Abstract
Two general pathways of mRNA decay have been characterized in yeast. In one pathway, the mRNA is degraded by the cytoplasmic form of the exosome. The exosome has both 3′ to 5′ exoribonuclease and endoribonuclease activity, and the available evidence suggests that the exonuclease activity is required for the degradation of mRNAs. We confirm here that this is true for normal mRNAs, but that aberrant mRNAs that lack a stop codon can be efficiently degraded in the absence of the exonuclease activity of the exosome. Specifically, we show that the endo- and exonuclease activities of the exosome are both capable of rapidly degrading nonstop mRNAs and ribozyme-cleaved mRNAs. Additionally, the endonuclease activity of the exosome is not required for endonucleolytic cleavage in no-go decay. In vitro, the endonuclease domain of the exosome is active only under nonphysiological conditions, but our findings show that the in vivo activity is sufficient for the rapid degradation of nonstop mRNAs. Thus, whereas normal mRNAs are degraded by two exonucleases (Xrn1p and Rrp44p), several endonucleases contribute to the decay of many aberrant mRNAs, including transcripts subject to nonstop and no-go decay. Our findings suggest that the nuclease requirements for general and nonstop mRNA decay are different, and describe a molecular function of the core exosome that is not disrupted by inactivating its exonuclease activity.
Keywords: Dis3, Saccharomyces cerevisiae
The core eukaryotic exosome contains nine subunits that are essential for viability, but catalytically inactive (1–4). The catalytic activity is provided by a 10th essential subunit, Rrp44p (2, 5, 6). This protein is similar to RNase II in that it contains three putative RNA binding domains that flank an RNB domain (3, 7–10). The RNB domain is responsible for the 3′ exonuclease activity of the exosome (3). In addition, the N terminus of Rrp44p contains an endonucleolytic PIN domain. Either active site is sufficient for viability; however, simultaneous inactivation of both nuclease activities results in a lack of cell growth (11–13). Although the biological substrates of the Rrp44p endonuclease have not been fully elucidated, the synthetic lethality observed upon inactivation of the Rrp44p nucleases implies that they have overlapping functions. The exosome is involved in RNA processing and RNA degradation, and several of these reactions have been shown to be defective if the exonuclease activity of the exosome is disrupted by a point mutation [including 5.8S rRNA and snoRNA processing and 5′ external transcribed spacer (ETS) and cryptic unstable transcripts (CUT) degradation]. In contrast, mutating the endonuclease active site of Rrp44p has minor or no effect on the exosome functions that have been tested (11–13).
The exosome is present in both the nucleus and the cytoplasm, and the cytoplasmic exosome plays a dual role in gene expression. First, the exosome is involved in one of two redundant decay pathways for normal mRNAs. The initiating step for decay of normal mRNAs is shortening of the poly(A) tail (14, 15). Removal of the poly(A) tail predominantly triggers cytoplasmic 5′-to-3′ decay in yeast (16–19). Deadenylation can also trigger the degradation of an mRNA from its 3′ end, in a process catalyzed by the cytoplasmic exosome (20).
The second role of the cytoplasmic exosome is to maintain the fidelity of gene expression by degrading aberrant mRNAs. Aberrant transcripts arise through mistakes in gene expression, including genetic mutations, defects in transcription or splicing, or premature polyadenylation at incorrect or cryptic sites. In one of the exosome-mediated mRNA surveillance pathways, mRNAs that lack in-frame termination codons are targeted to the nonstop decay pathway (21, 22). In the current model of nonstop decay, a translating ribosome stalls at the 3′ end, which triggers exosome-mediated decay (22).
Whereas normal mRNAs are degraded mainly by exonucleases, a number of specialized mRNA decay pathways can be initiated by endonuclease cleavage [e.g., no-go decay, RNAi, and nonsense-mediated mRNA decay (23–31)]. For example, in no-go decay, mRNAs that have stalled ribosomes within their coding region are cleaved by an unknown endonuclease (25, 29). Similar cleavage products can be generated by inserting a ribozyme into an mRNA (32). Though these pathways are initiated by a variety of endonucleases, the fragments resulting from this cleavage are degraded by common exonucleases. Specifically, the 5′ fragments are degraded by the cytoplasmic exosome, and the 3′ fragments are degraded by the 5′-to-3′ exonuclease Xrn1p (25–28, 32–34).
