Abstract
According to the multistep model of cell migration, chemokine receptor engagement (step 2) triggers conversion of rolling interactions (step 1) into firm adhesion (step 3), yielding transendothelial migration. We recently reported that glycosyltransferase-programmed stereosubstitution (GPS) of CD44 on human mesenchymal stem cells (hMSCs) creates the E-selectin ligand HCELL (hematopoietic cell E-selectin/L-selectin ligand) and, despite absence of CXCR4, systemically administered HCELL+hMSCs display robust osteotropism visualized by intravital microscopy. Here we performed studies to define the molecular effectors of this process. We observed that engagement of hMSC HCELL with E-selectin triggers VLA-4 adhesiveness, resulting in shear-resistant adhesion to ligand VCAM-1. This VLA-4 activation is mediated via a Rac1/Rap1 GTPase signaling pathway, resulting in transendothelial migration on stimulated human umbilical vein endothelial cells without chemokine input. These findings indicate that hMSCs coordinately integrate CD44 ligation and integrin activation, circumventing chemokine-mediated signaling, yielding a step 2–bypass pathway of the canonical multistep paradigm of cell migration.
Keywords: G-protein, selectin, cell therapeutics, regenerative medicine
The successful application of adoptive cellular therapies, including stem cell-based regenerative medicine, critically depends on delivering relevant cells to target sites (1). Four distinct steps have been described in the process of cell migration: tethering and rolling mediated principally by the selectin group of adhesion molecules (step 1), chemokine receptor engagement and resultant G-protein-coupled “inside-out” activation of integrins (step 2), firm adhesion by integrins (step 3), culminating in transendothelial migration (TEM) (step 4) (2, 3). The conventional multistep paradigm holds that step 2 is critical for TEM, with engagement of discrete chemokine receptors on the cell surface triggering subsequent integrin-dependent steps. For recruitment of circulating cells to bone marrow, the CXCL12/CXCR4 chemokine axis plays a central role (4). Notably, specialized sinusoidal vessels constitutively express CXCL12 and E-selectin at sites where cells extravasate into the marrow parenchyma (5). Hematopoietic stem cells express multiple E-selectin ligands and abundant CXCR4 (1), and are thus equipped with relevant effectors of step 1 and step 2 events mediating osteotropism.
Mesenchymal stem cells (MSCs) can differentiate into a variety of tissues (6, 7) and display potent immunomodulatory effects (8, 9). Although MSCs have shown therapeutic potential in skeletal diseases (10), myocardial injury, and immunologic disorders (11, 12), a critical limitation to the therapeutic use of MSCs is their modest tissue colonization upon systemic administration (13–15). Importantly, human MSCs (hMSCs) lack expression of various adhesion receptors that mediate step 1 interactions, particularly E-selectin ligands (16). Moreover, in contrast to hematopoietic cells, hMSCs display a rather limited repertoire of chemokine receptors, with variable evidence of functional receptors (17), particularly CXCR4 (16).
The paucity of effectors of cell migration on hMSCs has prompted investigations to develop strategies to promote hMSC TEM and tissue-specific trafficking. In a prior study, we glycan-engineered CD44 on hMSCs to express the E-selectin ligand HCELL (hematopoietic cell E-selectin/L-selectin ligand) (16). Following enforced HCELL expression, intravital microscopy in immunodeficient murine hosts showed that systemically administered hMSCs displayed robust extravasation (TEM) within marrow microvasculature, despite the absence of CXCR4 expression on these cells (16). This striking finding raised the possibility that hMSC/endothelial interactions may be encoded by chemokine-independent pathways. Our investigations here show that engagement of CD44 via interactions with E-selectin (through HCELL) or with hyaluronic acid (HA) markedly up-regulates binding of VLA-4 to VCAM-1 and fibronectin (FN), inducing hMSC firm adherence and subsequent TEM in the absence of chemokine input. CD44 ligation triggers inside-out up-regulation of VLA-4 adhesiveness via CD44-mediated G-protein-dependent signal transduction. These findings unveil a dimension of mechanosignaling within the multistep cascade, establishing an expanded role for CD44 receptor/ligand interactions in directing cell trafficking via a step 2–bypass pathway of integrin activation.
Results
Effect of Fucosyltransferase VI Treatment on hMSC Expression of Homing Molecules.
To determine whether stereospecific exofucosylation of the cell surface affects expression of key membrane structures involved in cell migration, hMSCs were analyzed for expression of CD44, α4, β1, αL, β2, CXCR4, sialyl Lewis X (sLex), and PSGL-1 using specific monoclonal antibodies (mAb), and probed for E-selectin ligand activity using E-selectin-Ig chimera (E-Ig). Comparative flow cytometric analysis before and after fucosyltransferase VI (FTVI) treatment showed that although ex vivo α(1,3)-fucosylation renders sLex expression and E-Ig reactivity on hMSCs, it does not alter cell-surface expression of CD44, VLA-4 (α4β1; CD49d/CD29), LFA-1 (αLβ2; CD11a/CD18), CXCR4, or PSGL-1 (Fig. 1).
