Abstract
In mammals, skin begins as a single-layered epithelium, which, through a series of signals, either stratifies and differentiates to become epidermis or invaginates downward to make hair follicles (HFs). To achieve and maintain proper tissue architecture, keratinocytes must intricately balance growth and differentiation. Here, we uncover a critical and hitherto unappreciated role for Yes-associated protein (YAP), an evolutionarily conserved transcriptional coactivator with potent oncogenic potential. We show that YAP is highly expressed and nuclear in single-layered basal epidermal progenitors. Notably, nuclear YAP progressively declines with age and correlates with proliferative potential of epidermal progenitors. Shortly after initiation of HF morphogenesis, YAP translocates to the cytoplasm of differentiating cells. Through genetic analysis, we demonstrate a role for YAP in maintaining basal epidermal progenitors and regulating HF morphogenesis. YAP overexpression causes hair placodes to evaginate into epidermis rather than invaginate into dermis. YAP also expands basal epidermal progenitors, promotes proliferation, and inhibits terminal differentiation. In vitro gain-and-loss of function studies show that primary mouse keratinocytes (MKs) accelerate proliferation, suppress differentiation, and inhibit apoptosis when YAP is activated and reverse these features when YAP is inhibited. Finally, we identify Cyr61 as a target of YAP in MKs and demonstrate a requirement for TEA domain (TEAD) transcriptional factors to comediate YAP functions in MKs.
Keywords: Hippo signaling, gene expression, proliferation and cancer, skin cancer
During tissue and organ development, progenitors must balance proliferation and differentiation in a temporally and spatially controlled fashion. Understanding the molecular mechanisms involved has attracted intense research interest, because too little proliferation contributes to aging and too much predisposes tissues to proliferative disorders, including cancers. Accumulating evidence has implicated an oncogene, Yes-associated protein (YAP), in this process. YAP is a transcriptional coactivator that lacks a DNA-binding motif but has a potent transactivation domain at its C terminus (1). YAP's N terminus has an association domain for the TEA domain (TEAD) family of DNA-binding proteins, which have been linked to YAP's growth-promoting function (2). Depending on the splicing variant, one or two protein–protein interacting (WW) domains yield the potential for additional interactions between YAP and other proteins. These include other possible transcriptional partners for YAP (ErbB, Runx2, and chromatin modeling proteins) as well as the negative YAP regulator large tumor suppressor (LATS) kinase (3–6).
YAP's importance was discovered in Drosophila, where its homolog, Yorkie, was shown to promote tissue growth by promoting cell proliferation and inhibiting apoptosis (6). Genetically, Yorkie is the ultimate effector of the evolutionarily conserved Hippo tumor suppressor pathway (6). Upon Hippo signaling in mice, YAP is phosphorylated at a key serine (S127), confining it to the cytoplasm, where it can no longer function in target gene expression (7).
Several lines of evidence suggest that YAP participates in regulating stem cell (SC) behavior. In cultured ES cells, YAP is highly expressed (8) and required for self-renewal and suppression of differentiation (9). In mouse intestine, YAP's overexpression causes an expanded pool of undifferentiated progenitors in the crypt (10). In chick, YAP functions through TEAD to regulate neural progenitor cell number (11), and in Drosophila, Yorkie maintains neuroepithelial cells in an undifferentiated state (12). The mechanisms underlying YAP regulation in progenitors remain limited, however, particularly within the physiological context of tissues.
In this study, we investigate YAP's function during mouse epidermal development. Skin begins as a single layer of multipotent progenitors. In response to extrinsic cues, embryonic epidermal cells either stratify to produce the protective barrier at the skin surface or invaginate to form hair follicles (HFs) (13). An underlying basement membrane (BM) rich in extracellular matrix and growth factors maintains the epithelial progenitors that adhere to it and also separates the epidermis and its appendages from surrounding dermis. We show that YAP is highly expressed in the most proliferative of these progenitors, where it functions in controlling their self-renewal and maintaining their undifferentiated state. We further implicate TEAD as a cofactor for YAP and uncover some of their possible targets in regulating the process.
Results
Nuclear YAP Marks the Progenitors in Developing Epidermis and Correlates with Proliferation.
Microarray analyses detected Yap transcripts in embryonic epidermis (14). Using a pan-antibody (Ab) that recognizes all forms of YAP, we confirmed YAP protein expression as early as embryo day 12 (E12), when epidermis exists as a single layer. At this time, immunoreactivity was predominantly nuclear, suggesting that the majority of YAP is in its active form (Fig. 1A).