Understanding the molecular mechanisms that are responsible for the degradation of aberrant transcripts is needed to understand how these mRNAs are preferentially targeted for rapid degradation and how the fidelity of gene expression is maintained. Additionally, the recent identification of a second nuclease active site in the exosome means that the role of the Rrp44p endonuclease must be examined in the known functions of the exosome. To address these issues, we tested the role of the Rrp44p nuclease activities in general mRNA degradation and in mRNA surveillance. Here we report that nonstop mRNAs and ribozyme-cleaved mRNAs can be degraded by either of the Rrp44p nuclease activities. Additionally, we show that the Rrp44p endonuclease is not responsible for endonucleolytic cleavage in no-go decay, which suggests that endonuclease-mediated nonstop decay is distinct from no-go decay. Our results indicate that the exonuclease activity of Rrp44p is needed for the cytoplasmic exosome-mediated turnover of normal cellular transcripts, but not for the degradation of nonstop mRNAs.
Results
Individual Mutations That Disrupt the Endo- or Exonuclease Activity of Rrp44p Do Not Affect Expression of Nonstop Reporters.
Mutations that inactivate the cytoplasmic exosome stabilize transcripts that lack stop codons, suggesting that the exosome degrades such mRNAs (21, 22). However, the cytoplasmic exosome contains two RNase domains, and it is unknown which domain degrades nonstop transcripts. To investigate this question we initially analyzed expression of a his3-nonstop reporter mRNA. Cells that have a functional nonstop decay pathway rapidly degrade the his3-nonstop transcript, resulting in a lack of growth on media lacking histidine. In contrast, in a strain with a defect in nonstop decay, the his3-nonstop reporter is stable, which allows cells to grow on media lacking histidine (22). To determine whether the nuclease activities of Rrp44p are required for nonstop decay, a plasmid encoding a his3-nonstop reporter was transformed into an rrp44Δ strain that was complemented with a catalytically inactive endonuclease point mutant (rrp44-D171A, hereafter called rrp44-endo−) or a catalytically inactive exonuclease point mutant (rp44-D551N, hereafter called rrp44-exo−). Similar to the wild-type strain, both the rrp44-endo− and rrp44-exo− point mutants failed to grow on media lacking histidine, indicating that the his3-nonstop mRNA was unstable. In contrast, as previously reported, deletion of the gene for the cytoplasmic exosome cofactor Ski7 allowed for growth on media lacking histidine, indicating that the his3-nonstop mRNA was stable in this strain (Fig. 1A) (22). These findings suggest that inactivation of the nuclease activities of Rrp44p, individually, does not affect the expression of a his3-nonstop allele.
Fig. 1.
Mutations that disrupt the endo- or exoribonuclease activity of Rrp44p do not affect expression of nonstop reporters. (A) Strains containing the rrp44-endo− or rrp44-exo− mutations were transformed with a his3-nonstop reporter. Each of the indicated strains were serially diluted and spotted onto media lacking histidine to assay suppression of the his3-nonstop allele. (B and C) Strains containing the rrp44-endo−, rrp44-exo−, or rrp44-CR3 mutations (B) or completely lacking the exonucleolytic RNB domain of Rrp44p (C) were transformed with a PGK1pG-nonstop reporter under the control of a galactose-inducible promoter. Expression of the reporter was repressed by the addition of glucose. Total RNA was isolated and PGK1pG-nonstop mRNA levels were analyzed by Northern blot analysis. Plotted is the mRNA remaining at each time point after correcting for loading differences using a probe specific for the RNA subunit of the signal recognition particle (SCR1).
To examine a second nonstop mRNA and more directly determine whether the nuclease activities of Rrp44p are required to degrade nonstop mRNAs, we measured PGK1pG-nonstop mRNA decay rates in the rrp44-endo− and rrp44-exo− mutants. In this experiment, transcription of the PGK1pG-nonstop reporter was repressed by the addition of glucose, and RNA was isolated at various time points. These and subsequent mRNA stability measurements were done using at least two independent time-course experiments, and the average value at each time point is plotted in Fig. 1B. Similar to what was observed in the his3-nonstop growth assay, the PGK1pG-nonstop transcript was as unstable in the rrp44-endo− and rrp44-exo− mutants, as it was in the wild-type strain. In both the his3-nonstop and PGK1-nonstop assays, the rrp44-endo− and rrp44-exo− strains resemble a wild-type strain, and this phenotype is distinct from that of a ski7Δ strain. These results indicate that mutations that individually disrupt either of the nuclease activities of Rrp44p do not affect the stability of nonstop transcripts.