Fig. 1.
FTVI treatment of hMSCs does not affect surface protein expression. Flow cytometric analysis of CD44, α4/β1 (VLA-4), αL/β2 (LFA-1), CXCR4, HECA-452 determinants (sLex), PSGL-1, and E-Ig reactivity on untreated (broken lines) and FTVI-treated hMSCs (shaded region). The dotted line indicates isotype control. FTVI treatment induced HECA-452 and E-Ig reactivity but did not change surface expression of any protein analyzed.
Enforced HCELL Expression on hMSCs Induces E-Selectin- and VCAM-1-Dependent TEM.
To examine the molecular basis of the previously reported extravasation of HCELL+hMSCs at marrow endothelium, we used a well-established endothelial primary culture model, human umbilical vein endothelial cells (HUVEC), to analyze hMSC/endothelial interactions. In all assays, serum-free conditions were used to avoid application of exogenous chemokine(s). Because marrow microvessels constitutively express E-selectin and VCAM-1, studies were performed using HUVEC stimulated to express these structures; as shown in Fig. 2A, treatment with IL-1β and TNF-α up-regulated both E-selectin and VCAM-1 expression on HUVEC.
Fig. 2.
HCELL+hMSCs undergo markedly enhanced TEM on stimulated HUVEC independent of CXCR4 signaling. (A) Expression of E-selectin and VCAM-1 on HUVEC before (broken lines) and after (shaded region) stimulation using IL-1β and TNF-α. The dotted line indicates isotype control. (B) HCELL expression enhances hMSC TEM: hMSCs were untreated or FTVI-treated, followed by incubation with function-blocking anti–VLA-4 mAb HP2/1 or PTX. In some cases, HUVEC were preincubated with function-blocking anti–E-selectin mAb clone 68-5H11 or mIg isotype Ab. FTVI-treated hMSCs underwent approximately fourfold higher transmigration compared with untreated; increased transmigration was dependent on HCELL/E-selectin engagement, VLA-4/VCAM-1 interactions, and PTX-sensitive G-protein signaling. Values are means ± SD (n = 6, duplicate wells of three donor hMSC cultures). Statistical significance (P ≤ 0.05) is indicated by asterisks. (C) PTX treatment (shaded region) does not change HECA-452 reactivity (Left), E-Ig reactivity (Center), or VLA-4 expression (Right) of HCELL+hMSCs compared with untreated control (broken lines). The dotted line indicates isotype control. (D) Real-time PCR analysis of hMSCs (two different donors, passage numbers 2 and 3, respectively) and of lymphocytes (PBMCs) shows that lymphocytes express ∼4,500-fold more CXCR4 mRNA than do hMSCs. Endogenous β-actin(ACTB) was used to normalize data. (E) Western blot analysis of CXCR4 expression on whole-cell lysates of hMSCs trypsin-lifted (MSC-T) and EDTA-lifted (MSC-E) and of lymphocytes. All lanes were normalized for total protein (25 μg), confirmed by equivalent β-actin staining (loading control) across all samples. hMSCs show nominal CXCR4 protein expression compared with lymphocytes. (F) Transmigration of HCELL+hMSCs in the presence or absence of AMD3100 (5 μg/mL) on a stimulated HUVEC monolayer. Values are means ± SD (n = 3 different donor hMSC cultures). Statistical significance (P ≤ 0.05) is indicated by brackets and asterisks.
TEM of hMSCs in transwell assays was scored as “migration index,” calculated as the ratio of transmigrated FTVI-treated (HCELL+hMSCs) or -untreated hMSCs (HCELL−hMSCs) on cytokine-stimulated HUVEC compared with transmigrated untreated hMSCs on unstimulated HUVEC. As shown in Fig. 2B, on stimulated HUVEC, HCELL+hMSCs consistently transmigrated a minimum of threefold more than HCELL−hMSCs. Transmigration of HCELL+hMSCs was critically dependent on engagement of both E-selectin and VLA-4, as pretreatment with function-blocking mAb to either E-selectin (clone 68-5H11) or VLA-4 (clone HP2/1) abrogated transmigration (Fig. 2B). G-protein-mediated signaling was requisite for HCELL+hMSC transmigration, as pretreatment with pertussis toxin (PTX), a specific inhibitor of Gαi, inhibited TEM (Fig. 2B). Importantly, decreased TEM upon PTX treatment did not result from alterations in surface expression of sLex, E-selectin ligand activity, or VLA-4 (Fig. 2C). Altogether, these data indicate that TEM of HCELL+hMSCs on stimulated HUVEC is mediated by E-selectin engagement, VLA-4/VCAM-1 interactions, and PTX-sensitive G-protein signaling.
