Abstract
Digital reduction is a striking evolutionary phenomenon that is clearly exemplified in theropod dinosaurs by the functionally didactyl manus of tyrannosaurids, the flight-adapted manus of birds (Aves), and the tridactyl but digit II-dominated manus of alvarezsauroids. The enlargement of manual digit II in alvarezsauroids and the concurrent reduction of the lateral digits have been interpreted as adaptations for digging, although no detailed biomechanical analysis of hand function has so far been carried out for this group. In the derived alvarezsauroid clade Parvicursorinae, the lateral digits are so small as to be presumably vestigial. Here we report a new alvarezsauroid, Linhenykus monodactylus gen. et sp. nov., based on a specimen from the Upper Cretaceous Wulansuhai Formation of Inner Mongolia, China. Cladistic analysis identifies Linhenykus as the most basal parvicursorine, and digit II of the manus retains a slender morphology and other primitive features. However, Linhenykus is also highly apomorphic in exhibiting the most extreme reduction of the lateral manual digits seen in any alvarezsauroid. Phalanges are retained only on the most medial digit (digit II), making Linhenykus the only known functionally monodactyl nonavian dinosaur. Other parvicursorines are more primitive in retaining a tridactyl manus but more derived in that digit II is highly robust and shows other apomorphic features in both of its phalanges. The unexpected combination of features seen in the hand of Linhenykus points to a complex mosaic pattern of manual evolution in alvarezsauroids, with loss of the presumably vestigial outer digits being decoupled from change in the form of digit II.
Keywords: mosaic evolution, Theropoda, Late Cretaceous, biogeography
Modifications of the hand were commonplace in the evolution of theropod dinosaurs. Primitive theropods had five metacarpals, although the lateralmost of these lacked phalanges and therefore was not a functional digit. A reduced count of three manual digits is typically present in members of the derived theropod clade Tetanurae, which includes the birds, and several tetanuran subgroups underwent further modification of the manus. Tyrannosaurids present a widely known example of reduction to two functional digits, and in Aves the phalanges of the three digits are reduced in number and partially fused to strengthen the distalmost part of the wing. Another striking example of digital reduction in theropods occurred in the Alvarezsauroidea, within which the manus became reduced to one functional medial digit and two very small, and presumably vestigial, lateral digits.
Alvarezsauroids were originally considered to be a group of flightless birds, but it is now widely accepted that they are not nested within Aves (1–3) and instead represent a basal maniraptoran lineage. Three functional digits are present in the basalmost known alvarezsauroid, the Asian Jurassic taxon Haplocheirus (1). In other members of the group, however, the outer digits are reduced to at least some degree (1, 4, 5). Derived members of the Alvarezsauroidea form a monophyletic group known as the Parvicursorinae (here defined as the most inclusive group including Parvicursor but not Patagonykus). The most distinctive part of the parvicursorine skeleton is perhaps the manus, in which digits III and IV are drastically reduced and digit II is normally widened and lengthened relative to the other forelimb elements. [We refer to the digits of the tetanuran hand as II–IV in this article (6, 7), although we recognize that some evidence favors identifying them as I–III (8–12). This issue of homology is immaterial to the present study.]
Here we report a new parvicursorine based on a specimen (Figs. 1 and 2) from the Upper Cretaceous Wulansuhai Formation of Inner Mongolia, China. Unlike other parvicursorines, this taxon retains phalanges only on digit II, the phalanges of the other manual digits having been entirely lost. As a functionally monodactyl nonavian dinosaur, the new parvicursorine provides important information on the phenomenon of digit reduction in the evolution of the alvarezsauroid hand.
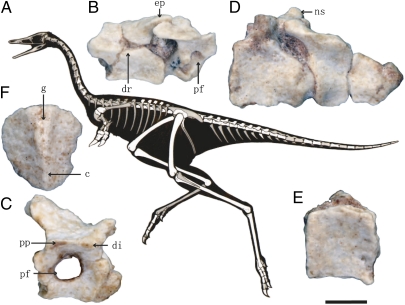
Fig. 1.
