Abstract
Proceeding on the assumption that all cancer cells have equal malignant capacities, current regimens in cancer therapy attempt to eradicate all malignant cells of a tumor lesion. Using in vivo targeting of tumor cell subsets, we demonstrate that selective elimination of a definite, minor tumor cell subpopulation is particularly effective in eradicating established melanoma lesions irrespective of the bulk of cancer cells. Tumor cell subsets were specifically eliminated in a tumor lesion by adoptive transfer of engineered cytotoxic T cells redirected in an antigen-restricted manner via a chimeric antigen receptor. Targeted elimination of less than 2% of the tumor cells that coexpress high molecular weight melanoma-associated antigen (HMW-MAA) (melanoma-associated chondroitin sulfate proteoglycan, MCSP) and CD20 lastingly eradicated melanoma lesions, whereas targeting of any random 10% tumor cell subset was not effective. Our data challenge the biological therapy and current drug development paradigms in the treatment of cancer.
The melanoma is an outstanding example of those tumors that are composed of phenotypically and genetically diverse tumor cells, are notoriously resistant to a variety of chemotherapeutic drugs, and relapse with high frequency after initial therapeutic remission (1). Standard chemotherapeutic regimens including dacarbazine have not provided substantial survival benefits in melanoma therapy. This indicates that there is a need for a biological therapy including antibodies to block CTLA-4 function (2), high-dose IFN-α2b, which has exhibited a significant relapse-free survival benefit in the adjuvant therapy of high-risk stage IIB and III melanomas (3), and IL-2, which has yielded rare but reproducible high-quality responses (4). A milestone in melanoma treatment was the report by the Rosenberg group demonstrating a 50% response rate in stage IV melanoma patients treated with ex vivo expanded tumor-infiltrating lymphocytes (TILs) and high-dose IL-2 administered after nonmyeloablative conditioning (5). Although the efficacy of TILs with obviously diverse specificities was high, adoptively transferred monospecific T cells induced regression of melanoma lesions in only 2 out of 15 patients (6), this could be improved by IL-2 and IFN-α (7). Increasing lymphodepletion before T-cell transfer improved objective response rates by up to 70%; this correlated well with the number and persistence of transferred T cells (8). Surviving tumor cell variants are thought to be the cause of tumor relapse despite initial reduction in tumor mass. If melanoma cells survive treatment in a random manner and tumor growth is reinitiated by any surviving melanoma cell, eradication of tumor lesions will require the elimination of all melanoma cells and will not be possible by targeting of any tumor cell subsets. Conversely, if melanoma growth depends on a defined tumor cell subpopulation, their specific elimination will effectively eradicate tumor lesions without targeting the bulk of the tumor cells.
We addressed the scenario by adoptive transfer of cytotoxic T cells (CTLs) with redirected specificities toward the bulk of melanoma cells and toward specific melanoma cell subsets, respectively, of an established tumor lesion. T cells were engineered with a chimeric antigen receptor (CAR, immunoreceptor) whose extracellular antibody domain binds to a predefined antigen in a MHC-independent manner and triggers T-cell activation upon antigen engagement via the intracellular CD3ζ signaling domain (9). By adoptive transfer of CAR-redirected CTLs, we revealed that established tumor lesions can be efficiently eradicated by targeted elimination of the less than 2% subset of melanoma cells with the CD20+ high molecular weight melanoma-associated antigen (HMW-MAA)+ phenotype without targeting the tumor cell mass, whereas targeted elimination of any minor subset is less effective. These data provide a strategy for improving the biological therapy of melanomas and, moreover, for redesigning current drug development paradigms used in the treatment of cancer.