Although all transwell assays were performed in the absence of exogenous chemokine input, it is well-recognized that hMSCs produce CXCL12 (18), and that the CXCL12/CXCR4 axis plays a pivotal role in recruiting circulating cells to marrow (1). Thus, we specifically sought to analyze whether this chemokine signaling pathway contributes to the observed TEM of HCELL+hMSCs. The expression of CXCR4 on hMSCs was determined at mRNA and protein levels by real-time PCR and Western blotting, respectively. As control, comparative analysis was performed on human lymphocytes (peripheral blood mononuclear cells; PBMC), which are known to express abundant levels of functional CXCR4. As seen in Fig. 2D, lymphocytes express ∼4,500-fold more CXCR4 mRNA compared with that of hMSCs. Western blot analysis of whole-cell lysates of trypsin-lifted and EDTA-lifted hMSCs (hMSC-T and hMSC-E, respectively) and lymphocytes, each normalized for equivalent input protein, shows significantly more CXCR4 protein in lymphocytes than in hMSCs (Fig. 2E); the low CXCR4 expression in EDTA cell lifts indicates that CXCR4 expression is not artifactually diminished by trypsin proteolysis, and confirms prior evidence by in situ immunofluorescence that hMSCs lack CXCR4 expression (16). To directly assess whether the CXCL12/CXCR4 axis functions in the observed TEM of HCELL+hMSCs, TEM assays were performed under serum-free conditions in the presence or absence of the CXCR4 antagonist AMD3100 at 5 μg/mL, a dose experimentally validated to block CXCL12-induced TEM (19). As seen in Fig. 2F, HCELL+hMSC transmigration was equivalent in the presence or absence of AMD3100, indicating that CXCR4 signaling is not necessary for HCELL-mediated TEM.
HCELL/E-Selectin Interactions Up-Regulate Adhesion of hMSCs to VCAM-1 Under Hemodynamic Flow Conditions.
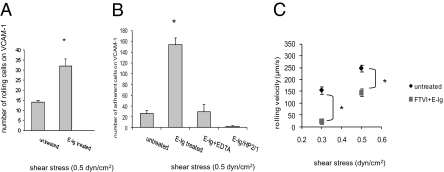
To assess the impact of HCELL/E-selectin interactions on VLA-4 adhesiveness to VCAM-1 under physiologic shear conditions, parallel-plate flow-chamber studies were performed. Untreated (HCELL−) or FTVI-treated hMSCs (HCELL+) were preincubated or not with E-Ig, and perfused over recombinant human VCAM-1 (rhVCAM-1) immobilized on plastic. The total number of rolling cells, firmly adherent cells, and rolling velocity were calculated. E-selectin binding to HCELL on hMSCs significantly increased the number of rolling and adherent cells on VCAM-1 (Fig. 3 A and B). Disruption of HCELL-E-selectin binding by EDTA prevented subsequent enhanced binding to VCAM-1 (Fig. 3B). VCAM-1 interactions were specific for VLA-4, as shown by abolishment by HP2/1 mAb (Fig. 3B). Analysis of rolling velocities showed that untreated hMSCs rolled at an average of 192.3 μm/s (at 0.3 dyn/cm2) to 241.5 μm/s (at 0.5 dyn/cm2) on VCAM-1. However, following E-Ig engagement, HCELL+hMSC rolling velocity on VCAM-1 decreased significantly, averaging 24.1 μm/s (at 0.3 dyn/cm2) and 138.89 μm/s (at 0.5 dyn/cm2) (Fig. 3C). These results indicate that HCELL+hMSC binding to E-selectin markedly induces shear-resistant adhesive interactions between VLA-4 and VCAM-1.
Fig. 3.
HCELL/E-selectin engagement mediates increased binding to VCAM-1 under flow conditions. (A) hMSCs after respective treatments were stimulated or not with E-Ig and perfused over VCAM-1 substrate at 0.5 dyn/cm2 and the total number of rolling cells was counted. (B) In some instances, E-Ig engagement was performed in the presence of 10 mM EDTA or followed by HP2/1 mAb treatment, and quantified for the number of adherent cells. All interactions were VLA-4-mediated shown by abrogation in the presence of HP2/1 mAb. (C) Rolling velocity on VCAM-1 of hMSCs (FTVI-treated or not), followed by E-Ig engagement or not, was calculated at shear stresses of 0.3 dyn/cm2 and 0.5 dyn/cm2. Values are means ± SD (n = 3–5 different donor hMSC cultures). Statistical significance (P ≤ 0.05) is indicated by asterisks.
CD44 Coassociates with VLA-4 in hMSCs.