Axial skeletal morphology of Linhenykus monodactylus holotype. (A) Skeletal silhouette showing preserved bones (missing portions shown in gray). (B) Two middle cervical vertebrae in left lateral view. (C) Middle dorsal vertebra in left lateral view. (D) Second and third caudal vertebrae in left lateral view. (E) First caudal vertebra in right lateral view. (F) Sternum in dorsal view. c, carina; di, diapophysis; dr, diapophyseal ridge; ep, epipophysis; g, groove; ns, neural spine; pf, pneumatic foramen; pp, parapophysis. (Scale bar, 5 mm.)
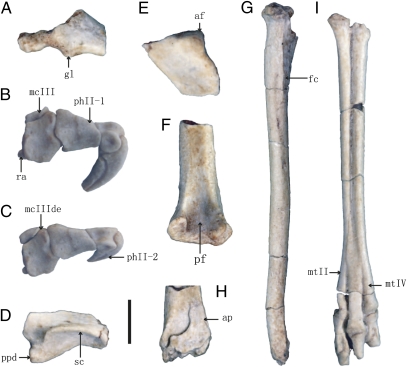
Fig. 2.
Appendicular skeletal morphology of Linhenykus monodactylus holotype. (A) scapulocoracoid in lateral view. Left manus of IVPP V17608 in medial (B) and dorsal (C) views (both slightly oblique). (D) Left ilium in lateral view. (E) Left pubis in lateral view. (F) Right femur in posterior view. (G) Right tibiotarsus in lateral view. (H) Distal end of right tibiotarsus in anterior view. (I) Left pes in anterior view. af, acetabular fossa; ap, ascending process; fc, fibular crest; gl, glenoid lip; mcIII, metacarpal III; mcIII de, distal end of metacarpal III; mtII, metatarsal II; mtIV, metatarsal IV; pf, popliteal fossa; ph II-1, phalanx II-1; ph II-2, phalanx II-2; ppd, pubic peduncle; ra, radiale; sc, supracetabular crest. (Scale bar, 5 mm for A–E, 10 mm for F, G, and I, 12 mm for H.)
Systematic Paleontology
The specimen described in this article is referable to the following nested clades: Theropoda Marsh, 1881; Coelurosauria Huene, 1914; Alvarezsauroidea Bonaparte, 1991; Parvicursorinae Karhu and Rautian, 1996; Linhenykus monodactylus gen. et sp. nov.
Etymology
The generic name is a combination of Linhe (a city in Inner Mongolia near the area where the specimen was found), and onyx (Greek, “claw”); the specific name refers to the presence of a single finger in this animal.
Holotype
IVPP (Institute of Vertebrate Paleontology and Paleoanthropology) V17608, a partial postcranial skeleton including cervical, dorsal, sacral, and caudal vertebrae, the left scapulocoracoid, a nearly complete sternum, much of the forelimbs, a partial pelvis, nearly complete hindlimbs, and some unidentified fragments.
Locality
Fine-grained nodular sandstone layer above bioturbated strata in the “Gate area” at Bayan Mandahu, north of the city of Linhe, Inner Mongolia, China (detailed locality information is available from the authors upon request); Wulansuhai Formation, Campanian, Upper Cretaceous (13).
Diagnosis
A small parvicursorine apomorphically possessing a transversely compressed metacarpal III without a distal articular surface; also differs from all other parvicursorines in having a longitudinal ventral furrow along the entire length of each cervical centrum, diapophyseal ridges on each cervical vertebra that extend to the posterodorsal rim of the centrum, extremely weak, ridge-like epipophyses on the postzygapophyses of the middle cervical vertebrae, large pneumatic foramina in the middorsal vertebrae, and anteriormost caudal vertebrae whose centra are amphiplatyan and whose neural spines are located completely posterior to the neural pedicles.
Description and Comparisons
All preserved vertebrae of Linhenykus have completely closed neurocentral sutures. However, intercentral sutures are present between successive sacral vertebrae, metacarpal III is not fused to metacarpal II, and the proximal tarsals are not completely coossified with the tibiae. Although neurocentral fusion is not an infallible indicator of ontogenetic stage (14), this combination of vertebral and appendicular features suggests a relatively late, probably subadult, ontogenetic stage for Linhenykus.
The holotype specimen is small, in that the estimated femoral length of ≈70 mm would imply a body mass of ≈450 g (15). Thus, Linhenykus was smaller and lighter than Mononykus olecranus (16, 17) but larger and heavier than Parvicursor remotus (18) (SI Appendix).
