Abstract
Whereas the role of NogoA in limiting axonal fiber growth and regeneration following an injury of the mammalian central nervous system (CNS) is well known, its physiological functions in the mature uninjured CNS are less well characterized. NogoA is mainly expressed by oligodendrocytes, but also by subpopulations of neurons, in particular in plastic regions of the CNS, e.g., in the hippocampus where it is found at synaptic sites. We analyzed synaptic transmission as well as long-term synaptic plasticity (long-term potentiation, LTP) in the presence of function blocking anti-NogoA or anti-Nogo receptor (NgR) antibodies and in NogoA KO mice. Whereas baseline synaptic transmission, short-term plasticity and long-term depression were not affected by either approach, long-term potentiation was significantly increased following NogoA or NgR1 neutralization. Synaptic potentiation thus seems to be restricted by NogoA. Surprisingly, synaptic weakening was not affected by interfering with NogoA signaling. Mechanistically of interest is the observation that by blockade of the GABAA receptors normal synaptic strengthening reoccurred in the absence of NogoA signaling. The present results show a unique role of NogoA expressed in the adult hippocampus in restricting physiological synaptic plasticity on a very fast time scale. NogoA could thus serve as an important negative regulator of functional and structural plasticity in mature neuronal networks.
Changes in the connectivity of neurons—synaptic plasticity—regulate the fine-tuning of neuronal networks during development and during adult learning. Synaptic plasticity includes functional and structural modifications at neurons and may be the underlying mechanism for learning and memory processes (1). The storage of new information therefore might depend on ever changing neuronal networks. On the other hand, recent data indicate that the large scale organization of neuronal networks is kept remarkably stable to maintain a constant flow of information and to support long-term memory storage (reviewed in ref. 2).
In the CA1 region of the hippocampus, changes in neuronal activity can lead to changes in synaptic weight. Molecular mechanisms include here changes in the number or properties of neurotransmitter receptors, retrograde messengers, structural changes at synapses, and activation of transcription/translation (3). What is less clear is whether molecular mechanisms restricting changes in synaptic weight and thus stabilizing the synapse also play a role as well. In this context it is interesting to note that preventing further potentiation of a given set of synapses in a neuronal network can be induced by a homeostatic shutdown of long-term potentiation (LTP) after intense stimulation (4). In the search for such molecular stabilizers, we investigated the protein NogoA, which has been identified as a negative regulator of structural changes in the CNS (5). NogoA prevents neurite outgrowth in the adult CNS after injury (6) and regulates the progressive restriction of plasticity during development (7–9). In the adult CNS, the bulk of NogoA is found in myelin, but interestingly, neuronal NogoA expression persists in those regions of the CNS that are known to be particularly plastic, e.g., the hippocampus and the olfactory system (10, 11, see also ref. 12). In the mature CNS both known receptors for NogoA, Nogo66 receptor 1 (NgR1) and the paired Ig-like receptor B (PirB), negatively modulate activity-dependent synaptic plasticity. In ngr1 knockout (9) and in pirB knockout mice (13), ocular dominance plasticity continues after the end of the critical period, suggesting that NgR1 and PirB signaling stabilizes the neural circuitry and limits experience-dependent plasticity. In addition, NgR1 signaling can influence LTP in concert with FGF2 (14) as well as long-term memory (15).
It is noteworthy that NogoA/NgR1 are expressed in pyramidal cells of the hippocampus (12), that their expression is regulated by neuronal activity (16, 17), and that NgR1 is located at synapses in the adult CNS (18). However, the physiological role of neuronal NogoA in the hippocampus of adult animals has remained largely unexplored (for a review see ref. 5).
Here we report a unique, acute physiological function of NogoA in the mature hippocampus acting on a fast time scale. Our results suggest that NogoA is involved in specifically stabilizing synaptic weight.
Results
Hippocampal Slices Treated with Function Blocking Antibodies Against NogoA.
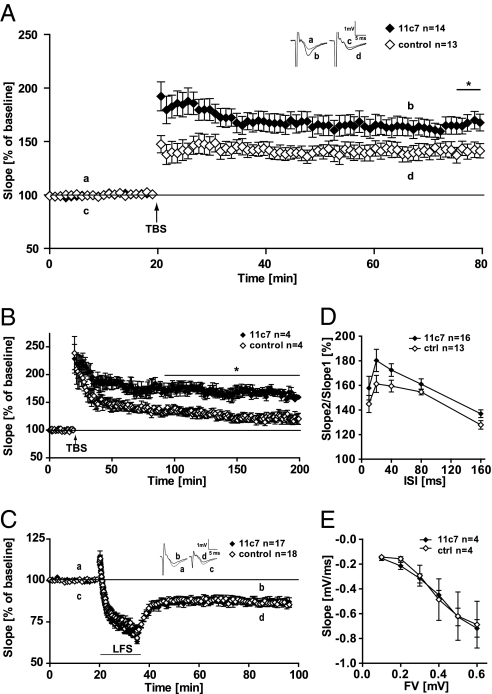
To study possible acute effects of NogoA in regulating synaptic plasticity, we treated wild-type (WT) mouse acute hippocampal slices with the NogoA-specific function blocking antibody (Ab) 11c7 (19) or control Ab (anticyclosporin) for 1 h and induced under these conditions LTP at the CA3-CA1 Schaffer-collateral pathway. Theta burst stimulation (TBS) to hippocampal slices of adult mice (P40–P60) was applied 20 min after baseline recording. The 11c7 Ab-treated slices showed already in the induction phase a higher LTP, which resulted in a significant difference 55–60 min after TBS (Fig. 1A; P = 0.02, t test). The average potentiation in 11c7-treated WT slices was 168 ± 7.4% (n = 14 slices per 7 animals), whereas control Ab-treated slices showed a potentiation of 141 ± 8.0% (n = 13 slices per 6 animals) 55–60 min after the TBS.