Fig. 1.
Expression of YAP during embryonic skin development. (A–C) Immunofluorescence microscopy of frozen back-skin sections labeled with pan-YAP Ab. (D and E) Progressive decline in nuclear YAP correlates with an age-related reduction in the proliferative potential of basal epidermal progenitors. EdU was administered 4 h before tissue processing and quantifications (n = 8). Primary Abs are color-coded according to their secondary Abs, and nuclei are counterstained with DAPI (blue). (Scale bar, 10 μm.) β4, β4 integrin; B, basal; Der, dermis; DP, dermal papilla; Epi, epidermis; K5, keratin 5 (a pan-marker of basal keratinocytes); SB, suprabasal. Dotted lines denote the dermoepidermal border.
On stratification, YAP distributed differentially, concentrating in the basal layer, where the majority of YAP was still nuclear (Fig. 1B). An Ab specific for the LATS phosphorylation site (Ser127) of YAP also showed some cytoplasmic staining at this time, however, suggestive that the Hippo signaling pathway was active (Fig. S1A).
As HFs initiated, YAP immunolabeling diminished but was still nuclear in the early placode (Fig. S1B). During maturation, nuclear YAP was detected in the outer root sheath (ORS) and transit-amplifying matrix (Mx) cells at the HF base (Fig. 1C). Notably, both of these populations are proliferative and adherent to the surrounding BM. Conversely, as shown by both pan-YAP and phospho-specific YAP Abs, YAP shifted mainly to the cytoplasm in terminally differentiating lineages of the inner root sheath (IRS) and hair shaft (Fig. 1C and Fig. S1).
Taken together, these developmental patterns revealed a correlation between nuclear YAP and either proliferative potential and/or proliferation (Fig. 1C). To explore the association between nuclear YAP and proliferation directly, we administered a pulse of nucleotide analog 5′-ethynyl-2′-deoxyuridine (EdU) and monitored S-phase cells and YAP during skin development. Although YAP's expression extended beyond that of S-phase cells, a good correlation existed between the timing at which the intensity of nuclear YAP waned in basal cells of developing epidermis and a decline in S-phase cells within this layer (Fig. 1 D and E). By postnatal day 11 (P11), proliferative activity in the basal layer was markedly diminished, as was nuclear YAP. These findings suggest that the cycling activity of epidermal progenitors may be dependent on the degree to which YAP is nuclear.
Increasing Nuclear YAP Levels in Cells with Proliferative Potential Causes Dramatic Imbalances in Tissue Organization Within Developing Skin.
To address the physiological significance of nuclear YAP in developing epidermis, we conducted genetic studies. Transgenic (Tg) mice expressing YAP(S127A) under the control of a tetracycline regulatory element (TRE) (10) were bred to mice expressing tetracycline-sensitive transactivator, rtTA2SM2-VP16 (rtTA), under the control of the keratin 14 (K14) promoter active in embryonic and postnatal basal cells (15). K14-rtTA/TRE-YAP(S127A) mice exhibited strict and rapid doxycycline (Dox)-mediated induction of YAP (Fig. S2). Because YAP(S127A) is refractory to LATS phosphorylation, it markedly enhanced nuclear YAP levels, particularly in basal epidermal cells.
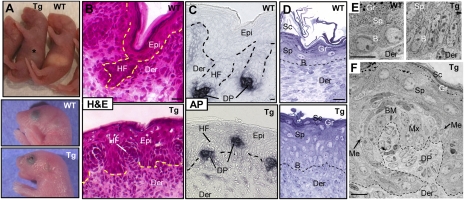
When YAP(S127A) was induced during embryogenesis (from E1 on; Materials and Methods), newborn (P0) K14-rtTA/TRE-YAP(S127A) Tg mice lacked milk in their stomach, displayed open eyes and mouth, and died within hours after birth (Fig. 2A). Histological analyses revealed a hyperthickened oral epithelium and poorly differentiated dorsal tongue epithelium, possibly contributing to these phenotypes and the early death of these animals (Fig. S3). Most notable, however, were dramatic skin abnormalities, exemplified by a hyperthickened epidermis and what appeared to be evaginated HFs (Fig. 2B). Closer inspection revealed that the HF dermal papillae (DP) had formed, as depicted by alkaline phosphatase (AP) activity (Fig. 2C). In contrast to the normal epidermal cross-talk that leads to DP downgrowth, however, the DP had moved up into the epidermis. These evaginations appeared shortly after YAP(S127A) induction, at the earliest stages of HF formation (Fig. S3).