The axial skeleton more closely resembles those of other parvicursorines than those of more basal alvarezsauroids in the following characteristics (Fig. 1): cervical centra strongly opisthocoelous and each bearing a longitudinal ventral furrow (19, 20); dorsal vertebrae opisthocoelous, lacking hyposphene–hypantrum articulations; dorsal parapophyses elevated to level of diapophyses, and dorsal postzygapophyseal articular facets oriented medially; posteriormost dorsal centrum biconvex; and anterior caudal vertebrae with transverse processes anteriorly displaced. In contrast, however, to both other parvicursorines and more basal alvarezsauroids (19), some dorsal centra have pneumatic foramina, and the anteriormost caudal centra are amphiplatyan. The small sternum generally resembles those of other parvicursorines but differs from them in numerous details, including much greater proportional transverse width, convexity of the anterior margin, medial displacement of the articular facet for the coracoid, and a much weaker carina that bears a proportionally longer medial groove (Fig. 1F).
Uniquely among alvarezsauroids (19), the scapula and coracoid of Linhenykus are fused together. The scapulocoracoid bears a weakly developed glenoid lip (Fig. 2A), a feature present in basal alvarezsauroids (1) but lost in other parvicursorines. The posterior surface of the distal end of the humerus is slightly concave, representing a condition intermediate between other Asian alvarezsauroids and Patagonykus (19).
The Linhenykus holotype includes a small, rounded radiale (Fig. 2 B and C), a bone previously only known in Haplocheirus among alvarezsauroids (1). The manus has a general similarity to those of other parvicursorines (19, 21), reflected in such features as hypertrophied manual digit II subequal to humerus in thickness, metacarpal II dorsoventrally compressed, transversely broad, and with highly modified proximal end, and metacarpal III much smaller than metacarpal II (Fig. 2 B and C). However, Linhenykus differs from other parvicursorines in many other manual features (19). Digit II of Linhenykus is conspicuously more primitive than those of other parvicursorines in being more slender, in that the proximal phalanx is less dorsoventrally compressed and bears a less developed laterodorsal process, and in that manual phalanx II-2 is less hypertrophied, less dorsoventrally compressed, and characterized by lateral grooves that are only partly enclosed in bone. By contrast, the more lateral part of the manus of Linhenykus is highly derived relative to the condition in other parvicursorines. Digit III bears no phalanges, as indicated by the fact that metacarpal III is a very small element whose distal end is strongly compressed in the transverse direction and lacks a distal articular surface (Fig. 2 B and C). Metacarpal IV is not preserved in the Linhenykus holotype. Given that digit III is a reduced structure lacking phalanges, it is probable that metacarpal IV is entirely absent in Linhenykus. Even if metacarpal IV is present, it is highly unlikely to bear phalanges given the prevailing patterns of digital reduction in tetrapods. Consequently, digit II clearly represents the only phalanx-bearing digit in the manus.
The preacetabular process of the ilium is nearly vertical in orientation, but the lateral surface of the iliac blade above the pubic peduncle faces somewhat dorsally as in other parvicursorines (19). The supracetabular crest is more prominent anteriorly than posteriorly (Fig. 2D), as in Mononykus (17). Unlike in other parvicursorines (19, 22), the pubis lacks a preacetabular tubercle, the proximal articular surface is subtriangular in outline rather than kidney-shaped, and the lateral margin of the proximal surface is not concave (Fig. 2E).
The femur is more primitive in general morphology than those of most other parvicursorines. Unlike in Mononykus (17), but resembling the condition seen in Parvicursor (18) and Patagonykus (5), the popliteal fossa is widely open distally (Fig. 2F). The medial condyle is transversely narrow in distal view and subtriangular in posterior view (Fig. 2F). The tibiotarsus is nearly identical in general morphology to those of other parvicursorines (Fig. 2 G and H). As in other parvicursorines (2, 23), the metatarsus is longer than the femur and exhibits a specialized arctometatarsalian condition in which metatarsal III terminates well short of the proximal end of the metatarsus (Fig. 2I). The pedal phalanges are relatively long and slender compared with those of Mononykus (17).