Fig. 1.
NogoA blocking functional antibody experiment. (A–C) Hippocampal slices were treated with NogoA blocking antibody (Ab) 11c7 (filled diamonds) or control (open diamonds). (A) LTP was induced by application of TBS, 10 trains of four pulses at 100 Hz, interburst interval 200 ms, repeated three times (arrow). At 60 min after TBS, a significant difference between 11c7 and control Ab could be observed indicated by the asterisk (P = 0.027, t test). (B) This significant difference became more pronounced and remained stable for a further 3 h (P = 0.01, t test). (C) LTD with 11c7 Ab revealed no difference compared with control Ab treatment (60 min after LFS: 11c7, 87 ± 2.2%; control, 86 ± 1.9%). (A and C) Insets show original traces from representative individual experiments; letters correspond to the time point when traces were taken. (D) PPF in 11c7-treated slices is slightly, but not significantly increased. (E) Input-output strength in 11c7-treated slices revealed no alterations compared with control Ab treatment. For the corresponding immunohistochemistry, see Fig. S1.
To investigate the role of NogoA in controlling protein-synthesis dependent l-LTP, we recorded the post-TBS potentiation for 3 h in 11c7-treated WT slices compared with control Ab-treated ones (Fig. 1B). Indeed the difference between both treatments became even more pronounced and was highly significant 3 h after TBS (P = 0.01, t test). The average potentiation in 11c7-treated WT slices was 158 ± 6.7% (n = 4/2) and 126 ± 5.7% (n = 4/2) in control Ab-treated slices, respectively.
We next investigated the effect of 11c7 treatment on the expression of long-term depression (LTD), induced by a 15-min low-frequency (1 Hz) stimulus (LFS) protocol. NogoA neutralization did not induce any differences in LTD induction or maintenance compared with control Ab-treated slices (Fig. 1C). At 55–60 min after LFS, the average depression was 85 ± 2.2% in 11c7 (n = 17/8) and 84 ± 1.9% in control (n = 18/10) Ab-treated slices.
To investigate whether the strengthening of LTP upon NogoA neutralization is due to changes in presynaptic components necessary to support LTP, we probed whether synaptic fatigue was altered under 11c7 conditions. We therefore analyzed for the first burst of the three TBS trains the ratio of the fourth field excitatory postsynaptic potential (fEPSP) to the first fEPSP in each burst. The results were as follows: first train 11c7, 204 ± 26%; control, 175 ± 14%, P = 0.35; second train 11c7, 128 ± 20%; control, 97 ± 7%, P = 0.15; 11c7, 134 ± 24%; control, 107 ± 6%, P = 0.28; 11c7, n = 10; control, n = 12. Overall, we could not find any significant difference between control Ab- and 11c7-treated slices. In addition, we examined short-term plasticity by measuring paired-pulse facilitation (PPF), characterized by a strong presynaptic component (20). PPF was induced by applying two stimuli separated by different time intervals (10, 20, 40, 80, and 160 ms) and recording the evoked fEPSPs. Fig. 1D shows a small increase in 11c7-treated slices, which is, however, not significantly different at any interstimulus interval (ISI) measured.
Changes in the height of potentiation during the induction and maintenance of LTP could also be due to changes in baseline synaptic transmission. We therefore recorded an input-output curve, using the fiber volley as an indicator of presynaptic stimulation strength and plotted a given fiber volley size against the fEPSP slope (Fig. 1E). With this approach we could not find any significant differences between 11c7 or control Ab-treated slices.
To confirm the penetration of the NogoA blocking Ab and identify its binding sites, the recorded hippocampal slices underwent immunostaining for mouse antibodies. We observed high levels of 11c7 Ab bound to possibly Mossy cells of the polymorphic layer of the dentate gyrus (Fig. S1 A and F, open arrows) and also especially to pyramidal cells of the CA3 region (Fig. S1 A, C, and D, closed arrows) up to 200 μm into the slice. In the latter, both the cell bodies and the dendrites were labeled (Fig. S1C). In the CA1 region, strongly labeled cells were sparser and only the cell bodies were stained (Fig. S1 A and E, arrowheads). No staining could be observed in slices treated with control antibodies (Fig. S1 B and G–I) indicating a specific binding of the 11c7 Ab. This is in line with EM data and biochemical evidence (14, 21, 22).