Fig. 2.
Elevated nuclear YAP results in hyperthickening of interfollicular epidermis and evagination of HFs. (A) Dox-induced Tg pups were distinguished from WT by their lack of a milk spot (*) and open eyes and mouth. (B) H&E staining reveals hyperthickening of Tg interfollicular epidermis accompanied by thinner stratum corneum. (C) HF evaginations in Tg skin are evident by alkaline phosphatase (AP) enzymatic activity (black) in DP. (D) Semithin 1-μm sections of P0 back-skins were stained with toluidine blue, and basal cell density was quantified (as discussed in the text). (E and F) Ultrastructure reveals aberrant cellular morphology in the basal layer and evaginated HFs in Tg mice. B, basal; Der, dermis; DP, dermal papilla; Epi, epidermis; Gr, granular layer; Me, melanin granules (denoted with arrows in F, prevalent in hair shaft and its progenitor Mx cells); Sc, stratum corneum; Sp, spinous layer. (Scale bar, 10 μm.)
Semithin and ultrastructural analyses revealed further insights into the unusual skin morphology. The Tg basal layer showed a marked increase in cell density over WT littermate epidermis (Fig. 2D) (12.0 ± 1.7 nuclei per 100 μm of BM, Tg; 7.4 ± 1.4 nuclei per 100 μm of BM, WT; n = 42; P < 0.05). In addition, Tg basal cells were elongated and columnar, in contrast to the round morphology typical of WT basal cells (Fig. 2E). Interestingly, a BM still separated the DP from surrounding skin epithelium, whose morphology resembled that of HF matrix (Fig. 2F). Moreover, surrounding these Mx-like cells were some cells with melanin granules, typical of hair shaft precursor cells, and other cells that were flatter and more electron-dense, characteristic of IRS precursors. Mature hair shaft and IRS cells were noticeably absent.
Increasing Nuclear YAP Markedly Increases Epidermal Proliferation at the Expense of Terminal Differentiation.
The increased cell density within the epidermal basal layer of YAP(S127A)-induced skin suggested that enhanced nuclear YAP may elevate cell proliferation. Consistent with this notion was a marked increase in Ki67 (S + M phase) and phosphorylated histone H3 (pH3; M phase) labeling within the basal layer, concomitant to the increase in nuclear YAP (Fig. 3 A and B). The layer of cells with YAP+ nuclei surrounding the DP also showed signs of enhanced proliferation, consistent with their Mx-like morphology.
Fig. 3.
YAP(S127A) induction leads to an expansion of basal-like layers, increased proliferation, and diminished terminal differentiation. (A–C) Immunofluorescence (IF) reveals enhanced proliferation in P0 YAP Tg epidermis. Note enhanced Ki67- and pH3-positive cells mostly in the basal layer of the epidermis and evaginating HFs but also in some suprabasal cells (arrows in expanded boxed areas). Note also the expanded layers of basal markers K5 and p63 in YAP Tg skin. (D–F) IF shows aberrant terminal differentiation in YAP Tg epidermis. B, basal; Der, dermis; Epi, epidermis; K1, keratin 1; Lor, loricrin; SB, suprabasal. Dotted lines denote the dermoepidermal border. (Scale bar,10 μm.)
Although the majority of nuclear YAP, Ki67, and pH3 labeling occurred in cells adjacent to the BM, some suprabasal cells also displayed these markers (Fig. 3 A and B). This contrasted with WT skin, whose cycling cells were exclusively found in basal progenitors. Moreover, immunolabeling for basal markers K5 and p63 revealed an expansion in the layers expressing these markers (Fig. 3 A–C). Correspondingly, the terminal differentiation program appeared to be perturbed, as judged by (i) an overlap in expression of basal (K5) and spinous (K1) keratins and (ii) a decrease in loricrin, a late-stage differentiation event (Fig. 3 D and E).