Our finding that the rrp44-exo− mutant did not affect stability on nonstop mRNAs is surprising, because published results indicate this mutation stabilizes normal transcripts (see below). One possibility is that the rrp44-exo− point mutation strongly reduces exonuclease activity but does not completely eliminate it. If this is true, then this strongly reduced activity may be sufficient for nonstop decay, but not for regular mRNA decay. To test this possibility we used a strain that completely lacks the RNB domain of Rrp44p. In this strain (rrp44-Δexo), the PGK1pG-nonstop transcript was also unstable, ruling out the possibility that any theoretical residual activity of the RNB domain in the rrp44-exo− mutant is sufficient for nonstop decay (Fig. 1C).
Either the Endo- or Exonuclease Activity of Rrp44p Can Efficiently Degrade Nonstop mRNAs.
Because disrupting the catalytic activities of Rrp44p individually does not abrogate nonstop decay, our results suggest that neither of the catalytic activities are required for nonstop mRNA degradation. This could be because the two activities are redundant for nonstop decay, or because Rrp44p plays no role in nonstop decay. To test whether Rrp44p had any role in nonstop mRNA degradation, we analyzed the expression of nonstop reporters in one additional RRP44 allele, rrp44-CR3. In this mutant, the three conserved cysteine residues of the N-terminal CR3 motif of Rrp44p have been mutated to serine. Although the biological function of this CR3 motif is not yet known, the rrp44-CR3 mutant has a slow growth phenotype (12). As shown in Figs. 2A and 1B, the rrp44-CR3 mutant suppressed the his3-nonstop allele and stabilized the PGK1pG-nonstop transcript. The Rrp44p-CR3 protein expression level is somewhat reduced compared with wild-type Rrp44p (Fig. 2B), and the defect in nonstop decay may be due to the sequence changes or the reduced expression of the Rrp44p-CR3 protein. In either case, this finding indicates that Rrp44p is required for nonstop decay.
Fig. 2.
Either the endo- or exoribonuclease activity of Rrp44p can efficiently degrade nonstop mRNAs. (A) Mutation of the CR3 region of Rrp44p affects expression of a his3-nonstop reporter. A strain containing the rrp44-CR3 mutation was transformed with a his3-nonstop reporter. Each of the indicated strains were serially diluted and spotted onto media lacking histidine. (B) Protein expression of the Rrp44p-CR3 mutant was analyzed using Western blot analysis with antibodies specific for Protein A, and Pgk1p as a loading control. (C) The nonstop mRNA decay defect seen in the rrp44-CR3 strain can be complemented by wild-type RRP44 or by the rrp44-endo− or rrp44-exo− allele, but not by the double-mutant rrp44-endo−exo−.
The rrp44-endo− exo− double mutant is inviable, making it impossible to analyze nonstop decay in this strain. However, knowing that the rrp44-CR3 mutation stabilizes nonstop mRNAs allowed us to test whether the endo- and exonuclease activities are redundant for nonstop decay. Specifically, we attempted to complement the rrp44-CR3 strain with wild-type RRP44, the rrp44-endo− mutant, the rrp44-exo− mutant, or the rrp44-endo− exo− double mutant. As shown in Fig. 2C, expressing rrp44-endo− or rrp44-exo− restored his3-nonstop mRNA decay to the rrp44-CR3 strain, confirming that both of these alleles were functional in nonstop mRNA decay. Importantly, the rrp44-endo− exo− double mutant failed to restore nonstop decay (Fig. 2C), despite being expressed at levels similar to wild-type Rrp44p (11). We conclude that either the endo- or exonuclease activity of Rrp44p can efficiently degrade nonstop mRNAs.
Exonuclease Activity of Rrp44p Is Required for the Exosome-Mediated Decay of Normal mRNAs.