Taken together, these results clearly show that NogoA is specifically involved in restricting the height of synaptic potentiation on a fast time scale. On the other hand, NogoA signaling is not mediating or preventing mechanisms of synaptic weakening and it does not significantly change properties of baseline synaptic transmission.
Role of the Nogo Receptor NgR1 on Processes of Synaptic Plasticity.
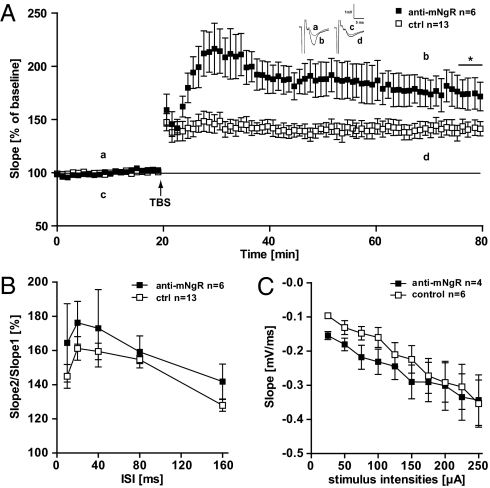
To analyze whether the role of NogoA on hippocampal LTP involves the known Nogo receptor (NgR) pathway, we tested whether the blockade of the NgR would have an effect on the induction and maintenance of LTP. We applied a function blocking anti-NgR Ab (23) to WT acute slices with the same procedures we used for the anti-NogoA Ab. Fig. 2A shows that the blockade of NgR also leads to higher levels of potentiation following TBS compared with control Ab treatment. At 60 min after LTP induction, we observed a significant difference between anti-NgR Ab-treated slices and controls, with an average potentiation of 175 ± 14.6% (n = 6/2) in the anti-NgR Ab-treated slices and 141 ± 8% in controls (n = 13/6) (P = 0.012).
Fig. 2.
Nogo-receptor blocking Ab experiments. (A) Hippocampal slices were treated with anti-mNogo receptor (anti-mNgR; filled squares) or control Ab (open squares). LTP was induced by application of TBS (arrow). At 60 min after TBS, a significant difference between anti–mNgR- and control Ab-treated slices could be observed, indicated by the asterisk (P = 0.033, t test). Inset shows original traces from representative individual experiments; letters correspond to the time point when traces were taken. (B) PPF in 11c7-treated slices is slightly, but not significantly increased. (C) Input-output strength in anti–mNgR-treated slices revealed no significant changes compared with control Ab-treated slices (P > 0.1).
Again paired-pulse facilitation and baseline synaptic transmission in the anti-NgR Ab experiments revealed no significant differences compared with control Ab-treated slices (Fig. 2 B and C).
Synaptic Plasticity in NogoA KO Mice.
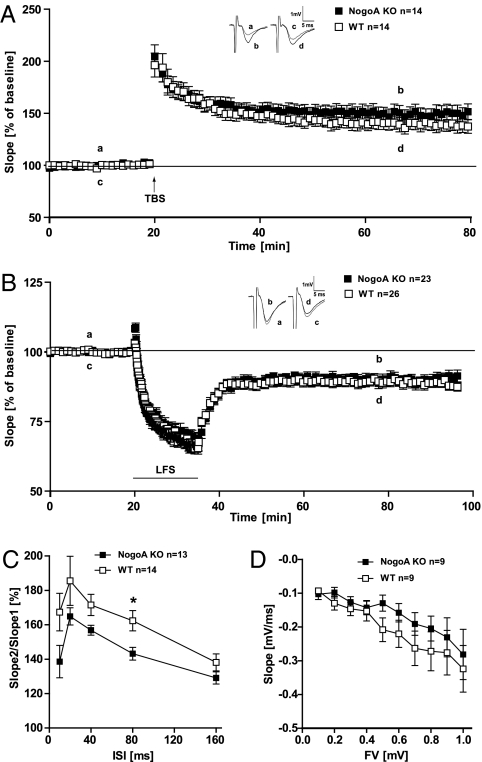
To have an Ab-independent approach to investigate the role of NogoA in mediating synaptic plasticity, we investigated whether LTP is affected as well in NogoA KO mice. We applied a TBS to hippocampal slices of NogoA KO and WT adult mice (P40–P60). Both genotypes showed an initially equally high posttetanic potentiation (PTP) and at up to 40 min, both curves overlaid (Fig. 3A). Then the fEPSPs from the KO slices became visibly larger than those in the WT slices (Fig. 3A). The average degree of potentiation was 150 ± 7.5% for the NogoA KO mice (n = 14/5) and 136 ± 6.8% for WT animals (n = 14/5) (P = 0.1). Although we could not find a significant difference between the two genotypes, we found a tendency for a higher LTP induction in NogoA KO mice in comparison with WT-derived slices resembling the effect of the acute treatment by the 11c7 Ab.