Notch signaling functions prominently in controlling the fate switch between undifferentiated basal progenitors and spinous cells that commit to the terminal differentiation program (16). Thus, it was intriguing that Hes1, a transcription factor (TF) directly regulated by Notch signaling, was markedly suppressed in the YAP(S127A)-induced epidermis (Fig. 3F). Signs of Notch signaling were also absent in the immature evaginated HFs (Fig. 3F). By contrast, the occasional mosaic HF exhibiting normal invagination and differentiation also showed normal Hes1 staining and served as an internal control.
Consistent with our morphological and Notch signaling data, markers of terminally differentiated HF cells were either delayed or inhibited. This included markers of the companion layer (K6), hair shaft (AE13 Ab+ hair keratins), and IRS (AE15 Ab+ trichohyalin) (Fig. S4). In contrast, biochemical signs of HF cell fate appeared to be intact in YAP Tg skin. The ORS/Mx marker K17 was expressed by evaginating HFs, thereby distinguishing them from the surrounding epidermis. Similarly, the early markers of HF progenitors, including TFs Sox9, Lhx2, and Lef1, were all expressed by evaginating HFs (Fig. S4). Overall, these results provide functional evidence that nuclear YAP regulates the proliferative activity of skin progenitors and that its transition to the cytoplasm is required for cells to execute terminal differentiation properly and to balance growth and lineage commitment.
Elevated Nuclear YAP Inhibits Apoptosis in Addition to Promoting Proliferation and Suppressing Differentiation in Keratinocytes.
To dissect further the mechanisms by which nuclear YAP controls tissue development in skin, we cultured primary mouse keratinocytes (MKs) from basal epidermal progenitors of P0 K14-rtTA/TRE-YAP(S127A) mice. When Dox was added to the culture medium, nuclear YAP was markedly elevated (Fig. S2). To determine how YAP activation affects primary MKs, we quantified the ability of K14-rtTA/TRE-YAP(S127A) MKs to form colonies (more than four cells) in the presence and absence of Dox. As shown in Fig. 4A, a nearly 10-fold increase in colony-forming efficiency was observed when YAP(S127A) was induced. Notably, most of these colonies grew to be >2 mm in size, a characteristic typical of SCs (17). Moreover, these MKs displayed an approximately twofold decrease in the percentage of activated Caspase 3 (Ac-Cas3, a key apoptotic marker)-positive MKs (Fig. 4B). These data showed that the dramatic increase in colony formation and size was reflected not only in the enhanced proliferation of basal progenitors but also in their survival.
Fig. 4.
YAP(S127A) induction in MKs in vitro increases proliferation potential and inhibits apoptosis and differentiation. Dox-induced WT and K14-rtTA/TRE-YAP(S127A) Tg MKs were assayed 13 d and 1 d later, respectively, for differences in efficiency to form colonies (A, n = 3) and apoptosis (B, n = 3). Images are phase-contrast. (C) Calcium-induced activation of terminal differentiation genes is suppressed by YAP(S127A) induction in MKs. mRNAs were isolated 24 h after the switch from low to high Ca+, and real-time RT-PCR assays were performed (n = 3). F, feeders. *, statistical significance at the level of P < 0.05.
Finally, and consistent with our observations in vivo, augmenting nuclear YAP specifically restricted calcium-induced differentiation in vitro. RT-PCR analyses showed marked reductions in transcripts encoding early (K1 and K10) and late (involucrin, filaggrin, loricrin, Lce1a2, Lce1d, and Lce1m) differentiation markers (18) (Fig. 4C). Taken together, these findings suggested that nuclear YAP promotes proliferation and survival of basal progenitors and maintains the cells in an undifferentiated state.
Knockdown of YAP Impedes Growth and Initiates Differentiation in MKs.