Results
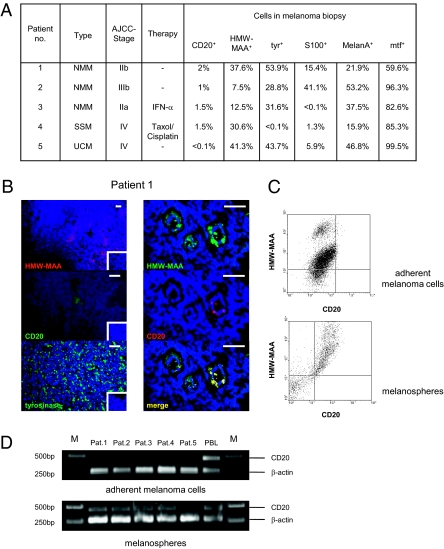
Flow cytometric analyses of five melanoma biopsies revealed a differential expression profile of the classical melanoma markers tyrosinase (tyr), S100, and Melan-A (Fig. 1A). Melanotransferrin (mtf) was expressed by nearly all melanoma cells and HMW-MAA, also referred to as melanoma-associated chondroitin sulfate proteoglycan (MCSP), by a substantial number of melanoma cells; this is in accordance with published data (10). Moreover, we detected small islets of CD20+ cells that coexpressed HMW-MAA (Fig. 1B). In four out of five biopsies, CD20+ HMW-MAA+ cells were detected by flow cytometry and found to represent less than 2% of cells in the melanoma lesion. The rareness of melanoma cells expressing CD20 was further reconfirmed by immunohistochemical staining of primary biopsies (Fig. S1). CD20+ melanoma cells from biopsies grown to spheroids in embryonic stem cell media coexpress HMW-MAA, whereas HWM-MAA+ melanoma cells propagated long term in adherent cultures in vitro do not express CD20 (Fig. 1C). CD20+ melanoma cells moreover coexpress CD44 and CD61 and lack CD24 and CD34 (Fig. S2), thereby displaying a phenotype similar to known cancer stem cells. CD20 expression of spheroid cells in contrast to adherent melanoma cells was confirmed by RT-PCR as shown in Fig. 1D. Of note, no CD20+ melanoma cells could be detected in biopsies of patient 5 by flow cytometry, immune histology, or RT-PCR.
Fig. 1.
Melanoma lesions harbor melanoma cells, which coexpress CD20 and HMW-MAA. (A) Melanoma cell suspensions were obtained from cutaneous melanoma biopsies, stained for CD20, HMW-MAA, tyrosinase (tyr), S100, Melan-A, and melanotransferrin (mtf), respectively, and analyzed by flow cytometry. No CD20+ cells were detected in biopsy from patient 5. NMM, nodular malignant melanoma; UCM, unclassified melanoma; SSM, superficial spreading melanoma. (B, Left) Cryostat sections of cutaneous melanoma lesions from patient 1 were stained for HMW-MAA using the PE-conjugated anti–HMW-MAA antibody EP-1 (red), for tyrosinase using the antityrosinase mAb T311, and for CD20 using the anti-CD20 mAb L27, respectively, followed by incubation with the biotinylated antimurine IgG1 antibody and streptavidin-conjugated Alexa Fluor 488 (green). Staining with isotype-matched PE-conjugated antimurine antibodies were used as controls (Insert). Cell nuclei were visualized by DAPI staining (blue). (Right) Coexpression of CD20 and HMW-MAA was detected by the anti-CD20 mAb L27 and the anti–HMW-MAA mAb 763.74 and visualized by the Alexa Fluor 488 (green) and Alexa Fluor 555 (red) conjugated secondary antibodies, respectively. Photographs were merged using Adobe Photoshop software. (Scale bars, 20 μm.) (C) Melanoma cells from patient 1 biopsy were cultivated in adherent cultures or grown to melanospheres in human embryonic stem cell medium as described (21). Spheroidal and adherent cells were analyzed for expression of CD20 and HMW-MAA by flow cytometry using the PE-conjugated anti–HMW-MAA mAb EP-1 and the FITC-conjugated anti-CD20 mAb L27. (D) RT-PCR analysis for CD20 mRNA expression of adherently growing melanoma cells (number of passages > 50) and of melanosphere cells derived from the same biopsy. A 459-bp fragment was derived from CD20 mRNA and a 267-bp fragment from β-actin mRNA as control. No CD20 mRNA was detected in cells from patient-5 biopsy.
To explore whether isolated melanoma cells exhibit the capacity to establish melanoma, cell suspensions from primary and metastatic cutaneous melanoma biopsies were s.c. transplanted in NIH-III mice (Crl:NIH-LystbgFoxn1nuBtkxid), which lack T, B, and NK cells. Transplanted tumors grew progressively and displayed the same histological morphology and melanoma cell subpopulations as the respective parental melanoma biopsy. HMW-MAA+ cells gave rise to melanomas upon transplantation, whereas sorted HMW-MAA− cells from the same melanoma did not (Fig. S3A). Overall survival of mice transplanted with a 1:1 mixed HMW-MAA+ and HMW-MAA− cell population was the same as mice transplanted with HMW-MAA+ cells. HMW-MAA+ cells isolated from first passage melanoma induced melanoma of the same phenotype upon second transplantation (Fig. S3B), indicating that these cells exhibit melanoma-initiating capacities.