Studies in activated lymphoid cells have revealed a physical association between CD44 and VLA-4 (20). To determine whether this coassociation occurs in hMSCs, hMSCs were incubated with E-Ig or HA, or activated using phorbol myristate acetate (PMA)/ionomycin, and analyzed by coimmunoprecipitation assay. VLA-4 immunoprecipitates probed for CD44 on Western blot revealed an ∼100 kDa band corresponding to the standard form of CD44 (Fig. 4 A–C). Western blots revealed a baseline coassociation of CD44 and VLA-4 in resting hMSCs (Fig. 4 A–C, lane 1); however, marked increased association was observed upon engagement of HCELL+hMSCs with E-selectin (Fig. 4A) or with HA (Fig. 4B). PMA/ionomycin stimulation also increased CD44-VLA-4 coassociation (Fig. 4C).
Fig. 4.
hMSC activation or CD44 ligation increases CD44-VLA-4 coassociation. hMSCs untreated (A–C, lane 1) or FTVI-treated followed by E-Ig binding (A, lane 2), or stimulated with HA (B, lane 2) or with PMA/ionomycin (C, lane 2), were subjected to immunoprecipitation (IP) with anti–VLA-4 Ab followed by Western blotting (immunoblotting; IB) for CD44 (∼100 kDa). Marked increased association of CD44 and VLA-4 was induced following ligation of CD44. The figure is representative of results of hMSC cultures from multiple donors.
E-Selectin Engagement of HCELL on hMSCs Activates G-Protein Signaling.
The observed blunting of TEM following PTX treatment (Fig. 2B) indicates that Gαi-protein signaling is requisite for CD44-mediated hMSC TEM. To identify downstream effectors, we analyzed GTPases such as Rap1 and a member of the Rho GTPases, Rac1, previously shown to participate in cell migration. We observed that HCELL/E-selectin engagement or CD44/HA engagement increased levels of active Rac1 and Rap1 without changes in total Rac or Rap levels (Fig. 5 A and B). To determine the role of these GTPases in facilitating HCELL+hMSC TEM, specific Rac1 and Rap1 inhibitors were used in TEM assays. Preincubation of HCELL+hMSCs with Rac1 inhibitor NSC23766 significantly inhibited transmigration to 50% of the levels observed without inhibitor (Fig. 5C). Rap1 inhibitor GGTI-298 by itself did not significantly affect TEM, but GGTI-298 combined with NSC23766 inhibited TEM of HCELL+hMSCs to levels observed in HCELL−hMSCs (Fig. 5C). Importantly, the effects of Rac1 and Rap1 inhibitors on hMSC TEM were not due to changes in E-selectin ligand activity or VLA-4 levels (Fig. 5D). To assess the roles of Rac and Rap GTPases in CD44-mediated shear-resistant integrin adhesiveness, cells were preincubated with GTPase inhibitors or with PTX before use in flow-based VCAM-1 binding assays. Preincubation with PTX, Rac1 inhibitor, or a combination of Rac1 and Rap1 inhibitors, significantly reduced the binding of HCELL+hMSCs to rhVCAM-1-Fc at 0.5 dyn/cm2 (Fig. 5E). These results indicate that G-protein signaling through Rac1 and Rap1 GTPases itself contributes to activating VLA-4 adhesiveness of hMSCs following HCELL/E-selectin engagement under flow conditions.
Fig. 5.
HCELL/E-selectin engagement triggers Rac1/Rap1 GTPase activation that promotes hMSC TEM. hMSCs after respective treatments were subjected to GTPase IP assays. Immunoblotting for active Rac1 GTPase (A) or Rap1 GTPase (B) shows increased levels of active Rac1 GTPase and Rap1 GTPase upon E-selectin or HA engagement compared with untreated control. Analysis of total Rac1 and Rap1 is also shown. The figure is representative of results of hMSCs from multiple donors. (C) FTVI-mediated hMSC TEM is dependent on Rac1 and Rap1 GTPases. Use of Rac1 inhibitor alone or in combination with Rap1 inhibitor significantly inhibited TEM of HCELL+hMSCs. Values are means ± SD (n = 3). Statistical significance (P ≤ 0.05) is indicated by brackets and asterisks. The figure is representative of results of hMSC cultures from multiple donors. (D) Rac1 and Rap1 inhibitors do not affect E-Ig reactivity or VLA-4 expression on HCELL+hMSCs. HCELL+hMSCs untreated (dotted lines), incubated with Rac1 inhibitor (broken lines), or a combination of Rac1 and Rap1 inhibitors (shaded region) were assayed for E-Ig reactivity or VLA-4 expression by flow cytometry. (E) Inhibition of G-protein signaling affects binding of HCELL+hMSCs to VCAM-1. hMSCs after respective treatments were stimulated or not with E-Ig and perfused on immobilized VCAM-1 at 0.5 dyn/cm2. Use of PTX, Rac1 inhibitor alone, or in combination with Rap1 inhibitor significantly inhibited binding of HCELL+hMSCs to VCAM-1. Values are means ± SD (n = 3). Statistical significance (P ≤ 0.05) is indicated by asterisks. The figure is representative of results of hMSC cultures from multiple donors. (F) Inhibition of G-protein signaling does not affect CD44-VLA-4 bimolecular association: hMSCs untreated (a and b, lane 1), FTVI-treated followed by E-Ig binding (a and b, lane 2), FTVI-treated followed by PTX treatment and E-Ig binding (a, lane 3), or FTVI-treated followed by a combination of Rac1 and Rap1 inhibitors and E-Ig binding (b, lane 3) were subjected to IP with anti–VLA-4 mAb followed by Western blotting for CD44 (∼100 kDa).