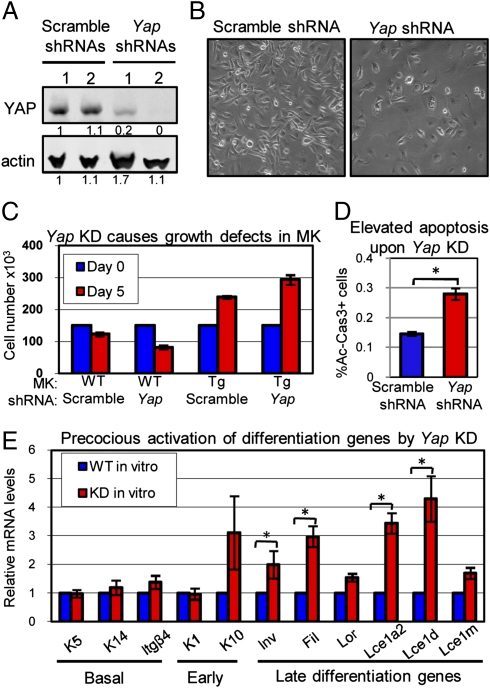
To examine whether YAP is essential for promoting MK growth and suppressing differentiation, we conducted loss-of-function studies. Primary MKs were infected with lentivirus carrying either Yap shRNA or scramble shRNA as a control, and efficient knockdown (KD) of YAP was validated by Western blot (WB) analysis (Fig. 5A). In comparison to the scramble control, YAP KD restricted the growth of MKs (Fig. 5B). This feature could be rescued by expressing the YAP(S127A) mutant, which was not targeted by the Yap shRNAs used (Fig. 5C). In addition, as opposed to our gain-of-function studies, Yap KD resulted in elevated cell death as shown by the flow analysis of Ac-Cas3 in MKs (Fig. 5D).
Fig. 5.
Loss of YAP leads to impaired growth, enhanced cell death, and precocious differentiation of epidermal MKs. Cultured WT and/or K14rtTA/TRE-YAP(S127A) Tg MKs were infected with lentivirus carrying either Yap shRNA or scramble shRNA as a control. Infected MKs were selected with puromycin (2 μg/mL) 2 d after infection and harvested 5 d later. (A) WB analysis reveals efficient KD of YAP expression (β-actin control). (B and C) Differences in cellular morphology (phase-contrast microscopy) and cell growth on Yap KD. Note that the abnormalities can be rescued by activating YAP(S127A). (D) Apoptosis assays. (E) Precocious induction of late-differentiation genes by Yap KD in MKs cultured in low-Ca2+ medium. mRNAs were isolated from FACS-purified MKs 5 d after infection with lentivirus carrying either Yap shRNAs or scramble shRNAs as well as red fluorescent protein (RFP) and were analyzed with real-time RT-PCR (n = 3 in C–E). *, statistical significance at the level of P < 0.05.
Importantly, under conditions that maintain WT MKs in an undifferentiated state, some of the MKs in which Yap was knocked down displayed a flattened squamous-like morphology typical of MKs exposed to high calcium and induced to terminally differentiate (Fig. 5B). In contrast to basal genes whose expression was not significantly affected, most of the differentiation genes examined were significantly up-regulated by Yap KD (Fig. 5E). This was accompanied by an elevation in expression of AP1 family members known to govern the expression of these late-differentiation genes (14) (Fig. S5). Together, these findings indicate that YAP deficiency increases the propensity of basal cells to undergo differentiation and/or apoptosis.
Identification of Cyr61 as a Target of YAP in MKs and TEAD as a Critical DNA-Binding Partner for YAP.
To investigate further how YAP controls this balance, we conducted microarray analysis to identify the transcriptional changes that occur in basal epidermal cells and/or MKs when (i) endogenous Yap is knocked down and (ii) nuclear YAP is enhanced (Dataset S1). Because YAP is thought to act predominantly as a coactivator, we focused on the 21 probes that scored as being down-regulated by greater than twofold in duplicate arrays when Yap was knocked down in MK (Dataset S1). These probes corresponded to nine known genes, one of which was Yap itself. We focused on one of these genes, Cyr61, because it was also up-regulated by greater than twofold in vivo and in vitro within 24 h after YAP(S127A) induction and its expression was among the most markedly reduced by Yap KD. Real-time PCR confirmed the changes in Cyr61 that had surfaced from the array data (Fig. 6A).
Fig. 6.
Identification of a target of YAP and of a role for TEADs in mediating YAP function in MKs. (A) Real-time RT-PCR shows that Cyr61 expression is elevated by increased nuclear YAP (ind., induced) in basal epidermal MKs in vivo and in vitro and is repressed by Yap KD (n = 3). (B) Growth defects conferred by Cyr61 KD in MKs. MKs were analyzed 5 d after Cyr61 KD by lentiviral shRNAs (n = 3). (C) ChIP of YAP and IgG (negative control) at the Cyr61 promoter (n = 2). (D and E) Growth defects caused by Tead KD in WT (uninduced, −Dox) and YAP(S127A) overexpressing (induced, +Dox) MKs (n = 3). (F) Effects of Tead KD on Cyr61 expression (n = 3). *, statistical significance at the level of P < 0.05.