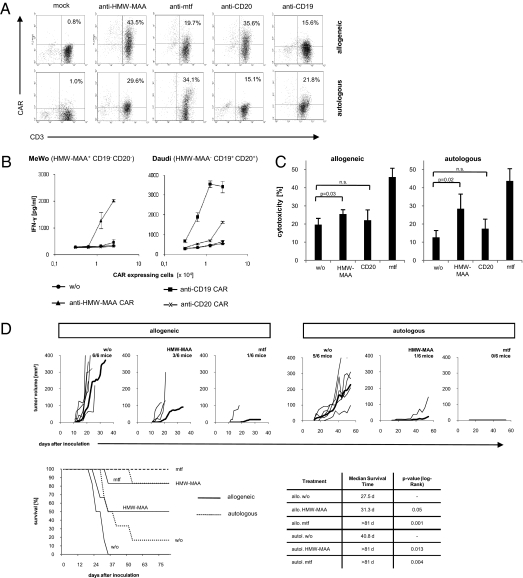
To specifically eliminate tumor cell subsets from a tumor lesion, we used redirection of CTLs with predefined specificity by expression of a CAR. We engineered CTLs with specificity for mtf, HMW-MAA, CD20, and for control CD19, respectively, by retroviral expression of a CAR with the respective single-chain antibody binding domain (Fig. 2A). The redirected T-cell specificity is defined by the extracellular scFv domain because engineered T cells with the anti–HMW-MAA CAR were activated by HMW-MAA+ cells, but not by HMW-MAA− cells (Fig. 2B). Conversely, T cells with the CD20- or CD19-specific CAR were activated by CD20+ CD19+ HMW-MAA− cells but not by CD20− CD19− HMW-MAA+ cells. Of note, differences in T-cell activation are likely to be due to different expression levels of targeted antigens because both anti-CD20 and anti-CD19 CARs exhibit nearly identical binding affinities.
Fig. 2.
Human T cells can be specifically redirected toward melanoma cells. (A) CD3+ T cells obtained from a healthy volunteer (allogeneic) and from melanoma patient 1 (autologous) were engineered by retroviral gene transfer to express the HMW-MAA, the melanotransferrin (mtf), and the CD20-specific CAR, respectively. Anti-CD19 CAR and mock-transduced T cells were used as controls. CAR expression on the cell surface was monitored by flow cytometry using a PE-conjugated antihuman IgG1 mAb, which binds to the CAR extracellular spacer region, and a FITC-conjugated anti-CD3 mAb. (B) To record antigen specificity of CAR-mediated T-cell activation, engineered CD3+ T cells with the HMW-MAA, CD19, and the CD20-specific CAR, respectively, and unmodified T cells without CAR (w/o) were coincubated for 48 h in serial dilutions with MeWo cells and Daudi cells (each 2.5 × 104 cells per well), respectively. IFN-γ secreted into the culture supernatant, was recorded by ELISA. (C) Primary melanoma cells (5 × 104 cells per well) from a melanoma lesion of patient 1 were coincubated with 2.5 × 104 allogeneic CAR-expressing T cells per well or 0.625 × 104 autologous CAR-expressing T cells per well, both without (w/o) or with CARs specific for mtf, HMW-MAA, and CD20, respectively. The ratios of engineered T cells: tumor cells were 1:2 in the allogeneic and 1:8 in the autologous settings. Specific cytotoxicity was recorded after 48 h. Data represent the mean of triplicates ± SEM. (D) Engineered T cells are cytolytic against HMW-MAA+ tumor cells in an in vivo coincubation assay. Allogeneic or autologous T cells engineered to express the HMW-MAA– or the mtf-specific CAR (1 × 106 CAR-expressing T cells) or mock-modified T cells without CAR (w/o) were s.c. inoculated together with 1 × 106 melanoma cells in NIH-III mice. Growth of the individual tumors was recorded; the bold line represents the mean tumor growth. The overall survival of treated mice is shown in a Kaplan–Meier plot. The median survival time and the P values of significance compared with mice treated with T cells without CAR (w/o) were calculated using the log-rank test and the XLSTAT2010 software.