G-Protein Signaling Does Not Regulate Formation of CD44-VLA-4 Bimolecular Complex.
The finding that CD44 coassociates with VLA-4 in hMSCs prompted us to investigate whether G-protein-mediated signaling through Rac1 and Rap1 GTPases regulates formation of this complex, and whether this complex is sufficient to induce CD44-VLA-4 cross-talk. To this end, HCELL+hMSCs were pretreated with PTX or a combination of Rac1 and Rap1 GTPase inhibitors, and exposed to E-Ig. Use of PTX (Fig. 5Fa) or Rac1/Rap1 GTPase inhibitors (Fig. 5Fb) had no effect on the physical association of CD44 and VLA-4 following E-selectin binding to HCELL+hMSCs; that is, CD44 ligation induces coassociation with VLA-4, and G-protein signaling is not required to maintain/create the physical association between CD44 and VLA-4. Although increased coassociation between CD44 and VLA-4 is correlated with increased VLA-4 adhesiveness, this complex formation does not alone confer increased adhesiveness of VLA-4 for VCAM-1. Collectively, our findings illustrate the role(s) of downstream G-protein signaling in mediating CD44-VLA-4 cross-talk triggering VLA-4 adhesion.
HCELL/E-Selectin Engagement Programs a Step 2–Bypass Pathway of VLA-4 Activation.
To examine the effect(s) of HCELL engagement in provoking hMSC binding to E-selectin and to VCAM-1, each natively expressed on human endothelial cells, experiments were performed using stimulated HUVEC monolayers under physiological shear stress conditions of 1 dyn/cm2. Under flow, untreated hMSCs displayed minimal firm adherence on HUVEC, and FTVI treatment dramatically increased (∼10-fold) firm adherence of hMSCs (Fig. 6). Pretreatment of HUVEC with anti–E-selectin mAb prevented firm adherence of FTVI-treated hMSCs, indicating that HCELL/E-selectin interaction(s) is prerequisite for step 3 firm adherence under hemodynamic shear (Fig. 6). Pretreatment of HCELL+hMSCs with HP2/1 mAb or with Rac/Rap inhibitors markedly reduced firm adherence (Fig. 6); the >50% decrement observed indicates that VLA-4 binding to VCAM-1 is the principal mediator of firm adherence between HCELL+hMSCs and the endothelium (Fig. 6). The residual firm adherence observed in the presence of HP2/1 mAb likely reflects resistance to blockade in the setting of markedly enhanced VLA-4 activity consequential to more complete engagement of HCELL coincident with rolling interactions along the entire hMSC membrane; under static conditions, complete inhibition of CD44 ligation-induced VLA-4 binding to ligands VCAM-1 and fibronectin-40 (FN-40, a chymotryptic fragment of FN), was observed using HP2/1 (Fig. S1). Altogether, these results provide evidence for direct transition of step 1 events (mediated by HCELL-E-selectin binding) to step 3 (mediated by activated VLA-4 binding to endothelial VCAM-1) under flow without the need for an intervening chemokine-mediated step 2 in the cell-migration pathway.
Fig. 6.
HCELL/E-selectin engagement translates into direct activation of VLA-4 adhesiveness for VCAM-1. hMSCs were perfused over cytokine-stimulated HUVEC monolayers at 1 dyn/cm2. HCELL/E-selectin engagement led to enhanced firm adherence between hMSCs and HUVEC. Anti–E-selectin mAb prevented any firm adherence, whereas HP2/1 or a combination of Rac1 and Rap1 inhibitors significantly reduced the number of HCELL+hMSCs arresting and interacting with stimulated HUVEC. Values are means ± SD (n = 3). Statistical significance (P ≤ 0.05) is indicated by asterisks. The figure is representative of results of hMSC cultures from three different donors.