Fig. 3.
NogoA KO experiments. (A) LTP was induced by TBS (arrow) in NogoA KO (filled squares) and WT mice (open squares). No significant difference between NogoA KO and WT mice could be observed 60 min after TBS (P > 0.1, t test). (B) LTD was induced by LFS (900 pulses at 1 Hz) stimulation. After LFS, there is no significant difference between transgenic and WT mice (P > 0.1, t test) (A and B) Insets show original traces from representative individual experiments; letters correspond to the time point when traces were taken. (C) PPF in NogoA KO mice is decreased compared with WT, but only shows a significant difference at an interstimulus interval (ISI) of 80 ms. (D) Input-output strength shows no significant difference between WT and NogoA KO mice.
Next we induced LTD in hippocampal slices of P14–P20 old NogoA KO and WT mice. LTD in the two genotypes showed exactly the same time course and no difference in fEPSP size could be observed (Fig. 3B). At 55–60 min after LFS, the average of both genotypes revealed a depression of 87 ± 1.9% for NogoA KO animals (n = 23/6) and 89 ± 1.8% for WT mice (n = 26/6), respectively.
To examine the presynaptic function, we again analyzed PPF. All intervals tested revealed an overall reduction in PPF in NogoA KO compared with WT mice, which was, however, not significant, resembling the acute treatment with the 11c7 Ab. Only at an ISI of 80 ms, a significant difference was found in the comparison between WT and KO mice (P < 0.05, t test; Fig. 3C). In addition we recorded an input-output curve. At higher stimulus strength a slight reduction in the size of NogoA KO mice fEPSPs in comparison with WT animals could be observed, but this was also not significantly different from WT mice (Fig. 3D). Taken together, NogoA KO mice show a similar phenotype as acute blockade of NogoA, but the effect is weaker, possibly due to compensatory mechanisms.
Application of the Active NogoΔ20 Peptide.
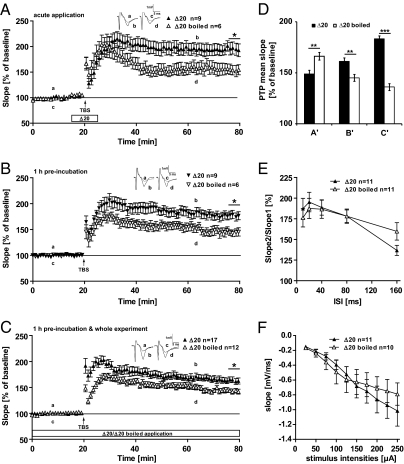
Having observed the pronounced NogoA loss-of-function effects elicited by the anti-NogoA Ab treatment and the NogoA KO, we asked whether gain-of-function effects can also be seen. The 11c7 Ab was raised specifically against an 18-aa peptide of the so-called NogoΔ20 fragment in the NogoA-specific inhibitory domain (19). The NogoΔ20 domain is a key active site of NogoA; NogoΔ20 strongly inhibits fibroblast spreading and neurite outgrowth. Following binding of NogoΔ20, however, the peptide is known to be rapidly internalized into neurons, forming first a receptor-containing signaling endosome followed by rapid degradation (24). We therefore first applied the NogoΔ20 for a short time—5 min before TBS and 5 min following TBS—and monitored the earliest synaptic plastic events, PTP (Fig. 4D), as well as LTP as a long-lasting effect (Fig. 4A). Indeed, the NogoΔ20-treated slices showed a significantly decreased PTP value compared with controls (Δ20, 148.9 ± 3.7; Δ20 boiled, 166.3 ± 3.8; P = 0.002; t test; Fig. 4D, columns in A′). However, Fig. 4A also shows that this small time interval of NogoΔ20 application was sufficient to induce a significantly higher increase in LTP 1 h after TBS compared with the boiled peptide-treated slices. Already 10 min after TBS, the NogoΔ20 peptide-treated slices reached a stable level of potentiation. At the end of the experiments the average potentiation stabilized at 193 ± 10.8% in NogoΔ20 (n = 9/3) and 154 ± 9.7% in control-treated slices (n = 6/2) (Fig. 3A). The observed difference between the two treatments was significant 60 min after TBS (P = 0.034).
Fig. 4.