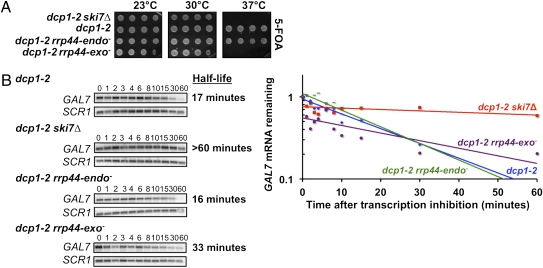
The lack of a requirement for the Rrp44p exonuclease in nonstop decay is surprising given that two experiments implicate that this activity is required for general mRNA degradation. First, it was shown that the rrp44-exo− allele is synthetically lethal with an xrn1Δ (13). Mutations that block exosome-mediated mRNA decay are synthetically lethal with defects in the 5′-to-3′ mRNA decay pathway, such as an xrn1Δ (20, 35, 36). Thus, a likely explanation for the rrp44-exo− xrn1Δ synthetic lethality is that combining these mutations blocks both general mRNA decay pathways. However, Xrn1p and Rrp44 exonuclease activities are also both required for rRNA processing (3, 11–13), which suggests that other possible explanations for the reported synthetic lethality may exist. Unlike Xrn1p, the Dcp1p/Dcp2p decapping enzyme is thought to only be required for mRNA degradation (37, 38). Thus, to confirm and expand on the previous results, we tested synthetic lethality with a dcp1 mutation. Because of its role in 5′-to-3′ decay, a temperature-sensitive allele of dcp1, dcp1-2, is synthetically lethal with mutations in exosome subunits and cytoplasmic exosome cofactors at the nonpermissive temperature of 37 °C (20). Similar to the results obtained with an xrn1Δ (13), the rrp44-exo− mutation was synthetically lethal with dcp1-2 at 37 °C (Fig. 3A). In contrast, the dcp1-2 rrp44-endo− double mutant is viable at 37 °C. This indicates that the exonuclease activity of Rrp44p is needed for general 3′-to-5′ mRNA decay.
Fig. 3.
The exoribonuclease activity of Rrp44p is required for exosome-mediated decay of normal mRNAs. (A) Strains containing either the rrp44-endo− or rrp44-exo− mutation in combination with dcp1-2 were serially diluted and grown at the indicated temperatures. (B) The stability of GAL7 mRNA in the dcp1-2 rrp44-endo− or dcp1-2 rrp44-exo− mutants was examined by growing cells at 23 °C in media containing galactose. The 5′-to-3′ decay pathway was inactivated by incubating cells for 1 h at 37 °C. Following the addition of glucose, RNA was isolated at the times indicated, and GAL7 mRNA levels were analyzed by Northern blot analysis. Plotted is the mRNA remaining at each time point after correcting for loading differences using a probe specific for the RNA subunit of the signal recognition particle (SCR1).
The second published experiment implicating the Rrp44p exoribonuclease activity in general mRNA decay is that MFA2pG mRNA is stabilized, and MFA2pG decay products accumulate in a strain that is defective in decapping, depleted of wild-type Rrp44p, and also expresses the rrp44-exo− mutant (3). This suggests that the exonuclease activity of Rrp44p is required for mRNA decay, but whether the endonuclease activity is also involved was not addressed in this assay. Furthermore, Dziembowski et al. (3) did not address whether the MFA2 decay intermediates were generated by the Rrp44p endo- or exonuclease activity. Therefore, we directly measured decay rates of the endogenous GAL7 mRNA. The GAL7 transcript is normally primarily degraded through the decapping pathway, but in a dcp1-2 strain at 37 °C, this transcript is degraded by the cytoplasmic exosome (20). We assayed the stability of GAL7 mRNA in dcp1-2 rrp44-endo− and dcp1-2 rrp44-exo− mutants. As expected, GAL7 was more stable in the dcp1-2 ski7Δ and dcp1-2 rrp44-exo− strains, compared with the dcp1-2 single mutant. In contrast, GAL7 was not stabilized in the dcp1-2 rrp44-endo− mutant (Fig. 3B). Overall, the dcp1 synthetic lethality and GAL7 mRNA stability results confirm that the exonuclease activity of Rrp44p is needed for the exosome-mediated decay of normal mRNAs, and thus that the nuclease requirements for nonstop and general mRNA decay are different.
Either the Endo- or Exonuclease Activity of Rrp44p Can Efficiently Degrade an Endonucleolytically Cleaved mRNA.
To determine whether the nuclease requirements for the degradation of other aberrant transcripts were similar to that of nonstop mRNAs, we analyzed the degradation of a ribozyme-cleaved transcript. Previous studies indicate that the 5′ cleavage product resulting from ribozyme cleavage is rapidly degraded by the cytoplasmic exosome (32). To determine whether the nuclease activities of Rrp44p were needed for this mRNA decay pathway, a plasmid containing a hammerhead ribozyme inserted into the HIS3 gene (his3-Rz) was transformed into the rrp44-endo− or rrp44-exo− mutants. Similar to the results obtained for nonstop decay, both the rrp44-endo− and rrp44-exo− mutants that contained his3-Rz failed to grow on media lacking histidine (Fig. 4), suggesting that inactivation of either of the nuclease activities of Rrp44p does not affect expression of his3-Rz. However, the rrp44-CR3 mutant supported growth on media lacking histidine, indicating that Rrp44p is required for the rapid degradation of this reporter mRNA. Therefore, the nuclease requirements for the degradation of this hammerhead-cleaved mRNA mirror those for nonstop decay, suggesting that the nuclease requirements for exosome-mediated decay of several kinds of aberrant transcripts differ from that of normal mRNAs.