Discussion
According to the canonical multistep model of cell migration, engagement of chemokine receptor(s) is obligatory to achieve (step 3) integrin activation and subsequent TEM (2, 3). It is well-known that recruitment to marrow is driven by engagement of CXCR4 on blood-borne cells with CXCL12 constitutively expressed on marrow microvascular endothelial cells (5). However, using real-time intravital microscopy in immunocompromised mice, we observed that enforced HCELL expression conferred extravasation (TEM) of intravenously infused CXCR4−hMSCs within marrow microvasculature (16). This striking physiologic finding prompted us to elucidate whether transendothelial migration of HCELL+hMSCs may be encoded by chemokine-independent effector pathways. Because E-selectin and VCAM-1 are natively expressed by bone marrow microvascular endothelium (5), we analyzed TEM of hMSCs across HUVEC monolayers stimulated to express E-selectin and VCAM-1. Our data show that HCELL+hMSCs undergo VLA-4-mediated firm adherence and TEM through stimulated HUVEC in the absence of chemokine administration; in particular, we observed heightened firm adherence of HCELL+hMSCs on HUVEC under flow conditions known to dissipate chemokine gradients (21). To specifically evaluate whether CXCR4 signaling contributes to the observed hMSC TEM, we performed PCR to measure CXCR4 mRNA levels and Western blot analysis of CXCR4 protein, together with TEM assays of HCELL+hMSCs treated with the CXCR4 antagonist AMD3100. Compared with human lymphocytes, our data show that hMSCs express very low levels of CXCR4, and, more critically, that inhibition of CXCR4 signaling has no effect on the HCELL-mediated increased transmigration of hMSCs. These results directly demonstrate that enhanced TEM of HCELL+hMSCs is not dependent on CXCR4 signaling. Collectively, our data show that CD44 engagement by E-selectin or by HA alone potentiates a heterodimeric association between CD44 and VLA-4, and triggers a Rac1/Rap1-dependent G-protein-coupled activation of VLA-4 adhesiveness. This previously uncharacterized GTPase-mediated CD44-VLA-4 cross-talk thereby licenses a step 2–bypass pathway capable of directing cell adhesion to, and subsequent transmigration through, vascular endothelium (Fig. S2). Our results thus uncover a mechanosignaling dimension to the “rules of the road” governing cell-navigation patterns in the vasculature, with broad implications for use of hMSCs and other cell types in adoptive therapeutics.
Functioning as step 2 effectors, chemokines contribute to integrin activation by binding to cognate ligands that are G-protein-coupled receptors (22). Upon activation by G-protein-coupled receptors, G-proteins undergo conformational changes to the active GTP-bound state, promoting downstream signaling leading to cellular processes including migration (23). Such activation of G-proteins can generate second messengers leading to selective activation of smaller GTPases such as Rap1 (24) or Rac1 (25) that serve as potent inside-out stimulators of integrin adhesiveness (26, 27). Rac and Rap GTPases have been implicated in the efficient homing and engraftment of hematopoietic stem/progenitor cells (28), and direct overexpression of GTPases can promote integrin-mediated firm adhesion and TEM without chemokine input (24).
CD44 was first identified by its role in binding to HA (29), and it is best-known for mediating cell-matrix interactions. An early study using a murine lymphoid cell line suggested that CD44 could function as a G-protein possessing both GTP binding and GTPase activity blocked by PTX, a specific inhibitor of Gαi (30). Another early study suggested a role for anti-CD44 mAb cross-linking in “outside-in” triggering of LFA-1 adhesiveness in human lymphocytes (31) and, more recently, a collaborating role for CD44 in VLA-4 adhesiveness was shown in murine T cells (20). Although these studies highlighted an association between CD44 engagement and integrin activation, they did not elucidate the signaling effector(s) of this process.
Our finding that PTX blocks activation of VLA-4 following CD44/HCELL ligation indicates that CD44-dependent G-protein signaling triggers the observed transition from step 1 to step 3 in hMSCs. Studies in murine cell lines have suggested that engagement of CD44 using anti-CD44 mAb activates the GTPases Rac1 (32) and Rap1 (33), and that signaling through G-proteins, including the PTX-sensitive Gαi, can activate GTPases such as Rap1 (34). Here, using human primary cells, we observed that CD44/HCELL binding to HA and E-selectin in each case results in up-regulation of Rac1 and Rap1 GTPases. Moreover, our studies using inhibitors of Rac1 and Rap1 GTPases provide direct evidence for active GTPases in promoting CD44-primed VLA-4 adhesiveness and subsequent TEM of hMSCs on stimulated HUVEC. Incubation of cells with Rac1 inhibitor NSC23766 resulted in significant inhibition of TEM of HCELL+hMSCs across stimulated HUVEC. The residual TEM observed with NSC23766 inhibition may be due to the contribution(s) of other GTPases and/or the incomplete effect of NSC23766 on all of the guanine-nucleotide exchange factors that promote GTP binding to Rac1 (35). Although the Rap1 inhibitor GGTI-298 alone did not affect TEM of HCELL+hMSCs, combination of GGTI-298 and NSC23766 led to a greater inhibition of TEM compared with NSC23766 alone. The observed synergism of NSC23766 and GGTI-298 in inhibiting HCELL+hMSC TEM is consistent with the ability of Rap1 to function upstream of Rac1 (36). Although further studies beyond the scope of this report are required to define the potential involvement of other GTPases, unmasking of the Rap1 contribution(s) in the presence of Rac1 inhibition suggests that signals through both Rac1 and Rap1 GTPases contribute to integrin adhesiveness and TEM consequent to engagement of CD44 on hMSCs.