Melanoma cells isolated from tumor biopsies were sensitive to a cytotoxic T-cell attack redirected by the mtf-specific CAR in vitro (Fig. 2C). Whereas those CTLs were from healthy volunteers, the same results were obtained using CTLs from melanoma patients redirected by the mtf-specific CAR against their autologous melanoma cells. HMW-MAA–specific CTLs also displayed redirected cytotoxicity toward melanoma cells in vitro, but the efficacy was lower; this result is to be expected due to the smaller number of HMW-MAA+ cells in the melanoma cell population. CD20-redirected CTLs did not significantly reduce the number of melanoma cells in the short-term cytotoxicity assay because CD20+ cells represented less than 2% of the melanoma cells in the assay.
The targeted cells have melanoma originating capacities because coinoculation of these cells with mtf-specific T cells in NIH-III mice prevented melanoma outgrowth, whereas coinoculation with unmodified T cells resulted in melanoma formation and progressive tumor growth (Fig. 2D). Redirected T cells targeting the HMW-MAA+ subpopulation of melanoma cells exhibited a similar prevention of tumor formation. The same melanoma repression was observed when redirecting the patients’ engineered T cells toward autologous melanoma cells, whereas coinoculation with the patients’ mock-modified T cells did not. Patients’ T cells seem to exhibit slightly higher antitumor activity; the effect, however, is not significant (P = 0.22) and was seen in some, but not all patients.
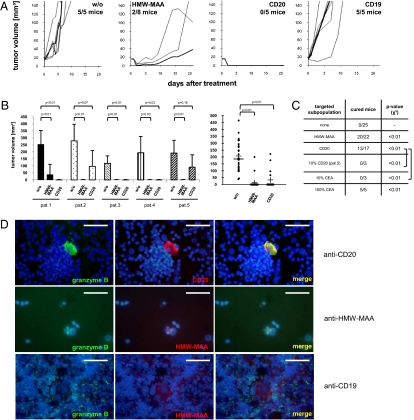
We then addressed whether an established tumor lesion can be eradicated by targeted elimination of a melanoma subset irrespective of the nontargeted cancer cell mass. Melanomas were induced by s.c. transplantation of biopsy cells and grown to sizes >15 mm3 when tumors were obviously established and vascularized. Engineered T cells were then administered by three consecutive injections into the tail vein. T cells with redirected specificity for CD20 eradicated established melanoma lesions in four out of five melanoma biopsies, although the targeted cells represented less than 2% of melanoma cells (Fig. 3A). It is noteworthy that transplanted tumors of patient 5, in which no CD20+ melanoma cells were detected, could not be eradicated by a CD20-redirected T-cell attack. Where tumors were eradicated, no tumor relapse occurred during the observation period of >36 wk. Targeting CD20+ melanoma cells significantly improved tumor-free survival compared with mice treated with redirected T cells of irrelevant specificity or to mice without treatment (P = 0.01) (Fig. 3B). Targeting by HMW-MAA–specific T cells likewise stopped tumor growth; this resulted in tumor eradication in six out of eight mice and the transient regression of two tumors. Considering all of the patients together, treatment with HMW-MAA–specific T cells significantly increased tumor-free survival compared with treatment with unmodified T cells (P = 0.00004). The controls—mock-modified T cells and T cells with redirected specificity for CD19, which is not expressed in melanoma cells—did not alter tumor growth. This once again demonstrates the redirected specificity of the T-cell attack and excludes the possibility that adoptively transferred T cells displayed a relevant but unknown antimelanoma specificity.
Fig. 3.