Fig. 4.
Mutations that disrupt the endo- or exoribonuclease activity of Rrp44p do not affect expression of a hammerhead ribozyme cleavage product. Strains containing the rrp44-endo−, rrp44-exo−, or rrp44-CR3 mutations were transformed with a reporter containing a hammerhead ribozyme inserted into the HIS3 gene. Each of the indicated strains were serially diluted and spotted onto media lacking histidine to assay suppression of the his3-ribozyme allele.
Endonuclease-Mediated Nonstop Decay Is Distinct from No-Go Decay.
Nonstop transcripts are thought to be recognized as aberrant because a ribosome is stalled at the 3′ end of an mRNA (22). This is conceptually similar to no-go decay, which is the rapid endonucleolytic cleavage of mRNAs with an internally stalled ribosome (25). Furthermore, nonstop decay and no-go decay require the paralogs SKI7 and HBS1, respectively (22, 25, 39). Given this similarity, we considered the possibility that Rrp44p may be the endonuclease responsible for no-go decay and that our nonstop mRNAs with stalled ribosomes at their 3′ ends were also susceptible to no-go decay. It has previously been shown that the 5′ cleavage product resulting from no-go decay of the PGK1-SL reporter mRNA accumulates in a ski7Δ strain and that 3′ no-go decay cleavage products accumulate in an xrn1Δ. Fig. 5 and Fig. S1A show that these cleavage products still accumulate when the endonuclease activity of Rrp44p is inactivated. These results suggest that the endonuclease activity of Rrp44p is not needed for the endonucleolytic cleavage in no-go decay. As detailed in SI Results and Fig. S1 B and C, we also show that unlike nonstop decay, the rrp44-CR3 mutation does not disrupt no-go decay. Combined, these findings suggest that endonuclease-mediated nonstop decay is distinct from the endonucleolytic cleavage in no-go decay.
Fig. 5.

Endoribonuclease-mediated nonstop mRNA decay is distinct from no-go decay. A wild-type strain, a ski7Δ strain, and a ski7Δ rrp44-endo− double-mutant strain were transformed with a PGK1 or PGK1-SL reporters under the control of a galactose-inducible promoter. Total RNA was isolated from cultures grown in galactose, and PGK1 mRNA levels were analyzed by Northern blot analysis. The RNA subunit of the signal recognition particle (SCR1) served as a loading control.
Endonuclease Activity of Rrp44p Is Not Redundant with the C-Terminal Domain of Ski7p in Nonstop Decay.
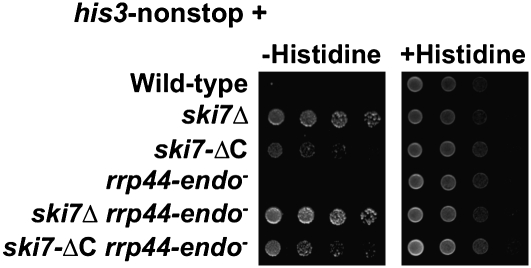
In the current model of nonstop mRNA degradation, the C terminus of Ski7p recognizes a stalled ribosome with an empty A-site and recruits the exosome to rapidly degrade the nonstop transcript from the 3′ end (22). One unresolved issue with this model is that the 3′ end of the mRNA should be inaccessible to the exosome because it is buried in the decoding center of the ribosome. One solution to this problem may be that the C terminus of Ski7p triggers ribosome disassembly (Fig. S2) (22). A second solution may be that the Rrp44p endonuclease cleaves the transcript near the stalled ribosome, thereby releasing the very 3′ end and its bound ribosome. As described in detail in SI Results, neither of these models is fully supported by published data (22) or the data described above. We therefore tested whether both mechanisms operate redundantly by introducing the his3-nonstop reporter into a strain containing a C-terminal truncation of Ski7p and the rrp44-endo− mutant (ski7-ΔC rrp44-endo−). As previously reported (22), the ski7-ΔC strain had a moderate effect on growth on media lacking histidine (Fig. 6, third row). Similarly, the ski7-ΔC rrp44-endo− mutant was able to only partially suppress of the his3-nonstop allele (Fig. 6, sixth row). In contrast, a complete deletion of SKI7 (ski7Δ) was able to more effectively suppress the his3-nonstop allele. This suggests that the endonuclease activity of Rrp44p is not specifically required to initiate nonstop decay, but that the endo- and exonuclease activities of Rrp44p act in the same step of nonstop decay.