Our studies using purified VCAM-1 as substrate under hemodynamic shear conditions provide direct evidence that engagement of HCELL on hMSCs markedly augments VLA-4/VCAM-1 adhesive interactions in the absence of exogenous chemokine input (Fig. 3). Importantly, our data illustrate that different ligands capable of engaging CD44 provoke increased binding of VLA-4 to VCAM-1, in each case funneling through identical intracellular signaling pathways (Fig. 5 A and B; Fig. S1). Studies of HCELL+hMSCs on stimulated HUVEC monolayers show that E-selectin/HCELL interactions are absolutely prerequisite for VLA-4-dependent firm adhesion and TEM (Figs. 2 and 6). Although a stimulated endothelium may secrete a restricted set of chemokines such as CCL2, CXCL8, and CCL5 (37, 38), chemotaxis across HUVEC monolayers would not occur in the absence of established gradients. However, in vivo, it is possible that hMSC chemokine receptors encountering cognate ligand(s) could act in a cooperative manner to activate VLA-4 following CD44 engagement. Low-level expression of a restricted repertoire of chemokine receptors, including CXCR1, CXCR2, CCR2, and CCR3, has been variably reported on hMSCs, with minimal chemotactic response to CXCL8 (ligand of CXCR1 and CXCR2) and CCL5 (ligand of CCR3) (39, 40). Wherever detected by flow cytometry, CXCR4 expression on hMSCs is typically nominal (41, 42) and, where more prominently observed, is always accompanied by relatively low staining intensity (17, 41, 43). In cases where CXCR4 expression has been reported, the chemotactic response of hMSCs to CXCL12 is modest (17). Our prior work has shown that chemokine gradients established across an endothelial interface are rapidly dissipated by hemodynamic shear forces (21), suggesting that cells bearing modest expression of chemokine receptors would be at a disadvantage in responding to perivascular chemokines in vivo. Collectively, these prior studies and our results here indicate that although chemokine(s) could cooperate in activating hMSC VLA-4 binding to endothelium and subsequent TEM in vivo, engagement of hMSC HCELL with E-selectin natively expressed on primary endothelial cells can drive this process directly.
In contrast to variable expression of chemokine receptors, hMSCs uniformly express CD44 and VLA-4. In this study, MSCs were expanded from marrow of dozens of donors, and CD44-VLA-4 cross-talk was observed across all cultures. Others have reported that enhanced osteotropism can be achieved by transducing murine MSCs with the α4 gene, resulting in increased VLA-4 expression and binding to VCAM-1 and FN (44), suggesting that increased VLA-4 adhesiveness mediates homing of cells to marrow. Notably, apart from constitutive expression on marrow sinusoidal endothelium, VCAM-1 is expressed among subsets of marrow myeloid cells and stromal cells (45, 46), and FN is prominently found in marrow. Thus, upon entry into the marrow parenchyma, CD44 binding to HA, an abundant marrow extracellular matrix component, could prime VLA-4-dependent VCAM-1/FN-based lodgement of MSCs within appropriate marrow microenvironments (47). Moreover, after transmigration, CD44 binding to HA may itself support MSC lodgement, as reported for hematopoietic stem cells (48).
Our results here provide an insight into the molecular basis of osteotropism previously observed with HCELL+hMSCs. Existence of such a step 2–bypass pathway on a glycan-engineered primary adult stem cell has profound implications for use of such cells in regenerative therapeutics, and in all adoptive cell therapeutics. Inflammatory cytokines such as TNF-α and IL-1 characteristically up-regulate expression of E-selectin and VCAM-1 on microvascular endothelial cells at sites of tissue injury/inflammation (49). Notably, prior studies have indicated that MSCs can localize to sites of inflammation in a CD44-dependent manner (50). Such recruitment may be mediated by CD44 binding to vascular deposits of HA triggering VLA-4 adhesiveness, as vascular lumen and perivascular HA deposition is characteristic of inflammation (51, 52). However, owing to prominent display of E-selectin on vascular endothelium at sites of inflammation, the capability to specifically enforce expression of the step 1 effector HCELL to engage E-selectin would markedly amplify trafficking of hMSCs to injury sites. The expression of HCELL and VLA-4 on a circulating cell encountering E-selectin and VCAM-1 at the endothelial interface could alone program that cell's “decision” to extravasate. Therefore, the capacity to custom-modify HCELL expression on cells expressing both CD44 and VLA-4 should promote cellular delivery to any site of tissue damage. Further studies are warranted to determine whether the observed CD44-VLA-4 cross-talk is a general property of all cells expressing these two adhesion molecules, and to scrutinize whether engagement of other step 1 effectors can trigger integrin activation sufficient to drive tissue-specific delivery of relevant cells for adoptive therapeutics.