Discussion
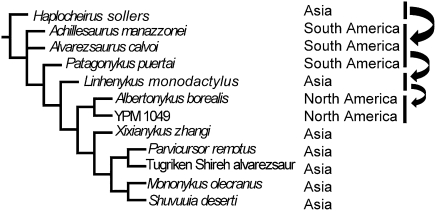
A cladistic analysis of alvarezsauroid relationships (SI Appendix) produced five most parsimonious trees, the strict consensus of which is shown in Fig. 3. Linhenykus is recovered at the base of the Parvicursorinae in all of the most parsimonious trees. Within the framework of the phylogeny proposed in Fig. 3, the biogeographic distribution of alvarezsauroids is best explained by a dispersal hypothesis (SI Appendix). Under this interpretation, the Alvarezsauroidea originated in Asia, and at least three dispersal events subsequently occurred: one from Asia to Gondwana, one from Gondwana to Asia, and finally one from Asia to North America. This dispersal hypothesis is consistent with faunal interchanges between Gondwana and other continents that have been suggested on the basis of the distributions of abelisauroid dinosaurs and some other reptilian groups (24). However, the hypothesis is inconsistent with the distributions of certain other theropod groups, which are better explained by vicariance (25, 26). Unfortunately, the Jurassic fossil record of alvarezsauroids is scant, and this group is currently unknown from the Lower Cretaceous. A more stringent test of the biogeographic hypothesis awaits further data from these poorly represented time periods and from additional geographic regions.
Fig. 3.
Suggested systematic position of Linhenykus monodactylus among the alvarezsauroids, based on a numerical cladistic analysis (SI Appendix). Continental distributions are listed after taxon names. Arrows indicate the directions of episodes of dispersal. The geographical distribution of alvarezsauroids is best explained by a dispersal hypothesis.
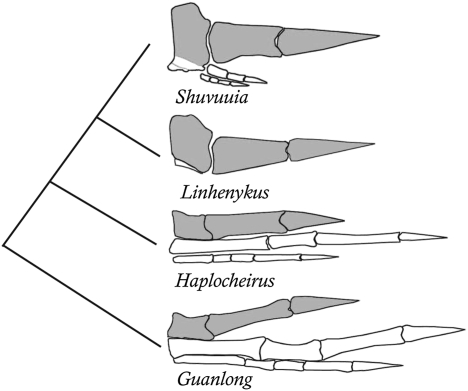
As a basal parvicursorine, Linhenykus provides important data on the evolution of the highly modified alvarezsauroid hand. In the evolution of theropod dinosaurs, digital reduction has occurred independently multiple times in different ways. In most cases, reduction of a given metacarpal has been accompanied by loss of the phalanges of the same digit, as best exemplified by the didactyl manus of tyrannosaurids (27). By contrast, the two lateral manual digits of derived alvarezsaurids have reduced metacarpals but still at least approximate a normal phalangeal formula even though the individual phalanges are relatively small (19, 28). Linhenykus bears phalanges on only one metacarpal, documenting an extreme degree of digital reduction within this group (Fig. 4). Furthermore, the single phalanx-bearing manual digit of Linhenykus (digit II) is more primitive than the corresponding digit in more derived parvicursorines (see above), suggesting that Linhenykus was less derived in having a relatively less hypertrophied digit II but more derived with respect to the loss of phalanges on digits III and IV (Fig. 4). This documents a decoupling, representing mosaic evolution on a small scale, between two different types of specialization in the parvicursorine hand: functional refinement of digit II and reduction of digits III and IV. Mosaic evolution on various scales has also been suggested in other dinosaur groups, such as the Tyrannosauroidea (29, 30) and the Sauropoda (31). The classic exemplar of mosaic evolution is the avialan dinosaur Archaeopteryx, which is bird-like in some details of the skeleton and in having asymmetric flight feathers but more typically reptilian in retaining such features as a long bony tail and unfused metapodial elements (32). Although the transition from nonavian dinosaurs to birds is now understood in far more detail (33), current evidence confirms that asymmetric feathers of modern aspect were in place long before many other aspects of derived avian morphology. The discovery of Linhenykus further extends the known distribution of the phenomenon of mosaic evolution within dinosaurs.
Fig. 4.
Simplified alvarezsauroid phylogeny showing enlargement of manual digit II (in gray) and reduction of manual digits III and IV. Linhenykus has a less specialized digit II but much more reduced lateral digits compared with other derived alvarezsaurs. The basal tyrannosauroid Guanlong is used to illustrate the typical tetenuran manus. Mani are not to scale.