To assess whether these changes in Cyr61 expression might be relevant to the proliferative differences we observed, we used lentiviral shRNA to knock down expression of Cyr61 in MK (Fig. S6A). Indeed, efficient KD of Cyr61 significantly inhibited MK growth (Fig. 6B). Furthermore, anti-YAP ChIP demonstrated an enrichment of YAP occupancy at the Cyr61 promoter (Fig. 6C), suggesting the possibility that Cyr61 may be a direct target of YAP and play a key role in mediating YAP function in cell growth regulation.
YAP does not bind DNA on its own. Among many TFs known to interact with YAP, the TEADs are the best studied genetically and biochemically as mediators of YAP's proliferation-inducing function (11, 19–21). By RT-PCR, all four TEAD members were detected in MKs (Fig. S6B). We therefore selected two shRNAs corresponding to sequences conserved among Tead1, Tead3, and Tead4 (21). RT-PCR of transduced MKs exhibited efficient KD of the three expected Teads and, surprisingly, a reduction in Tead2 as well (Fig. S6C). Additionally, both shRNAs potently inhibited cell growth, and, importantly, this growth defect could not be reversed by induction of YAP(S127A) (Fig. 6 D and E). Finally, Tead KD resulted in a significant reduction in Cyr61 expression, and induction of YAP(S127A) failed to rescue it (Fig. 6F). Together, these results suggest that YAP functions together with TEADs to promote cell growth and that this occurs, at least partially, through regulating Cyr61 expression.
YAP and Skin Cancers.
Mutations in the Hippo pathway have been linked to human cancers (22). Functioning at the end of the Hippo pathway, YAP is itself an oncogene and its overexpression promotes tissue overgrowth and tumorigenesis in Tg mice (7, 10). Amplification of the Yap gene locus and/or overexpression of Yap has been reported in many common human cancers (22, 23). Given our findings on the epidermis, we wondered whether this might also be the case for human skin cancers. Although a detailed investigation is beyond the scope of the present study, we conducted YAP immunohistochemical analyses on a panel of >50 epithelial skin cancers. A significant portion of these (22 of 40 squamous cell carcinomas and 10 of 14 basal cell carcinomas) showed an increase in YAP+ cells and/or elevation in nuclear YAP within the tumor masses (Fig. S7), supporting the oncogenic role of YAP and its growth-promoting function in epidermis reported in this work.
Discussion
Our study unveiled a critical role for YAP in maintaining epidermal progenitors during development. Through genetic studies, we showed that elevating nuclear YAP levels leads to massive expansion of proliferative basal epidermal cells. Many proliferation-related genes have been reported to be affected by YAP in mammalian cells (22), but no common targets of YAP have been reported to date. Because YAP lacks intrinsic DNA-binding activity, its targets are dictated by its transcription partners in a context-dependent manner. We identified Cyr61 as a potential direct target of YAP in epidermis, and its regulation appears to be mediated through TEAD TFs. Interestingly, connective tissue growth factor, which belongs to the same family of proteins as CYR61 (24, 25), has been reported to be a direct target of YAP in some cell types (21), suggesting the possibility that YAP may regulate related sets of genes to fulfill common functions in different cell types. In the future, it will be important to examine the physiological significance of these potential targets of YAP within intact mammalian tissues.
Our studies show that in addition to promoting cell growth, elevated nuclear YAP inhibits terminal differentiation both in vivo and in vitro, whereas endogenous YAP seems to function in suppressing precocious differentiation in basal epidermal keratinocytes. A similar role was recently reported for YAP in suppressing differentiation of mouse ES cells (9). As this burgeoning field continues to unfold, it will be interesting to see if YAP acts broadly as a key repressor of lineage commitment in SCs.
It is noteworthy that hyperproliferation and repressed terminal differentiation have also been described for epidermis of mice lacking WW45, an upstream component of the Hippo pathway (18). When taken together, these findings indicate that YAP is regulated by Hippo signaling in the epidermis. It is interesting that in WW45 null mice, HF morphogenesis appeared to be completely blocked (18), whereas in our conditional YAP(S127A)-induced animals, HF fates were still specified. A priori, this could reflect a broader role for Hippo-YAP signaling, perhaps in both dermis and epidermis. Alternatively, it could be that YAP elevation in skin epidermis on WW45 loss of function occurs earlier than in our K14-rtTA/TRE-YAP(S127A) Tg mice. Finally, these differences could reflect roles for WW45 that extend beyond YAP. Whatever the roots of the differences, the commonalities expose YAP as the primary mediator of the Hippo pathway and an important gatekeeper of the balance between proliferation and differentiation in the developing epidermis.