Targeted elimination of CD20+ melanoma cells eradicates established melanoma lesions in vivo. (A) Melanomas were induced in NIH-III mice by s.c. application of melanoma cell suspension from a biopsy of patient 1. When melanoma lesions had reached a size of ≥15 mm3 and were vascularized, engineered T cells (1 × 106 CAR-expressing T cells) were i.v. injected three times at 2-d intervals. Engineered T cells expressed CARs with specificity for HMW-MAA, CD20, and CD19, respectively. Mock-modified T cells without CAR (w/o) were used as control. Growth of the individual tumors was recorded (thin lines); the bold line in each diagram represents the mean of tumor volumes. (B) The same assays were performed with melanoma cells from biopsies of five randomly chosen patients with melanomas of different histologies and clinical stages (Fig. 1A). Tumor volumes of each individual patient at day 21 of treatment and tumor-free survival of treated mice are shown. Statistical analyses were performed using Student´s t test. (C) Summary of adoptive T-cell transfer data. Melanomas were treated by adoptive transfer of engineered T cells as described in A. Nonmodified T cells, which do not target a specific melanoma cell subset (none) or modified T cells with a CAR specific for HMW-MAA, CD20, or CEA, respectively, were administered. Patient 5’s melanoma cells, which lack CD20+ and CEA+ cells, were engineered by transfection with CD20 and CEA, respectively (Figs. S4 and S5), and added in a frequency of 10% to nonmodified melanoma cells. CEA+ melanoma cells were specifically eliminated by anti-CEA CAR T cells in vitro and in vivo (Figs. S5 and S6); melanoma spiked with 10% CEA+ melanoma cells, however, progressed in the presence of anti-CEA CAR T cells. As a control, melanomas with 100% CEA+ melanoma cells were eradicated by anti-CEA CAR T cells. Mice were assessed to be cured when no tumor lesion was detected at day 21 after treatment. Cured mice were found to be tumor-free during the observation period of >6 wk. Data were compared with treatment with nonmodified T cells using the χ2 test. (D) Melanoma cells from a biopsy take up granzyme B as revealed by coincubating with CD20 and HMW-MAA redirected cytotoxic T cells. Melanoma cells coincubated with CD19-specific T cells were used as the control. Granzyme B, CD20 and HMW-MAA were stained as described in Materials and Methods. Photographs were merged using Adobe Photoshop software. (Scale bars, 20 μm.)
We then asked whether targeted elimination of any small subpopulation of melanoma cells in the tumor lesion results in eradication of established melanomas. To address this question, melanoma cells of the biopsy were genetically marked by expression of carcinoembryonic antigen (CEA) (Fig. S4), which is expressed on the cell surface, can be specifically targeted by engineered T cells redirected with a CEA-specific CAR (Fig. S5), is not expressed by the mouse, and is not physiologically expressed in those human tumor lesions used in the study. Genetic marking, furthermore, did not alter tumor formation. Unmodified CEA− melanoma cells were spiked with CEA+ marked melanoma cells from the same patient providing up to 10% CEA+ melanoma cells in the tumor lesion. Although adoptive transfer of CEA-specific T cells specifically eliminated CEA+ melanoma cells (Fig. S6), tumor lesions progressed and could not be eradicated (Fig. 3A), whereas positive control-induced melanomas with 100% sorted CEA+ melanoma cells were efficiently eradicated. The data demonstrate that elimination of any random subpopulation of melanoma cells does not eradicate established tumor lesions.
To address whether CD20 is a target on a specific melanoma subpopulation or functionally crucial, we stably expressed CD20 in CD20− melanoma cells from patient 5. The engineered CD20+ melanoma population was mixed with unmodified melanoma cells to a frequency of 10% CD20+ cells. Application of anti-CD20 CAR-modified T cells did not reduce tumor growth; all mice developed tumors as did mice injected with unmodified T cells (Fig. 3C). There is no melanoma cure when targeting artificially marked melanoma cells. We conclude that CD20 is not functionally crucial but marks a specific cell population.
Redirected cytotoxic T cells mediate target cell lysis predominantly in a granzyme-dependent manner (11). By immunostaining tumors in mice treated with HMW-MAA redirected T cells, we thus revealed that HMW-MAA+ melanoma cells took up granzyme B as did CD20+ melanoma cells after a CD20-specific T-cell attack (Fig. 3D). In the control experiment, melanoma cells did not take up granzyme upon treatment with CD19-specific T cells. The observation is in accordance with in vitro data demonstrating lysis of HMW-MAA+, but not of HMW-MAA− melanoma cells attacked by redirected T cells.