Fig. 6.
The endoribonuclease activity of Rrp44p is not redundant with the C-terminal domain of Ski7p in nonstop mRNA decay. The indicated strains were transformed with a his3-nonstop reporter and analyzed as in Fig. 1A.
Discussion
Nuclease Requirements for Exosome-Mediated Degradation of Normal and Nonstop mRNAs Differ.
In this study we show that a mutation that inactivates the exonuclease activity of the cytoplasmic exosome inhibits its ability to degrade normal mRNAs, but has no effect on its ability to degrade nonstop mRNAs. We directly measured the ability of the cytoplasmic exosome to degrade the GAL7 mRNA and found that this normal mRNA is stabilized when the exonuclease domain of Rrp44p is inactivated. The conclusion that the rrp44p-exo− allele blocks exosome-mediated decay of normal mRNAs is further supported by the observations that this allele (i) is synthetically lethal with a decapping mutation (Fig. 3A) and an xrn1Δ (13), (ii) stabilizes MFA2pG mRNA in a decapping-defective strain, and (iii) causes accumulation of degradation intermediates of the MFA2pG mRNA (3). Although the rrp44-exo− allele blocks exosome-mediated decay of normal mRNAs, it has no effect on the expression of his3-nonstop and his3-Rz reporter genes or the degradation of a PGK1pG-nonstop reporter mRNA. Therefore, we conclude that the nuclease requirements for exosome-mediated mRNA decay depend on the mRNA substrate. Furthermore, the observation that rrp44-exo− and rrp44-endo− do not affect the expression of two different kinds of aberrant mRNA indicate that the degradation of several kinds of aberrant mRNAs are not affected by the rrp44-exo− mutation. More broadly, our findings describe a molecular function of Rrp44p that is exonuclease independent.
Contributions of the Endo- and Exonuclease Activities of Rrp44p to Nonstop Decay.
We show that mutations that disrupt the endo- or exonuclease activity of Rrp44p do not affect the stability of nonstop mRNAs, but that the rrp44-CR3 allele stabilizes nonstop transcripts. These data indicate that Rrp44p is required for nonstop decay, but that neither of its nuclease activities is required. Furthermore, the observation that rrp44-endo− or rrp44-exo−, but not the double rrp44-endo−exo− mutants, can complement rrp44-CR3 indicates that either ribonuclease activity can rapidly degrade nonstop mRNAs.
If the endo- and exonuclease activities of Rrp44p can both degrade nonstop mRNAs, they could have different roles or identical roles in the overall process. For example, we initially considered the possibility that nonstop decay is initiated by the endonuclease activity, and that the exonuclease activity degrades the cleavage products. This model would predict that the rrp44-exo− mutation should have no effect of the stability of the full-length nonstop mRNA, but should stabilize decay intermediates. We have not been able to detect such decay intermediates, and therefore this model is unlikely. Another possibility we considered is that the endonuclease activity of Rrp44p contributes to the removal of the ribosome stalled at the 3′ end, which makes the mRNA accessible to the exonuclease activity. However, this would predict that the rrp44-endo− allele stabilizes nonstop mRNAs either by itself or in combination with the ski7-ΔC allele, which we have shown does not occur (SI Results and Fig. S2). A third possibility is that the degradation of nonstop mRNAs by the Rrp44p endonuclease may mean that ribosomes stalled on nonstop mRNAs can also trigger no-go decay. However, this model predicts that the rrp44-endo− mutation blocks no-go decay, which we show is not true. Furthermore, the rrp44-CR3 allele blocks nonstop decay, but does not block the cleavage of no-go mRNAs (SI Results and Fig. S1). Having ruled out all of these alternatives, we propose that the two nuclease activities of the exosome act in the same step of nonstop decay.
In Vivo Endonuclease Activity of the Exosome.