Another intriguing parallel was the resemblance between the HF evagination seen in YAP Tg mice and that seen in skin conditionally targeted for Dicer, encoding a core component of the microRNA (miRNA) processing machinery (26, 27). One possibility is that YAP and certain miRNA(s) may antagonize each other's function in regulating expression of key proteins involved in HF morphogenesis. Alternatively, Yap mRNA itself might be modulated by miRNAs other than Hippo signaling. That said, hyperproliferation was not a feature of Dicer-null epidermis; hence, such interactions, if they exist, would likely be tissue-specific.
In searching for a possible mechanism underlying HF evagination, it is likely to be relevant that in WT embryos, endogenous YAP translocates from the nucleus to cytoplasm in the small cluster of suprabasal cells that appear within the developing hair placodes. It is tempting to speculate that nuclear YAP and expression of its targets in these suprabasal cells may need to be shut down to ensure the proper cell shape changes that adjacent basal cells need to invaginate. A role for YAP in regulating cytoskeleton, polarity, and/or adhesive properties might also explain the elongated columnar shapes of YAP Tg basal cells. Although the defects we observed could also be a reflection of cell crowding within the basal layer, the underlying connection between YAP and these changes in cellular and tissue architecture merits attractive avenues for future investigations.
Materials and Methods
Mice.
Mice were housed and bred in the Association for Assessment and Accreditation of Laboratory Animal Care International-Accredited Comparative Bioscience Center animal facility at The Rockefeller University in accordance with university and National Institutes of Health guidelines. TRE-YAP(S127A) mice (10) were mated with K14-rtTA mice (14), and the genotypes were confirmed by PCR of toe DNA. Mice were fed Dox-containing food (BIO-SERV) to induce YAP, or i.p. injections of Dox (100 μg) were administered for short-term induction (<3 d). EdU (Invitrogen) was administered to pregnant mice or to neonatal pups by i.p. injection (50 μg/g of EdU).
Lentivirus.
Vesicular Stomatitis Virus G-pseudotyped lentivirus was produced by calcium phosphate transfection of 293FT cells (Invitrogen) with pLKO.1 (carrying shRNA and either the puromycin or H2B-mRFP1 cassette) and helper plasmids pMD2.G and psPAX2 (Addgene plasmids 12259 and 12260) (28). Infected MKs were selected with puromycin (2 μg/mL) 2 d after infection or FACS-isolated with RFP for subsequent experiments. shRNA sequences are listed in Table S1.
Statistics.
For all graphs, data presented are mean value ± SE. P ≤ 0.05 was accepted for statistical significance, as determined by the Student's t test (indicated by a bracket and asterisk in the figures).
Supplementary Material
Acknowledgments
We thank F. D. Camargo for TRE-YAP(S127A) mice; N. Stokes, L. Polak, and D. Oristian for assistance in animal work; S. Williams, S. Beronja, and G. Livshits for reagents and advice on lentiviral experiments; the Bio-Imaging Resource Center, Flow Cytometry Resource Center, and Genomics Core Laboratory (Memorial Sloan–Kettering Cancer Center) for technical support; and members of the E.F. laboratory for advice and help. H.Z. was supported by National Research Service Award Training Grant CA 09673 and is an American Cancer Society Postdoctoral Fellow (PF-09-042-01-DDC). This work was supported by a grant from the Starr Foundation (to E.F.). E.F. is an investigator at the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019603108/-/DCSupplemental.
References
- 1.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudol M, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–14741. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- 4.Linn H, et al. Using molecular repertoires to identify high-affinity peptide ligands of the WW domain of human and mouse YAP. Biol Chem. 1997;378:531–537. doi: 10.1515/bchm.1997.378.6.531. [DOI] [PubMed] [Google Scholar]
- 5.Chen HI, Sudol M. The WW domain of yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci USA. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 9.Lian I, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy BV, Rauskolb C, Irvine KD. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137:2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezhkova E, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perbal B. CCN proteins: Multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 25.Leask A, Abraham DJ. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 26.Yi R, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 27.Andl T, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beronja S, Livshits G, Williams S, Fuchs E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat Med. 2010;16:821–827. doi: 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.