Discussion
In the present paper, we demonstrate that human melanoma lesions, which had been established in immunodeficient mice by means of biopsy cells, can efficiently be eradicated by targeted elimination of a definite, minor melanoma cell population without targeting the bulk of the tumor cell mass. The targeted melanoma cell population consists of CD20+ HMW-MAA+ melanoma cells and represents less than 2% of the melanoma cells in the tumor lesion. Elimination of the major HMW-MAA+ melanoma cell population, which incorporates CD20+ melanoma cells, was likewise effective. Targeted elimination of a randomly selected, 10% melanoma cell subpopulation did not eradicate melanoma lesions; targeting of randomly CD20-modified melanoma cells did not result in tumor eradication either. Data definitely demonstrate the specificity of the observation and indicate that CD20 is not functionally crucial but marks a specific melanoma cell population. Because no tumor relapse was observed in any case during the >36 wk observation period, such melanoma eradication is obviously long lasting. Targeted elimination that is based on the redirected, MHC-independent specificity of CAR engineered cytotoxic T cells is specific and does not affect the bulk of tumor cells. Conversely, unmodified allogeneic or autologous T cells do not exhibit an antitumor effect; this makes an intrinsic, unidentified antimelanoma capacity of the adoptively transferred cells unlikely. Redirected CD20 targeting does not affect other host cells because the NIH-III mouse used in the study is B-cell deficient and the targeting CAR domain specifically recognizes human, not murine, CD20. Redirected HMW-MAA targeting was as effective in tumor elimination as CD20 targeting because HMW-MAA is coexpressed on CD20+ melanoma cells, but also on a larger number of CD20− melanoma cells. In accordance with this observation, HMW-MAA has been discussed as a prospective target for the immunotherapy of melanoma for more than a decade and HMW-MAA–specific antibodies have been used in trials with some success (10). There is no difference in the efficacy of HMW-MAA– and CD20-targeted melanoma eradication. The unexpected observation, however, is that targeting the minor CD20+ melanoma cell population is as effective as targeting the majority of tumor cells. A caveat for a broad therapeutic application is that obviously some melanomas, in our cohort one out of five patients, do not harbor CD20+ melanoma cells, which may be due to lack of the CD20 molecule on the same melanoma cell subset or absence of those target cells.
The observation that the specific elimination of a minor subset of tumor cells results in eradication of established melanoma lesions is crucial for the understanding of the biology of melanoma. Continuous melanoma growth obviously requires the presence of a minor population of cells, which displays the CD20+ HMW-MAA+ phenotype; other minor subsets may additionally exist. Elimination of these cells is obviously required to eradicate the melanoma irrespective of the bulk of tumor cells. Although our data sustain the model that established melanoma is organized in a hierarchical manner to sustain tumor progression, data do not account for whether or not melanoma can be newly established by a specific, phenotypically stable melanoma cell after transplantation. In contrast, recent data imply a phenotypic plasticity driven largely by reversible changes within tumorigenic melanoma cell lineages (12). Our data are in accordance with the reversal phenotype model, assuming that those melanoma cells that sustain progression in an established tumor display a certain phenotype, although reversible, and represent a minority at a particular time, the elimination of which is sufficient to eradicate established tumor lesions.
Our conclusion has substantial implications for the future design of therapeutic strategies. In the case of melanoma, elimination of nearly all melanoma cells is not required as long as the cell subset with tumor-sustaining potential is eliminated. Apart from adoptive T-cell therapy, the minor melanoma cell population may also be targeted by other means including the anti-CD20 antibody rituximab, which is clinically used to induce regression of CD20+ non-Hodgkin's lymphoma and B-cell chronic lymphocytic leukemia (13). Anti-CD20 radioimmunoconjugates, including ibritumomab, tiuxetan, and tositumomab, also exhibit efficacy in eliminating B-cell lymphoma (14). Rituximab treatment is accompanied by rapid and sustained depletion of normal CD20+ B cells from circulation, which is tolerated without significant toxicity (15, 16). Similar depletion of circulating B cells is expected upon treatment with CD20-redirected T cells. However, in a previous small melanoma trial using rituximab as an adjunct to low-dose IL-2 therapy, no objective response was observed (17). The lack of decrease in circulating Ig levels and obviously incomplete therapeutic loss of B-lineage cells in that trial indicates a low efficacy of rituximab treatment in those patients. It therefore remains unclear whether rituximab or other CD20-targeting antibodies are effective in melanoma treatment. However, in this context it must be considered that antibodies penetrate solid tissues less efficiently than T cells, and such penetration is required to deplete the rare CD20+ melanoma cells. Recombinant single chain antibodies may have an advantage because they penetrate solid tissues more efficiently than full-length antibodies and, when combined with a T cell-engaging domain in a so-called bispecific T-cell engager (BiTE) antibody, have proved to be extremely effective in eliminating tumor cells in circulation as well as in solid tissues (18). Conversely, genetically redirected T cells have the advantage of continuous proliferation as long as cognate antigen is present, thereby multiplying their antitumor response.