NogoΔ20 application experiments. (A) Slices were incubated with the NogoΔ20 peptide 5 min before and 5 min after TBS. A significant difference in the levels of potentiation could be observed 60 min after TBS (indicated by the asterisk; P = 0.034, t test). (B) Slices were incubated for only 1 h before starting the experiment, whereas during the whole recording, no peptide was applied. With this approach a significant difference in LTP induction between NogoΔ20 incubation and control could be shown (indicated by the asterisk; P = 0.024, t test). (C) Slices were treated with NogoΔ20 peptide 1 h before and during the whole experiment. LTP was induced by application of a TBS (arrow). At 60 min after TBS, a significant difference between NogoΔ20 and the control could be observed (indicated by the asterisk; P = 0.008, t test). (A, B, and C) Insets show original traces from representative individual experiments; letters correspond to the time point when traces were taken. (D) Mean values of PTP 5 min after TBS; A′ corresponds to Fig. 3A, B′ to Fig. 3B, and C′ to Fig. 3C. (A′) acute application of NogoΔ20 resulted in a significantly lower PTP compared with control-treated slices (P = 0.002; t test), (B′) NogoΔ20 preincubation led to a significantly higher PTP compared with controls (P = 0.0019, t test); (C′) preincubation combined with application of NogoΔ20 during the whole experiment showed an even higher PTP compared with controls (P < 0.0001, t test). (E and F) Slices preincubated for 1 h with either NogoΔ20 or control peptide and during the whole experiment. (E) PPF in Δ20-treated slices showed no significant differences at any ISI in comparison with boiled Δ20 as control. (F) Input-output curve in Δ20-treated slices revealed no significant alterations compared with Δ20-boiled treated slices.
This finding is at first hand surprising. A possible explanation for these results could be that the application of NogoΔ20 leads first to a signaling event showing the expected opposite effect of that of NogoA neutralization (Fig. 1A, 1–5 min after TBS). Internalization of the ligand–receptor complex, as shown by Joset et al. (24), could then result in the clearance of the functional Nogo receptor from the surface and thus a longer-term dominant negative effect of NogoΔ20 (SI Materials and Methods and Fig. S2). To test this hypothesis, we preincubated the slices for 1 h with NogoΔ20 and performed the experiments completely without the peptide in normal artificial cerebrospinal fluid (ACSF). We then reprobed to see whether PTP and/or LTP would be changed in these preincubated slices. The time course of LTP is monitored in Fig. 4B; PTP is plotted in Fig. 4D. Here we saw that the 1-h preincubation led to an increase in PTP in the NogoΔ20 peptide-treated slices compared with controls (Δ20, 161.2 ± 3.5%; Δ20 boiled, 145.1 ± 3.3%; P = 0.0019, t test, Fig. 4D, columns in B′). This increase in PTP in NogoΔ20 peptide preincubated slices was stabilized 1 h after TBS at significantly higher LTP values compared with controls (Δ20, 175 ± 7.7% (n = 9/3); Δ20 boiled, 145 ± 7.0% (n = 6/4); P = 0.024). In line with these results is the interpretation that during the 1-h preincubation, the NogoΔ20 peptide with its receptor is already completely internalized from the surface of the cell and mimics therefore the effect of NogoA blockade via antibodies. This is in line with immunostaining data, where we could show that the activation with NogoΔ20 leads to a significant increase in the cytoplasmic immunostaining of NogoΔ20 already 10 min after peptide application (Fig. S2 and SI Materials and Methods).
Next we investigated the effect of the NogoΔ20 peptide under similar conditions as used for the 11c7-NogoA Ab treatment. We incubated the slices with NogoΔ20 peptide for 1 h before induction of LTP and until the end of the experiment. Fig. 4C shows the LTP time course and in Fig. 4D the PTP is shown in the third pair of columns (C′). This long treatment with NogoΔ20 peptide revealed an even higher increase in PTP than preincubation alone (Δ20, 183.4 ± 2.9%; Δ20 boiled, 136.1 ± 3.3%; P < 0.0001, t test). This again can most likely be explained by the fact that under these conditions, the receptor for NogoΔ20 is removed from the surface. In addition, Fig. 4C shows nicely that during the whole time course of the experiment the NogoΔ20 peptide-treated slices showed a significantly enhanced LTP, which stabilized 60 min after TBS (Δ20, 163 ± 5.4% (n = 17/12); Δ20 boiled, 142 ± 3.6% (n = 12/6); P = 0.008, t test).
PPF as well as the input-output curves revealed no significant differences between the NogoΔ20 and the inactivated peptide, independent of the duration of the treatment (Fig. 4 E and F).
Influence of the GABAergic System on the NogoA-Regulated LTP Enhancement.