Previous studies suggested that the exonuclease activity of the exosome predominates over the endoribonuclease activity in vivo because the rrp44-endo− mutation has, at best, minor effects on various nuclear RNA processing and degrading activities of the exosome, whereas the rrp44-exo− mutation has clear effects (11–13). This suggests that the endonuclease activity is normally not very active in these processes. Our direct measurement of endonuclease-mediated decay of the PGK1pG-nonstop mRNA in an rrp44-exo− mutant shows that this endonuclease is sufficient to rapidly degrade an mRNA in vivo and that the contributions of the endo- and exonuclease activities of Rrp44p to exosome function are substrate dependent. We conclude that even though the Rrp44p endonuclease activity in vitro requires nonphysiological conditions (i.e., 3 mM Mn2+), in vivo, the activity is sufficient to rapidly degraded mRNAs.
Multiple Endonucleases Are Involved in Specialized mRNA Decay Pathways.
Although most normal eukaryotic mRNAs are thought to be degraded by exonucleases (i.e., Xrn1p and the exonuclease activity of the exosome), several more-specialized mRNA decay pathways in diverse eukaryotes appear to use endonucleases. These pathways include no-go decay, which is initiated by an unknown endonuclease (25, 29); NMD, which can be initiated by SMG6 in Drosophila and human cells (26–28) or by PMR1 in human erythroid cells (23, 30); RNAi, which can be initiated by Argonaute (31); and the degradation of endoplasmic reticulum-localized transcripts, which is initiated by Ire1p during the human unfolded protein response (40). To this list of specialized endonuclease-mediated mRNA decay pathways, we can now add nonstop mRNA decay, although nonstop decay appears unique, in that in most of the above pathways an endonuclease appears to initiate mRNA degradation, but exonucleases degrade the bulk of the transcript.
Materials and Methods
The yeast strains and plasmids used in these studies were created using standard techniques and are described in Tables S1 and S2. Synthetic lethality and his3-nonstop growth assays were performed essentially as described (22, 36). The half-life of the PGK1pG-nonstop reporter was determined essentially as described (22). Northern blots were hybridized with 32P 5′ end-labeled oligonucleotides listed in Table S3. Signals were detected and quantitated using a STORM PhosphorImager, and corrected for loading by quantitating the RNA subunit of the signal recognition particle, SCR1, or ACT1. Each mRNA stability measurement was done at least twice, using independent cultures. Average values of replicate experiments were graphed.
Supplementary Material
Acknowledgments
We thank Dr. Roy Parker for providing the PGK1-SL plasmid and the A.v.H. laboratory for helpful discussions. Funding was provided by National Institutes of Health Grant GM069900 (to A.v.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013180108/-/DCSupplemental.
References
- 1.Allmang C, et al. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev. 1999;13:2148–2158. doi: 10.1101/gad.13.16.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: A conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 3.Dziembowski A, Lorentzen E, Conti E, Séraphin B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat Struct Mol Biol. 2007;14:15–22. doi: 10.1038/nsmb1184. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Greimann JC, Lima CD. Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell. 2006;127:1223–1237. doi: 10.1016/j.cell.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Staals RH, et al. Dis3-like 1: A novel exoribonuclease associated with the human exosome. EMBO J. 2010;29:2358–2367. doi: 10.1038/emboj.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomecki R, et al. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J. 2010;29:2342–2357. doi: 10.1038/emboj.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonneau F, Basquin J, Ebert J, Lorentzen E, Conti E. The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell. 2009;139:547–559. doi: 10.1016/j.cell.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Frazão C, et al. Unravelling the dynamics of RNA degradation by ribonuclease II and its RNA-bound complex. Nature. 2006;443:110–114. doi: 10.1038/nature05080. [DOI] [PubMed] [Google Scholar]
- 9.Lorentzen E, Basquin J, Tomecki R, Dziembowski A, Conti E. Structure of the active subunit of the yeast exosome core, Rrp44: Diverse modes of substrate recruitment in the RNase II nuclease family. Mol Cell. 2008;29:717–728. doi: 10.1016/j.molcel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Zuo Y, et al. Structural basis for processivity and single-strand specificity of RNase II. Mol Cell. 2006;24:149–156. doi: 10.1016/j.molcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Lebreton A, Tomecki R, Dziembowski A, Séraphin B. Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature. 2008;456:993–996. doi: 10.1038/nature07480. [DOI] [PubMed] [Google Scholar]