The differential effect of targeting tumor cell subsets with different proliferative and tumor-sustaining capacities was recently emphasized by mathematical modeling (19). The models predict that therapeutic strategies that increase the death rate or decrease the production of mature tumor cells will not eradicate progressively growing cancer lesions, whereas strategies that accelerate the death of tumor repopulating cells will be successful. Debulking tumor mass combined with targeting strategies specific for the minor subset of melanoma cells is therefore assumed to eradicate tumor lesions more rapidly while concurrently avoiding the risk of tumor relapse over time. The concept of selective targeting of tumor subpopulations is moreover supported by extensive clinical experience with germ-cell cancers where therapeutic elimination of the undifferentiated subset of cancer cells can cure patients even if differentiated cancer cells are left behind (20). These models together with the data presented in this paper provide a strong rationale for reexamining current strategies and drug development paradigms in melanoma treatment and in cancer therapy in general.
Materials and Methods
Cell Lines and Antibodies.
293T cells are human embryonic kidney cells that express the SV40 large T antigen. MeWo (ATCC; HTB-65) and Daudi (ATCC; CCL-213) cell lines are derived from human melanoma and Burkitt's lymphoma, respectively. All cell lines were cultured in RPMI medium 1640: 10% (vol/vol) FCS, 10 units/mL of penicillin and 200 units/mL of streptomycin (Invitrogen). The FITC–conjugated anti-CD3, the antityrosinase antibody mAb T311, the polyclonal anti-S100 Ab, and the anti–Melan-A mAb A103 were purchased from Dako; the PE–conjugated anti–HMW-MAA antibody EP-1, from Miltenyi Biotec. The APC–conjugated anti-CD3, the PE-Cy5.5–conjugated anti-annexin V, the FITC-conjugated antibodies with specificity for CD20 and granzyme B, respectively, and 7-AAD, were purchased from BD Pharmingen. The goat antihuman IgG antibody and its biotin-, FITC-, and PE-conjugated F(ab′)2 fragment derivatives were obtained from Southern Biotechnology. The Pacific Blue-conjugated goat antirabbit IgG antibody, the FITC, and Alexa Fluor 555-conjugated goat antimouse IgG antibody, and streptavidin-Alexa Fluor 488 were acquired from Invitrogen. The biotinylated anti-granzyme B antibody was purchased from Biozol. The anti–HMW-MAA antibody 763.64 was a kind gift from S. Ferrone (University of Pittsburgh, Pittsburgh, PA), the anti-CD20 scFv was from W. Wels (Georg-Speyer-Haus, Frankfurt, Germany), and the anti-CD19 scFv was from M. Topp (University Hospital, Würzburg, Germany).
Immunohistochemistry.
Skin biopsies from patients with metastatic melanoma were obtained from surgical excisions performed at the Department of Dermatology, University of Cologne. All patients signed the informed consent approved by the Institutional Ethics Committee (ref. no. 9645/96). The diagnosis was confirmed by a reference center. In addition, cryostat sections were stained using the PE-conjugated anti–HMW-MAA antibody (Miltenyi Biotec), the anti-CD20 antibody L27 (BD), the antityrosinase antibody (Dako), followed by the respective fluorochrom-conjugated secondary antibodies (Invitrogen) (5 μg/mL each). Slides were mounted with DAPI containing mounting medium (Vector Laboratories).
Isolation of Melanoma Cells.
To obtain melanoma cell suspensions, biopsies were disaggregated by incubation with collagenase I (1 mg/mL, pH 7.5) for 3–4 h at 37 °C and cultivated in RPMI medium 1640, 20% (vol/vol) FCS, 10 units/mL of penicillin and 200 units/mL of streptomycin. Melanoma cells were isolated with respect to HMW-MAA expression first by magnetic sorting to enrich HMW-MAA+ and HMW-MAA− cells using the PE-conjugated anti–HMW-MAA antibody and anti-PE MicroBeads in an AutoMACS device (Miltenyi Biotec). Additionally, cells were sorted by flow cytometry using FACSvantage (Becton Dickinson) cell sorter. CD20+ HMW-MAA+ spheroids were enriched by culture of melanoma biopsy cell suspensions in serum-free stem cell medium containing 8 ng/mL of bFGF (Stemcell Technologies) for 10–14 d as described (21). Melanoma cells were modified with CEA and CD20, respectively, by retroviral transduction.
RT-PCR.
RNA from cultivated melanoma cells and melanospheres was eluted using the RNeasy mini kit (Qiagen); the cDNA was synthesized using the SuperScript III kit (Invitrogen). CD20 and β-actin DNA fragments were amplified by PCR technique as described (22), using the following primer oligonucleotides: 5′-GGGGCTGTCCAGATTATGAA-3′ and 5′-TTCCTGGAAGAAGGCAAAGA-3′ for CD20 and 5′-TGCTATCCCTGTACGCCTCT-3′ and 5′-CCATCTCTTGCTCGAAGTCC-3′ for β-actin cDNA amplification.