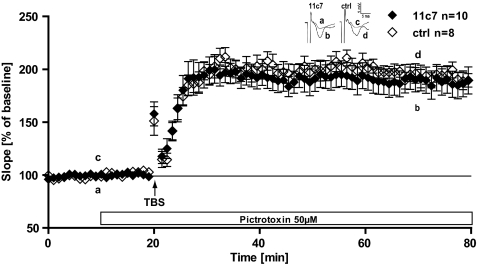
We found no evidence that excitatory baseline synaptic transmission is altered under blockade of NogoA. This prompted us to ask whether changes in the GABAergic system might contribute to the observed phenotype. GABAergic transmission has indeed been known for a long time to be implicated in the induction and early maintenance phase of LTP (25). To probe for a GABAergic involvement, we induced LTP in Ab-treated slices by application of picrotoxin (PTX, 50 μM), a GABAA receptor blocker. PTX was washed for 10 min before the induction of LTP by TBS and applied until the end of the experiments. Within 5 min from the TBS application, the fEPSP size of both Ab treatments (control and 11c7) increased and remained stable until the end of the experiments (Fig. 5). At 55–60 min after TBS, a very high level of LTP could be observed, higher than with NogoA inactivation; however, no difference between the 11c7 treatment and the control Ab was visible [P = 0.61, t test; 11c7, 188 ± 12% (n = 10/5); control, 192 ± 7.4% (n = 13/6)]. To check whether the effect of PTX on LTP induction may mask the Ab treatment, we also tested whether the 11c7 treatment alone would be significantly different from the 11c7 treatment plus PTX. We compared the data of Fig. 1A with Fig. 5A and found no significant difference between the two treatments (P = 0.2). Thus, blockade of GABAA receptors with PTX enhanced the level of LTP in WT slices, whereas it did not change the potentiation in slices where NogoA was blocked.
Fig. 5.
GABAergic influence on NogoA-mediated synaptic plasticity. Hippocampal slices treated with either NogoA blocking Ab 11c7 (filled diamonds) or control Ab (open diamonds). After 10 min of baseline recording, 50 μM of picrotoxin (PTX) was washed in until the end of the experiments. LTP was induced by application of TBS (arrow). At 60 min after TBS, no significant difference between 11c7 and control Ab treatment was found (P = 0.61, t test).
Discussion
NogoA is an inhibitor of axonal regeneration and sprouting following CNS injury (6, 26) and acts as a growth suppressor in the adult CNS (6, 8, 9, 26–28). These effects are thought to be mainly due to oligodendrocytes and myelin NogoA, whereas the functions of NogoA in subpopulations of neurons are largely unknown. To address this question, we have investigated whether interfering with NogoA expression and signaling restricts synaptic plasticity in the adult intact hippocampus where NogoA is expressed by pyramidal as well as interneurons (11, 29). We found a unique physiological role of NogoA in negatively regulating LTP in the hippocampus on a fast time scale. Our findings are overall in line with the observations of Lee et al. (14), who could show that in NgR1 KO mice FGF2-dependent hippocampal LTP was enhanced and LTD was decreased. The present results with acute, Ab-mediated neutralization of NogoA or NgR1 showed a more pronounced, FGF2-independent effect on LTP, but no effect on LTD. Similar, but less pronounced, effects were seen in the NogoA KO tissue. This is a phenomenon that is frequently observed when comparing acute interventions with constitutive KOs where compensatory mechanisms are often activated. These results are also in line with the proposed role of NogoA and NgR1 in regulating the stabilization of neuronal circuits and in inhibiting structural rearrangements and thereby limiting, e.g., spinal cord, or ocular dominance (OD) plasticity (9, 27, 30, 31), or regulating the formation of long-lasting memories (15).
NogoΔ20 Experiments.
To complement the Ab and KO loss-of-function experiments, we applied the active NogoA-specific NogoΔ20 fragment to the hippocampal slices. NogoΔ20 induces growth arrest of neurites and growth cone collapse via activation of RhoA (32, 33). NogoΔ20 is rapidly internalized following receptor binding, and its internalization is followed first by intracellular signaling and then degradation (24). We therefore studied the short-term application and effects separately from the long-term preincubations and synaptic effects. A 5-min preincubation of the tissue with NogoΔ20 significantly decreased PTP. This effect is in line with a synaptic weakening effect of NogoA. Sixty minutes after TBS, however, LTP was significantly enhanced, similar to what was seen after NogoA neutralization. Preincubation of hippocampal slices with the NogoA active fragment for 1 h always led to LTP enhancement. These results can be best explained by a diphasic effect: first fast signaling upon internalization occurred leading to synaptic weakening, and then NogoΔ20 induced down-regulation of the cell surface NogoA receptor complex (24), rendering the neurons temporarily insensitive to the NogoA present in the tissue, thereby causing the observed enhancement of LTP. This is supported by the observation that upon NogoΔ20 application, an increased cytoplasmic staining for NogoΔ20 in CA3 and CA1 pyramidal neurons can be observed in comparison to the use of boiled NogoΔ20 (SI Materials and Methods and Fig. S2).
Role of GABAergic Neurons.
Our analysis of hippocampal synaptic plasticity shows that blockade of NogoA signaling causes significant changes in LTP. This cannot be attributed to changes in baseline excitatory synaptic transmission, because our fEPSP analysis of fiber volley size and paired-pulse facilitation were not significantly different under conditions of NogoA or NgR suppression. Although blockade of GABAergic activity can facilitate NMDA receptor-dependent LTP (34), in its natural form it is a key feature of LTP induction. During regular synaptic transmission, a delicate balance between excitation and inhibition is crucial, which needs to be transiently shifted toward less inhibitory activity to allow induction of plastic rearrangements of connectivity. Therefore, it is intriguing to speculate that NogoA does not act only on excitatory pyramidal neurons, but also on inhibitory neurons, as it is suggested by our picrotoxin experiments.