Adaptation to a specialized function can lead to hypertrophy of some digits and reduction or loss of others (34). The highly modified manus of derived alvarezsauroids, in which digit II significantly widens and lengthens and acquires a large trenchant ungual whereas digits III and IV are reduced, has been suggested to reflect adaptation for digging (23, 35, 36). These manual features are accompanied by a suite of other forelimb characters, including a distally located deltopectoral crest of the humerus, a large olecranon process of the ulna, and a short forearm and manus, that collectively resemble the biomechanical tool kit of extant mammalian diggers in hard substrates, such as the giant armadillo Priodontes (23, 37). In this context, the robustness and derived morphology of digit II in typical parvicursorines are most straightforwardly interpreted as additional digging adaptations.
However, the combination in Linhenykus of a less specialized manual digit II and total absence of phalanges on more lateral digits demonstrates that manual evolution in alvarezsauroids did not follow a simple linear trend (Fig. 4). The presence of lateral digits with phalanges in derived parvicursorines and their absence in Linhenykus can potentially be explained by the likelihood that the tiny lateral digits of the typical parvicursorine manus are vestigial, as postulated for various other structures in dinosaurs (38). Because vestigial structures have little or no functional significance by definition and are typically small and biologically inexpensive to build and maintain, they experience low levels of stabilizing selection and tend to show high morphological variability. This phenomenon is most amenable to quantitative study at the intraspecific level (39, 40), but examples at higher taxonomic levels are also known. Amphisbaenians or “worm lizards,” a group of squamates uniformly lacking external hindlimbs, vary significantly at the interspecific and intergeneric levels with respect to the form of the vestigial pelvic skeleton (41, 42). A slender, degenerate ilium is present but varies from boomerang-shaped in Amphisbaena fuliginosa to splint-like in Amphisbaena ewerbecki and hatchet-like in Blanus cinereus (Fig. 9 in ref. 41). Blanus is apparently unique in retaining an additional calcified (although not ossified) pelvic element. Furthermore, Blanus and Bipes both retain internal rudiments of the hindlimb skeleton, whereas these are lacking in other amphisbaenians (41, 43). The presence of similar variability in the apparently vestigial lateral digits of alvarezsaurid dinosaurs is thus not surprising.
Materials and Methods
We investigated the systematic position of Linhenykus monodactylus using a dataset specifically designed to illuminate alvarezsaurid interrelationships (23), adding three taxa including Linhenykus monodactylus. The data matrix was analyzed using the NONA (version 2.0, S. M. de Tucuman, Argentina) software package, and matrix formatting and character exploration were performed in WinClada (Nixon, KC, Ithaca, NY). The analysis was run with the following search parameters: 1,000 replications, 15 starting trees per replication, and Multiple TBR+TBR (mult*max*) search strategy.
Supplementary Material
Acknowledgments
We thank Wang Jianming, Liu Jinsheng, Li Zhiquan, and Zhao Xinquan for coordinating the project; Wang Haijun for assistance in the field; and Yu Tao and Ding Xiaoqin for preparing the specimen. This work was supported by the Chinese Academy of Sciences, the Department of Land and Resources, Inner Mongolia, China, the Gloyne Outdoor Geological Research Fund of the Geological Society of London, and by a US National Science Foundation Office of International Science and Engineering grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011052108/-/DCSupplemental.
