Abstract
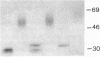
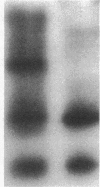
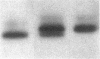
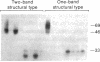
We characterized Fc receptor III (FcR III) on human neutrophils and found it to be heavily glycosylated and polymorphic. In some individuals, FcR III that had been digested with N-glycanase appeared after SDS-PAGE under reducing conditions as two bands with apparent molecular masses of 33 and 29 kD. In other individuals, N-glycanase-treated FcR III appeared as a single band with an Mr of either 33 or 29 kD. After SDS-PAGE of N-glycanase-treated FcR III under nonreducing conditions, the apparent Mr of each structural type was decreased, suggesting the presence of intramolecular disulfide bonds. Digestion of the 33-kD band and the 29-kD band with Staphylococcus aureus V8 protease yielded similar, but not identical, peptide maps. Thus, at least two polymorphic forms of FcR III are expressed on human neutrophils. The structural polymorphism of neutrophil FcR III correlated with previously described antigenic polymorphisms detected by monoclonal antibody Gran 11 and by alloantisera which recognize epitopes of the biallelic, neutrophil antigen (NA) system. Individuals whose neutrophils expressed the two-band structural type of FcR III were NA1NA2 heterozygotes. Individuals whose neutrophils expressed the single 33-kD band structural type were NA2NA2 homozygotes, and individuals whose neutrophils expressed the single 29-kD band structural type were NA1NA1 homozygotes. These findings indicate that antigenic and structural polymorphisms of human neutrophil FcR III are related and can be accounted for by differences at the level of primary protein structure.
Full text
PDF

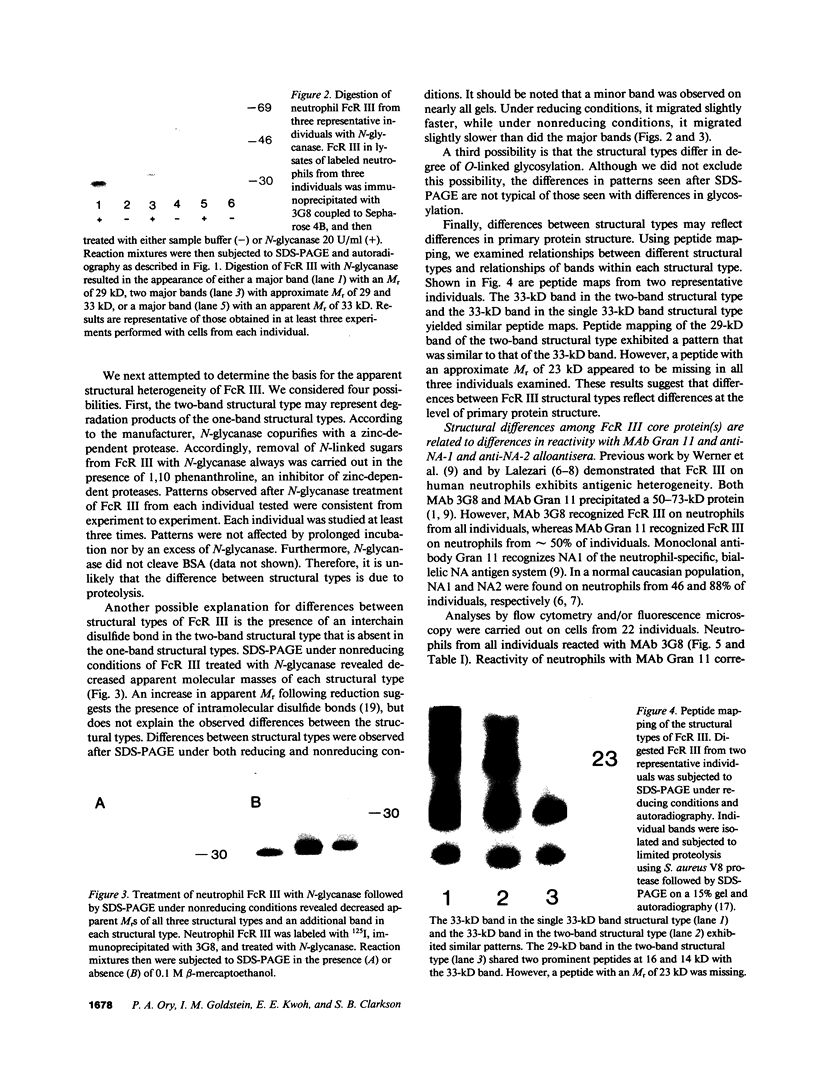



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Ryan D. H., Looney R. J., Leary P. C. Structural polymorphism of the human monocyte 40 kilodalton Fc receptor for IgG. J Immunol. 1987 Apr 1;138(7):2254–2256. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ceuppens J. L., Meurs L., Van Wauwe J. P. Failure of OKT3 monoclonal antibody to induce lymphocyte mitogenesis: a familial defect in monocyte helper function. J Immunol. 1985 Mar;134(3):1498–1502. [PubMed] [Google Scholar]
- Clarkson S. B., Bussel J. B., Kimberly R. P., Valinsky J. E., Nachman R. L., Unkeless J. C. Treatment of refractory immune thrombocytopenic purpura with an anti-Fc gamma-receptor antibody. N Engl J Med. 1986 May 8;314(19):1236–1239. doi: 10.1056/NEJM198605083141907. [DOI] [PubMed] [Google Scholar]
- Clarkson S. B., Kimberly R. P., Valinsky J. E., Witmer M. D., Bussel J. B., Nachman R. L., Unkeless J. C. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J Exp Med. 1986 Aug 1;164(2):474–489. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson S. B., Ory P. A. CD16. Developmentally regulated IgG Fc receptors on cultured human monocytes. J Exp Med. 1988 Feb 1;167(2):408–420. doi: 10.1084/jem.167.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Fleit H. B., Kuhnle M. Biochemical characterization of an Fc gamma receptor purified from human neutrophils. J Immunol. 1988 May 1;140(9):3120–3125. [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H., Frank M. M. A serial study of splenic reticuloendothelial system Fc receptor functional activity in systemic lupus erythematosus. Arthritis Rheum. 1982 Jan;25(1):48–54. doi: 10.1002/art.1780250108. [DOI] [PubMed] [Google Scholar]