Regeneration and Increase of Plasticity.
It is well established that severed adult mammalian CNS axons show very little, if any, regenerative axonal growth. Nevertheless, considerable spontaneous sensory-motor recovery can be accomplished in particular after smaller lesions (35, 36). Recovery is use dependent, and exercise substantially improves sensory and motor recovery after incomplete transection of the spinal cord (37). Cortical representations, for example, undergo well-adaptive changes (36, 38). Elimination of NogoA or of its receptor NgR1 in KO mice or blockade of their function with specific antibodies enhances anatomical regeneration, compensatory fiber growth, and functional recovery following lesions in the spinal cord or brain; for reviews see refs. 6, 26, 39. In addition to the decrease of inhibitory signals in the environment of the growing fibers and the hypothesized derepression of the neuronal growth program (28, 31, 40), our present data suggest that when NogoA signaling is blocked, the enhanced synaptic plasticity might promote, e.g., motor (re)learning at the synaptic and circuit level, thus improving intrinsic repair mechanisms of the adult CNS. Indeed, enhanced activity-dependent synaptic plasticity could be a crucial driving force for improved functional recovery in addition to fiber sprouting, regeneration, and structural plasticity. Interestingly and in line with such a model, NgR1 was shown to be down-regulated in those sensory-motor areas undergoing plastic changes after thoracic spinal cord injuries (41).
Mechanism of Action of NogoA in Modulating Synaptic Plasticity.
A crucial question is how the function of NogoA can mechanistically be explained in the context of our current knowledge about processes of synaptic plasticity. One conceptual view would be that NogoA counteracts growth factor-mediated promoting effects on synaptic plasticity. In line with this are the results of Lee et al. (14), which indicate that NgR1, as one of the interacting partners for NogoA, counteracts the FGF2 action on LTP. An alternative view would be that NogoA restricts synaptic plasticity via p75NTR, an NgR1-associated component of the Nogo receptor complex, which has been postulated as a mediator of negative synaptic plasticity (42–44). Because we do not see an effect on LTD after NogoA neutralization, however, it is unlikely that NogoA acts solely via p75NTR. It is intriguing to speculate that NogoA acts via the synaptic cytoskeleton to stabilize synaptic function and structure and puts a break on growth-promoting processes. NogoA is known to mediate neurite repulsion by negatively modulating actin assembly via small GTPases of the Rho family (28, 32, 45, 46). The synaptic actin cytoskeleton is important for the regulation of neurotransmitter release in the presynaptic compartment as well as spine shape changes and anchorage of postsynaptic density (PSD)-associated molecules in the postsynaptic compartment (reviewed in ref. 47). Downstream of RhoA and the RhoA kinase ROCK, NogoA may regulate effectors such as LimK and cofilin (28, 32), which are important regulators of F-actin dynamics (28). Indeed phosphocofilin+ PSDs were shown to be increased after LTP induction (48), and limk ko mice show impaired spatial learning capabilities, abnormal spine morphology, and an increase in LTP (49). This supports the concept that NogoA signaling negatively modulates synaptic plasticity via Rho, LimK, and cofilin and subsequent changes of the pre- and/or postsynaptic cytoskeleton.
In summary, our results strengthen the idea that neuronal NogoA and its receptors are important for upholding synaptic circuitry and regulating synaptic plasticity. They increase our understanding of how NogoA could mediate its effects in stabilizing neuronal networks, and how suppression of NogoA–Nogo receptor activity could enhance the formation of compensatory circuits and functional recovery after CNS injury. NogoA in the intact, adult hippocampus restricts physiological synaptic plasticity on a fast time scale. We therefore suggest that NogoA plays a crucial role in modulating the balance between plasticity and stability of the mature circuitry in the intact hippocampus thus ensuring the necessary level of stability in an ever changing neuronal network.
Materials and Methods
Slice Preparation and Electrophysiological Recordings.
Acute hippocampal slices were prepared from wild-type C57BL/6 mice and from NogoA KO mice according to standard procedures. For LTP experiments, 40- to 60-d-old mice (P40–P60) and for LTD experiments, 14- to 21-d-old mice (P14–P21) were used; for details see SI Materials and Methods. All procedures were approved by the guidelines from Animal Committee on Ethics in the Care and Use of Laboratory animals of TU Braunschweig.
Antibody Treatment.
For the Ab treatment, mouse and goat monoclonal Ab were used: A NogoA-specific blocking Ab (Ab 11c7, mIgG1), raised against an 18-aa peptide in the most active region of NogoA (NogoΔ20 region), see ref. 19; a control Ab (anticyclosporin, mIgG1, a gift from Novartis Pharma, Basel, Switzerland), and an Ab against the Nogo receptor, NgR (mNogo receptor affinity-purified goat IgG, R&D Systems). The Ab 11c7 was shown to block the NogoA-mediated neurite outgrowth inhibition in vitro and in vivo (6, 19, 37, 50); for details see SI Materials and Methods.