References
- 1.Choiniere JN, et al. A basal alvarezsauroid theropod from the early Late Jurassic of Xinjiang, China. Science. 2010;327:571–574. doi: 10.1126/science.1182143. [DOI] [PubMed] [Google Scholar]
- 2.Turner AH, Nesbitt SJ, Norell MA. A large alvarezsaurid from the Late Cretaceous of Mongolia. Am Mus Novit. 2009;3648:1–14. [Google Scholar]
- 3.Senter P. A new look at the phylogeny of Coelurosauria (Dinosauria:Theropoda) J Systematic Palaent. 2007;5:1–35. [Google Scholar]
- 4.Bonaparte JF. The vertebrate fossils of the Rio Colorado Formation, from the city of Neuquén and surrounding areas, Upper Cretaceous, Argentina (Translated from Spanish) Paleontología. 1991;4:17–123. [Google Scholar]
- 5.Novas FE. Anatomy of Patagonykus puertai (Theropoda, Maniraptora, Alvarezsauridae) from the Late Cretaceous of Patagonia. J Vertebr Paleontol. 1997;17:137–166. [Google Scholar]
- 6.Xu X, et al. A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature. 2009;459:940–944. doi: 10.1038/nature08124. [DOI] [PubMed] [Google Scholar]
- 7.Kundrát M, Seichert V, Russell AP, Smetana K., Jr Pentadactyl pattern of the avian wing autopodium and pyramid reduction hypothesis. J Exp Zool. 2002;294:152–159. doi: 10.1002/jez.10140. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier J. Saurischian monophyly and the origin of birds. Mem California Acad Sci. 1986;8:1–55. [Google Scholar]
- 9.Wagner GP, Gauthier JA. 1,2,3 = 2,3,4: A solution to the problem of the homology of the digits in the avian hand. Proc Natl Acad Sci USA. 1999;96:5111–5116. doi: 10.1073/pnas.96.9.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee S. Counting the fingers of birds and dinosaurs. Science. 1998;280:355a. [Google Scholar]
- 11.Vargas AO, Fallon JF. Birds have dinosaur wings: The molecular evidence. J Exp Zoolog B Mol Dev Evol. 2005;304:86–90. doi: 10.1002/jez.b.21023. [DOI] [PubMed] [Google Scholar]
- 12.Vargas AO, Kohlsdorf T, Fallon JF, Vandenbrooks J, Wagner GP. The evolution of HoxD-11 expression in the bird wing: Insights from Alligator mississippiensis. PLoS ONE. 2008;3:e3325. doi: 10.1371/journal.pone.0003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerzykiewicz T, et al. Djadokhta Formation correlative strata in Chinese Inner Mongolia: An overview of the stratigraphy, sedimentary geology, and paleontology and comparisons with the type locality in the pre-Altai Gobi. Can J Earth Sci. 1993;30:2180–2190. [Google Scholar]
- 14.Irmis RB. Axial skeletal ontogeny in the Parasuchia (Archosauria: Pseudosuchia) and its implications for ontogenetic determination in Archosaurs. J Vertebr Paleontol. 2007;27:350–361. [Google Scholar]
- 15.Christiansen P, Fariña RA. Mass prediction in theropod dinosaurs. Hist Biol. 2004;16:85–92. [Google Scholar]
- 16.Perle A, Norell MA, Chiappe L, Clark JM. Flightless bird from the Cretaceous of Mongolia. Nature. 1993;362:623–626. [Google Scholar]
- 17.Perle A, et al. Skeletal morphology of Mononykus olecranus (Theropoda: Avialae) from the Late Cretaceous of Mongolia. Am Mus Novit. 1994;3105:1–29. [Google Scholar]
- 18.Karhu AA, Rautian AS. A new family of Maniraptora (Dinosauria: Saurischia) from the Late Cretaceous of Mongolia. Paleont J. 1996;30:583–592. [Google Scholar]
- 19.Chiappe LM, Norell MA, Clark JM. In: Mesozoic Birds: Above the Heads of Dinosaurs. Chiappe LM, Witmer LM, editors. Berkeley: Univ California Press; 2002. pp. 87–120. [Google Scholar]
- 20.Novas FE. Alvarezsauridae, Cretaceous maniraptorans from Patagonia and Mongolia. Mem Queensl Mus. 1996;39:675–702. [Google Scholar]
- 21.Chiappe LM, Norell MA, Clark JM. Phylogenetic position of Mononykus (Aves: Alvarezsauridae) from the Late Cretaceous of the Gobi Desert. Mem Queensl Mus. 1996;39:557–582. [Google Scholar]
- 22.Hutchinson JR, Chiappe LM. The first known alvarezsaurid (Theropoda: Aves) from North America. J Vertebr Paleontol. 1998;18:447–450. [Google Scholar]