Materials and Methods
Fucosylation Reaction.
Fucosylation of hMSCs using FTVI to render HCELL expression was performed as described (16). In brief, cells were treated with 60 mU FTVI for 1 h at 37 °C in HBSS buffer (without Ca2+ and Mg2+) containing 20 mM Hepes, 0.1% human serum albumin, and 1 mM GDP-fucose, whereas control cells were treated in the same buffer without FTVI.
Additional Methods.
For all other methods please refer to SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors appreciate the helpful advice of Drs. Tanya Mayadas, Xavier Cullere, and Hans Widlund. This work was supported by National Institutes of Health R01 Grants HL073714 and CA121335, and is dedicated to the memory of Harold C. Sackstein.
Footnotes
Conflict of interest statement: In accordance with NIH guidelines, intellectual property related to HCELL expression is retained by R.S.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018064108/-/DCSupplemental.
References
- 1.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122:1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Peled A, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 8.Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 9.Krampera M, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 10.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda K. Application of mesenchymal stem cells for the regeneration of cardiomyocyte and its use for cell transplantation therapy. Hum Cell. 2003;16:83–94. doi: 10.1111/j.1749-0774.2003.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagaya N, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 13.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 15.Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Sackstein R, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 17.Sordi V, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 18.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber TH, Shinder V, Cain DW, Alon R, Sackstein R. Shear flow-dependent integration of apical and subendothelial chemokines in T-cell transmigration: Implications for locomotion and the multistep paradigm. Blood. 2007;109:1381–1386. doi: 10.1182/blood-2006-07-032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Buzney CD, Poznansky MC, Sackstein R. Dynamic alterations in chemokine gradients induce transendothelial shuttling of human T cells under physiologic shear conditions. J Leukoc Biol. 2009;86:1285–1294. doi: 10.1189/jlb.0309214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Bos JL. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimonaka M, et al. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Bernal D, et al. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin α4β1. Mol Biol Cell. 2005;16:3223–3235. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Wetering S, et al. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 27.Reedquist KA, et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancelas JA, et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 29.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 30.Lokeshwar VB, Bourguignon LY. The lymphoma transmembrane glycoprotein GP85 (CD44) is a novel guanine nucleotide-binding protein which regulates GP85 (CD44)-ankyrin interaction. J Biol Chem. 1992;267:22073–22078. [PubMed] [Google Scholar]
- 31.Koopman G, et al. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990;145:3589–3593. [PubMed] [Google Scholar]
- 32.Oliferenko S, Kaverina I, Small JV, Huber LA. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J Cell Biol. 2000;148:1159–1164. doi: 10.1083/jcb.148.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vachon E, et al. CD44-mediated phagocytosis induces inside-out activation of complement receptor-3 in murine macrophages. Blood. 2007;110:4492–4502. doi: 10.1182/blood-2007-02-076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weissman JT, Ma JN, Essex A, Gao Y, Burstein ES. G-protein-coupled receptor-mediated activation of Rap GTPases: Characterization of a novel Gαi regulated pathway. Oncogene. 2004;23:241–249. doi: 10.1038/sj.onc.1207014. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maillet M, et al. Crosstalk between Rap1 and Rac regulates secretion of sAPPα. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- 37.Läubli H, Spanaus KS, Borsig L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114:4583–4591. doi: 10.1182/blood-2008-10-186585. [DOI] [PubMed] [Google Scholar]
- 38.Øynebråten I, Bakke O, Brandtzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- 39.Ringe J, et al. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 40.Ponte AL, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 41.Hung SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi M, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 43.Honczarenko M, et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic α 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 45.Ulyanova T, et al. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulyanova T, Priestley GV, Banerjee ER, Papayannopoulou T. Unique and redundant roles of α4 and β2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Exp Hematol. 2007;35:1256–1265. doi: 10.1016/j.exphem.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matrosova VY, Orlovskaya IA, Serobyan N, Khaldoyanidi SK. Hyaluronic acid facilitates the recovery of hematopoiesis following 5-fluorouracil administration. Stem Cells. 2004;22:544–555. doi: 10.1634/stemcells.22-4-544. [DOI] [PubMed] [Google Scholar]
- 49.Sackstein R. Glycosyltransferase-programmed stereosubstitution (GPS) to create HCELL: Engineering a roadmap for cell migration. Immunol Rev. 2009;230:51–74. doi: 10.1111/j.1600-065X.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrera MB, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 51.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milinkovic M, Antin JH, Hergrueter CA, Underhill CB, Sackstein R. CD44-hyaluronic acid interactions mediate shear-resistant binding of lymphocytes to dermal endothelium in acute cutaneous GVHD. Blood. 2004;103:740–742. doi: 10.1182/blood-2003-05-1500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.