Generation of Chimeric Antigen Receptors and Redirected T-Cell Activation.
The retroviral expression cassettes for the chimeric antigen receptors used in this study were generated by replacing the scFv binding domain of the anti-CEA CAR BW431/26scFv-Fc-CD3ζ (439) (23), with the 61scFv to obtain the HMW-MAA–specific CAR (570), with the L49scFv to obtain the melanotransferrin-specific CAR (579), with the FMC63 scFv (24) to obtain the CD19-specific CAR, and with the Leu16 scFv (25) to obtain the CD20-specific CAR, respectively. CD3+ T cells were isolated by magnetic-activated cell sorting (MACS) to purities >98% using human anti-CD3 MicroBeads (Miltenyi Biotec). CD3+ T cells were retrovirally transduced to express the CAR as described earlier (23). Specific cytotoxicity of engineered T cells in vitro was monitored as described in ref. 23. IFN-γ was recorded by ELISA using matched pairs of antibodies specific for IFN-γ (clones NIB 42 and B133.5) (Becton Dickinson).
Tumor Growth in NIH-III Mice.
Tumor cells (1 × 106 cells per mouse) were s.c. injected in NIH-III mice (Crl:NIH-LystbgFoxn1nuBtkxid) (purchased from Charles River Laboratories) and melanomas were grown until a volume of 15–20 mm3 was reached. CD3+ T cells (1 × 106 T cells per mouse) with or without the respective CAR were i.v. applied three times at 2-d intervals. Tumor size was measured every 2 d and mice were killed when tumor growth reached an overall volume of 400 mm3. The observation period for mice with successfully cleared tumors was >36 wk.
Statistical Procedures.
Statistical analyses were performed using the χ2, two-tailed Student´s t test and log-rank analysis, respectively.
Supplementary Material
Acknowledgments
We thank Christoph Göttlinger for flow cytometry-based cell sorting, Jan Zamek for technical assistance, and Samir Tawardos for his help in animal experiments. We thank Drs. S. Ferrone, M. Topp, and W. Wels for supplying scFv antibodies and M. Grez for CD20 cDNA. Our work was supported by the Deutsche Krebshilfe and the Fortune program of the medical faculty of the University of Cologne.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009069108/-/DCSupplemental.
References
- 1.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 2.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheatley K, et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–252. doi: 10.1016/s0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 5.Dudley ME, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25(3):243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khammari A, et al. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. J Invest Dermatol. 2009;129:2835–2842. doi: 10.1038/jid.2009.144. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshhar Z. In: Therapeutic Antibodies. Chernajovsky Y, Nissim A, editors. Berlin Heidelberg: Springer; 2008. pp. 329–342. [Google Scholar]
- 10.Campoli MR, et al. Human high molecular weight-melanoma-associated antigen (HMW-MAA): A melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/critrevimmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 11.Koehler H, Kofler D, Hombach A, Abken H. CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res. 2007;67:2265–2273. doi: 10.1158/0008-5472.CAN-06-2098. [DOI] [PubMed] [Google Scholar]
- 12.Quintana E, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cvetković RS, Perry CM. Rituximab: A review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2006;66:791–820. doi: 10.2165/00003495-200666060-00005. [DOI] [PubMed] [Google Scholar]
- 14.Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin P, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 16.Coiffier B, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 17.Aklilu M, et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15(7):1109–1114. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 18.Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 19.Dingli D, Michor F. Successful therapy must eradicate cancer stem cells. Stem Cells. 2006;24:2603–2610. doi: 10.1634/stemcells.2006-0136. [DOI] [PubMed] [Google Scholar]
- 20.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- 21.Fang D, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 22.Sonoki T, et al. Establishment of a novel CD20 negative mature B-cell line, WILL2, from a CD20 positive diffuse large B-cell lymphoma patient treated with rituximab. Int J Hematol. 2009;89:400–402. doi: 10.1007/s12185-009-0295-4. [DOI] [PubMed] [Google Scholar]
- 23.Hombach A, et al. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 1999;6:300–304. doi: 10.1038/sj.gt.3300813. [DOI] [PubMed] [Google Scholar]
- 24.Cooper LJN, et al. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 25.Müller T, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–423. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.