- 12.Schaeffer D, et al. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat Struct Mol Biol. 2009;16:56–62. doi: 10.1038/nsmb.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider C, Leung E, Brown J, Tollervey D. The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res. 2009;37:1127–1140. doi: 10.1093/nar/gkn1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhlrad D, Parker R. Mutations affecting stability and deadenylation of the yeast MFA2 transcript. Genes Dev. 1992;6:2100–2111. doi: 10.1101/gad.6.11.2100. [DOI] [PubMed] [Google Scholar]
- 15.Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 16.Cao D, Parker R. Computational modeling of eukaryotic mRNA turnover. RNA. 2001;7:1192–1212. doi: 10.1017/s1355838201010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decker CJ, Parker R. A turnover pathway for both stable and unstable mRNAs in yeast: Evidence for a requirement for deadenylation. Genes Dev. 1993;7:1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CL, Stevens A. Yeast cells lacking 5′→3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol Cell Biol. 1993;13:4826–4835. doi: 10.1128/mcb.13.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhlrad D, Decker CJ, Parker R. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′→3′ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs Anderson JS, Parker RP. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 1998;17:1497–1506. doi: 10.1093/emboj/17.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frischmeyer PA, et al. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- 22.van Hoof A, Frischmeyer PA, Dietz HC, Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science. 2002;295:2262–2264. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- 23.Bremer KA, Stevens A, Schoenberg DR. An endonuclease activity similar to Xenopus PMR1 catalyzes the degradation of normal and nonsense-containing human beta-globin mRNA in erythroid cells. RNA. 2003;9:1157–1167. doi: 10.1261/rna.5720303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JM, Park EY, Kim JH, Chang SK, Cho Y. Probing the functional importance of the hexameric ring structure of RNase PH. J Biol Chem. 2004;279:755–764. doi: 10.1074/jbc.M309628200. [DOI] [PubMed] [Google Scholar]
- 25.Doma MK, Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–564. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberle AB, Lykke-Andersen S, Mühlemann O, Jensen TH. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat Struct Mol Biol. 2009;16:49–55. doi: 10.1038/nsmb.1530. [DOI] [PubMed] [Google Scholar]
- 27.Gatfield D, Izaurralde E. Nonsense-mediated messenger RNA decay is initiated by endonucleolytic cleavage in Drosophila. Nature. 2004;429:575–578. doi: 10.1038/nature02559. [DOI] [PubMed] [Google Scholar]
- 28.Huntzinger E, Kashima I, Fauser M, Saulière J, Izaurralde E. SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA. 2008;14:2609–2617. doi: 10.1261/rna.1386208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passos DO, et al. Analysis of Dom34 and its function in no-go decay. Mol Biol Cell. 2009;20:3025–3032. doi: 10.1091/mbc.E09-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens A, et al. Beta -Globin mRNA decay in erythroid cells: UG site-preferred endonucleolytic cleavage that is augmented by a premature termination codon. Proc Natl Acad Sci USA. 2002;99:12741–12746. doi: 10.1073/pnas.192442399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 32.Meaux S, Van Hoof A. Yeast transcripts cleaved by an internal ribozyme provide new insight into the role of the cap and poly(A) tail in translation and mRNA decay. RNA. 2006;12:1323–1337. doi: 10.1261/rna.46306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orban TI, Izaurralde E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA. 2005;11:459–469. doi: 10.1261/rna.7231505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souret FF, Kastenmayer JP, Green PJ. AtXRN4 degrades mRNA in Arabidopsis and its substrates include selected miRNA targets. Mol Cell. 2004;15:173–183. doi: 10.1016/j.molcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AW, Kolodner RD. Synthetic lethality of sep1 (xrn1) ski2 and sep1 (xrn1) ski3 mutants of Saccharomyces cerevisiae is independent of killer virus and suggests a general role for these genes in translation control. Mol Cell Biol. 1995;15:2719–2727. doi: 10.1128/mcb.15.5.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Hoof A, Staples RR, Baker RE, Parker R. Function of the ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol Cell Biol. 2000;20:8230–8243. doi: 10.1128/mcb.20.21.8230-8243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beelman CA, et al. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature. 1996;382:642–646. doi: 10.1038/382642a0. [DOI] [PubMed] [Google Scholar]
- 38.LaGrandeur TE, Parker R. Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J. 1998;17:1487–1496. doi: 10.1093/emboj/17.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Hoof A. Conserved functions of yeast genes support the duplication, degeneration and complementation model for gene duplication. Genetics. 2005;171:1455–1461. doi: 10.1534/genetics.105.044057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.