified CD8+ T cells were cultured for 2 d with 1 × 105 CD11c+ spleen DCs in the presence of 1 μg/mL previously defined H-2Kd restricted gag peptide (AMQMLKETI, p24 197–205), a 15-mer peptide mix containing the gag-p24 197–205 epitope (gag-p24 reactive pool 2), or gag-p17 nonreactive peptide mix. Plates were developed with α-IFN-γ biotinylated mAb (BD Biosciences), and spots were visualized with avidin-HRP (Vector Laboratories), followed by diaminobenzidine as a substrate (Invitrogen). Spots were counted in an ELISPOT reader (Autoimmun Diagnostika GmBH).
Leukocyte Preparation.
Spleens were harvested and digested for 25 min at 37 °C in Hanks’ buffer (Gibco) containing 400 units/mL Collagenase D (Roche) and 50 μg/mL DNaseI (Roche). A total of 5 mM EDTA (Gibco) was added for the last 5 min. For analysis of different leukocytes in the spleen, cells were selected with α-CD19 magnetic beads (Miltenyi Biotec), and for enrichment of DCs, splenocytes were incubated with α-CD11c beads (Miltenyi Biotec) following manufacturer's instructions.
Rapid Assay to Evaluate Cross-Presentation of HIV gag-p24 in Vivo.
To evaluate cross-presentation of HIV gag-p24 by DCs in vivo, antigen-primed T cells isolated from mice that had been primed and boosted 4 wk apart with adenovirus-gag or adenovirus-OVA were enriched from spleen cell suspensions by excluding MHC class II+ cells, macrophages, and B cells using a combination of α-MHCII (TIB120), α-B220, and α-F4/80 rat mAb and α-rat IgG Dynabeads (Invitrogen). T cells were subsequently labeled at 107 cells/mL with 5 μM CFSE (Invitrogen/Molecular Probes) for 10 min at 37 °C and adoptively transferred into Thy 1.1+ recipient mice (one spleen equivalent was transferred per mouse). One day later, targeting mAbs were injected i.p. in the presence or absence of a maturation stimulus, which was 25 μg of agonistic α-CD40 IC10 mAb. Four days later, the transferred T cells were evaluated for proliferation by CFSE dilution and IFN-γ production after surface staining for Thy 1.2 (transferred T cells), CD3, CD4, and CD8 and intracellular cytokine staining for IFN-γ as described above.
Statistical Analysis.
Data reported in the figures represent the average of at least three independent experiments. Error bars represent the SD. Data were analyzed and charts were generated using Prism 5 (GraphPad Software). Statistical significance was determined by an unpaired t test.
Supplementary Material
Acknowledgments
We thank J. Adams for graphics, M. Nulty for help with the manuscript, and L. Zbytnuik for technical support. Funding was provided by National Institutes of Health Grants AI40045, AI13013, and P01AI081677 and by Collaboration for AIDS Vaccine Discovery–HIV/AIDS Vaccine Enterprise Grant GH-HTR-05 (to R.M.S.). B.E.C. is a Fellow of the Netherlands Organization for Scientific Research (VIDI 917-76-365).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019547108/-/DCSupplemental.
References
- 1.Plotkin SA. Vaccines: The fourth century. Clin Vaccine Immunol. 2009;16:1709–1719. doi: 10.1128/CVI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumida SM, et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 4.Boscardin SB, Trumpfheller C, Nussenzweig MC, Steinman RM. Vaccines based on dendritic cell biology. In: Levine MM, editor. New Generation Vaccines. New York: USA: Informa Healthcare; 2009. pp. 327–339. [Google Scholar]
- 5.Bonifaz LC, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trumpfheller C, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozzacco L, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozzacco L, et al. HIV gag protein is efficiently cross-presented when targeted with an antibody towards the DEC-205 receptor in Flt3 ligand-mobilized murine DC. Eur J Immunol. 2010;40:36–46. doi: 10.1002/eji.200939748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 10.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 11.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 12.Lahoud MH, et al. Signal regulatory protein molecules are differentially expressed by CD8− dendritic cells. J Immunol. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- 13.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 14.Soares H, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do Y, et al. Targeting of LcrV virulence protein from Yersinia pestis to dendritic cells protects mice against pneumonic plague. Eur J Immunol. 2010;40:2791–2796. doi: 10.1002/eji.201040511. [DOI] [PubMed] [Google Scholar]
- 16.Reis e Sousa C, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochrein H, et al. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 18.Iyoda T, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pooley JL, Heath WR, Shortman K. Cutting edge: Intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 21.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifaz L, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnorrer P, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 26.Henri S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 27.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caminschi I, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancho D, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–16701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idoyaga J, et al. Cutting edge: Langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 32.Inaba K, et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. 1995;163:148–156. doi: 10.1006/cimm.1995.1109. [DOI] [PubMed] [Google Scholar]
- 33.Cheong C, et al. Production of monoclonal antibodies that recognize the extracellular domain of mouse langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flacher V, et al. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci USA. 1982;79:161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraal G, Breel M, Janse M, Bruin G. Langerhans’ cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang W, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 38.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mata M, Travers PJ, Liu Q, Frankel FR, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J Immunol. 1998;161:2985–2993. [PubMed] [Google Scholar]
- 40.Longhi MP, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl-Hennig C, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trumpfheller C, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi JH, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valladeau J, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 46.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heath AW, Wu WW, Howard MC. Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur J Immunol. 1994;24:1828–1834. doi: 10.1002/eji.1830240816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.