- Hamburger M. I., Moutsopoulos H. M., Lawley T. J., Frank M. M. Sjögren's syndrome: a defect in reticuloendothelial system Fc-receptor-specific clearance. Ann Intern Med. 1979 Oct;91(4):534–538. doi: 10.7326/0003-4819-91-4-534. [DOI] [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Lalezari P., Jiang A. F., Yegen L., Santorineou M. Chronic autoimmune neutropenia due to anti-NA2 antibody. N Engl J Med. 1975 Oct 9;293(15):744–747. doi: 10.1056/NEJM197510092931504. [DOI] [PubMed] [Google Scholar]
- Lalezari P., Khorshidi M., Petrosova M. Autoimmune neutropenia of infancy. J Pediatr. 1986 Nov;109(5):764–769. doi: 10.1016/s0022-3476(86)80690-4. [DOI] [PubMed] [Google Scholar]
- Lalezari P. Neutrophil antigens: immunology and clinical implications. Prog Clin Biol Res. 1977;13:209–225. [PubMed] [Google Scholar]
- Lalezari P., Radel E. Neutrophil-specific antigens: immunology and clinical significance. Semin Hematol. 1974 Jul;11(3):281–290. [PubMed] [Google Scholar]
- Lawley T. J., Hall R. P., Fauci A. S., Katz S. I., Hamburger M. I., Frank M. M. Defective Fc-receptor functions associated with the HLA-B8/DRw3 haplotype: studies in patients with dermatitis herpetiformis and normal subjects. N Engl J Med. 1981 Jan 22;304(4):185–192. doi: 10.1056/NEJM198101223040401. [DOI] [PubMed] [Google Scholar]
- Madyastha P. R., Fudenberg H. H., Glassman A. B., Madyastha K. R., Smith C. L. Autoimmune neutropenia in early infancy: a review. Ann Clin Lab Sci. 1982 Sep-Oct;12(5):356–367. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Priest J. R., Clay M. E., McCullough J., Ramsay N. K., Lalezari P., Krivit W. Transient autoimmune neutropenia due to anti-NA1 antibody. Am J Pediatr Hematol Oncol. 1980 Fall;2(3):195–199. [PubMed] [Google Scholar]
- Russo C., Callegaro L., Lanza E., Ferrone S. Re.: Purification of IgG monoclonal antibody by caprylic acid precipitation. J Immunol Methods. 1983 Dec 16;65(1-2):269–271. doi: 10.1016/0022-1759(83)90324-1. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Rosse W. F., Silber R., Springer T. A. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988 Jun 9;333(6173):565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- Simmons D., Seed B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature. 1988 Jun 9;333(6173):568–570. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- Stengelin S., Stamenkovic I., Seed B. Isolation of cDNAs for two distinct human Fc receptors by ligand affinity cloning. EMBO J. 1988 Apr;7(4):1053–1059. doi: 10.1002/j.1460-2075.1988.tb02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tax W. J., Hermes F. F., Willems R. W., Capel P. J., Koene R. A. Fc receptors for mouse IgG1 on human monocytes: polymorphism and role in antibody-induced T cell proliferation. J Immunol. 1984 Sep;133(3):1185–1189. [PubMed] [Google Scholar]
- Tax W. J., Willems H. W., Reekers P. P., Capel P. J., Koene R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature. 1983 Aug 4;304(5925):445–447. doi: 10.1038/304445a0. [DOI] [PubMed] [Google Scholar]