Pharmacology.
The GABAA-receptor antagonist PTX was added in a 50-μM concentration 10 min before LTP induction until the end of the experiments.
Statistical Analysis.
The average values of the slope function of the field EPSP (mV/ms) per time point were analyzed using a t test.
Supplementary Material
Acknowledgments
We thank Reinhard Huwe for his excellent technical assistance. The work was supported by the Deutsche Forschungsgemeinschaft ZA 554/2-1 (to M.Z. and M.K.) and by the Swiss National Science Foundation (to M.E.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013322108/-/DCSupplemental.
References
- 1.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 2.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 3.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Roth-Alpermann C, Morris RG, Korte M, Bonhoeffer T. Homeostatic shutdown of long-term potentiation in the adult hippocampus. Proc Natl Acad Sci USA. 2006;103:11039–11044. doi: 10.1073/pnas.0600894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 6.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Kapfhammer JP, Schwab ME. Increased expression of the growth-associated protein GAP-43 in the myelin-free rat spinal cord. Eur J Neurosci. 1994;6:403–411. doi: 10.1111/j.1460-9568.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 8.Gianola S, Savio T, Schwab ME, Rossi F. Cell-autonomous mechanisms and myelin-associated factors contribute to the development of Purkinje axon intracortical plexus in the rat cerebellum. J Neurosci. 2003;23:4613–4624. doi: 10.1523/JNEUROSCI.23-11-04613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, et al. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zagrebelsky M, Schweigreiter R, Bandtlow CE, Schwab ME, Korte M. Nogo-A stabilizes the architecture of hippocampal neurons. J Neurosci. 2010;30:13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, et al. Synaptic function for the Nogo-66 receptor NgR1: Regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlén A, et al. Nogo receptor 1 regulates formation of lasting memories. Proc Natl Acad Sci USA. 2009;106:20476–20481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josephson A, et al. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res. 2003;311:333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- 17.Bandtlow CE, et al. Increased expression of Nogo-A in hippocampal neurons of patients with temporal lobe epilepsy. Eur J Neurosci. 2004;20:195–206. doi: 10.1111/j.1460-9568.2004.03470.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 19.Oertle T, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YY, Jin WL, Liu HL, Ju G. Electron microscopic localization of Nogo-A at the postsynaptic active zone of the rat. Neurosci Lett. 2003;346:153–156. doi: 10.1016/s0304-3940(03)00508-1. [DOI] [PubMed] [Google Scholar]
- 22.Raiker SJ, et al. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrinovic MM, et al. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development. 2010;137:2539–2550. doi: 10.1242/dev.048371. [DOI] [PubMed] [Google Scholar]
- 24.Joset A, Dodd DA, Halegoua S, Schwab ME. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 2010;188:271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 26.Gonzenbach RR, Schwab ME. Disinhibition of neurite growth to repair the injured adult CNS: Focusing on Nogo. Cell Mol Life Sci. 2008;65:161–176. doi: 10.1007/s00018-007-7170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bareyre FM, Haudenschild B, Schwab ME. Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. J Neurosci. 2002;22:7097–7110. doi: 10.1523/JNEUROSCI.22-16-07097.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montani L, et al. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/cofilin in the unlesioned adult nervous system. J Biol Chem. 2009;284:10793–10807. doi: 10.1074/jbc.M808297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josephson A, Widenfalk J, Widmer HW, Olson L, Spenger C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp Neurol. 2001;169:319–328. doi: 10.1006/exnr.2001.7659. [DOI] [PubMed] [Google Scholar]
- 30.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 31.Buffo A, et al. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash M, Pribiag H, Fournier AE, Jacobson C. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 2009;40:224–235. doi: 10.1007/s12035-009-8083-y. [DOI] [PubMed] [Google Scholar]
- 33.Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- 35.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 36.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 37.Maier IC, et al. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132:1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh A, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 39.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craveiro LM, et al. Neutralization of the membrane protein Nogo-A enhances growth and reactive sprouting in established organotypic hippocampal slice cultures. Eur J Neurosci. 2008;28:1808–1824. doi: 10.1111/j.1460-9568.2008.06473.x. [DOI] [PubMed] [Google Scholar]
- 41.Endo T, Spenger C, Tominaga T, Brené S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain. 2007;130:2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- 42.Zagrebelsky M, et al. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo NH, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 44.Rösch H, Schweigreiter R, Barde YA, Bonhoeffer T, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci USA. 2005;102:7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh SH, Ferraro GB, Fournier AE. Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. J Neurosci. 2006;26:1006–1015. doi: 10.1523/JNEUROSCI.2806-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng K, et al. Overcoming amino-Nogo-induced inhibition of cell spreading and neurite outgrowth by 12-O-tetradecanoylphorbol-13-acetate-type tumor promoters. J Biol Chem. 2010;285:6425–6433. doi: 10.1074/jbc.M109.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cingolani LA, Goda Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 48.Rex CS, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng Y, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 50.Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.