- 23.Longrich NR, Currie PJ. Albertonykus borealis, a new alvarezsaur (Dinosauria: Theropoda) from the Early Maastrichtian of Alberta, Canada: Implications for the systematics and ecology of the Alvarezsauridae. Cretac Res. 2009;30:239–252. [Google Scholar]
- 24.Pereda-Suberbiola X. Biogeographical affinities of Late Cretaceous continental tetrapods of Europe: A review. Bull Soc Geol Fr. 2009;180:57–71. [Google Scholar]
- 25.Makovicky PJ, Apesteguía S, Agnolín FL. The earliest dromaeosaurid theropod from South America. Nature. 2005;437:1007–1011. doi: 10.1038/nature03996. [DOI] [PubMed] [Google Scholar]
- 26.Upchurch P, Hunn CA, Norman DB. An analysis of dinosaurian biogeography: Evidence for the existence of vicariance and dispersal patterns caused by geological events. Proc Biol Sci. 2002;269:613–621. doi: 10.1098/rspb.2001.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtz TR. In: The Dinosauria. 2nd Ed. Weishampel DB, Dodson P, Osmólska H, editors. Berkeley: Univ California Press; 2004. pp. 111–136. [Google Scholar]
- 28.Suzuki S, et al. A new specimen of Shuvuuia deserti Chiappe et al., 1998 from the Mongolian late Cretaceous with a discussion of the relationships of alvarezsaurids to other theropod dinosaurs. Contrib Sci. 2002;494:1–18. [Google Scholar]
- 29.Snively E, Henderson DM, Phillips DS. Fused and vaulted nasals of tyrannosaurid dinosaurs: Implications for cranial strength and feeding mechanics. Acta Palaeontol Pol. 2006;51:435–454. [Google Scholar]
- 30.Li DQ, et al. A longisrostrine tyrannosauroid from the Early Cretaceous of China. Proc R Soc B. 2010;277:183–190. doi: 10.1098/rspb.2009.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buffetaut E. A new sauropod dinosaur with prosauropod-like teeth from the Middle Jurassic of Madagascar. Bull Societe Geologique France. 2005;176:467–473. [Google Scholar]
- 32.Stebbins GL. Mosaic evolution: An integrating principle for the modern synthesis. Experientia. 1983;39:823–834. doi: 10.1007/BF01990398. [DOI] [PubMed] [Google Scholar]
- 33.Xu X. Feathered dinosaurs from China and the evolution of major avian characters. Integr Zool. 2006;1:4–11. doi: 10.1111/j.1749-4877.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 34.Liem KF, Bemis WE, Walker WF, Grande L. Functional Anatomy of the Vertebrates: An Evolutionary Perspective. 3rd Ed. Fort Worth, TX: Harcourt College; 2001. [Google Scholar]
- 35.Senter P. Function in the stunted forelimbs of Mononykus olecranus (Theropoda), a dinosaurian anteater. Paleobiology. 2005;31:373–381. [Google Scholar]
- 36.Zhou ZH. Is Mononykus a bird? Auk. 1995;112:958–963. [Google Scholar]
- 37.Hildebrand M, Goslow G. Analysis of Vertebrate Structure. 5th Ed. New York: John Wiley & Sons; 2001. [Google Scholar]
- 38.Senter P. Vestigial skeletal structures in dinosaurs. J Zool (Lond) 2010;280:60–71. [Google Scholar]
- 39.Tague RG. Variability of a vestigial structure: First metacarpal in Colobus guereza and Ateles geoffroyi. Evolution. 1997;51:595–605. doi: 10.1111/j.1558-5646.1997.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 40.Tague RG. Variability of metapodials in primates with rudimentary digits: Ateles geoffroyi, Colobus guereza, and Perodicticus potto. Am J Phys Anthropol. 2002;117:195–208. doi: 10.1002/ajpa.10028. [DOI] [PubMed] [Google Scholar]
- 41.Zangerl R. Contributions to the osteology of the post-cranial skeleton of the Amphisbaenidae. Am Midl Nat. 1945;33:764–780. [Google Scholar]
- 42.Renous S, Gasc JP, Reynaud A. Comments on the pelvic appendicular vestiges in an amphisbaenian: Blanus cinereus (Reptilia, Squamata) J Morphol. 1991;209:23–38. doi: 10.1002/jmor.1052090104. [DOI] [PubMed] [Google Scholar]
- 43.Kearney M, Stuart BL. Repeated evolution of limblessness and digging heads in worm lizards revealed by DNA from old bones. Proc Biol Sci. 2004;271:1677–1683. doi: 10.1098/rspb.2004.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.