Abstract
Objectives
To determine the risks and benefits associated with the transfusion of packed red blood cells (PRBCs) in extremely low birth weight (ELBW) infants. We hypothesized that when ELBW infants underwent transfusion with the University of Washington Neonatal Intensive Care Unit (NICU) 2006 guidelines, no clinical benefit would be discernible.
Study design
We conducted a retrospective chart review of all ELBW infants admitted to the NICU in 2006. Information on weight gain, apnea, heart rate, and respiratory support was collected for 2 days preceding, the day of, and 3 days after PRBC transfusion. The incidence, timing, and severity of complications of prematurity were documented.
Results
Of the 60 ELBW infants admitted to the NICU in 2006, 78% received PRBC transfusions. Transfusions were not associated with improved weight gain, apnea, or ventilatory/oxygen needs. However, they were associated with increased risk of bronchopulmonary dysplasia, necrotizing enterocolitis, and diuretic use (P < .05). Transfusions correlated with phlebotomy losses, gestational age, and birth weight. No association was found between transfusions and sepsis, retinopathy of prematurity, or erythropoietin use.
Conclusions
When our 2006 PRBC transfusion guidelines were used, no identifiable clinical benefits were identified, but increased complications of prematurity were noted. New, more restrictive guidelines were developed as a result of this study.
Preterm infants are among the most highly transfused patient populations. The most common rationale for transfusing with packed red blood cells (PRBCs) in preterm infants is to improve oxygen delivery. However, there is little evidence that oxygen delivery is compromised at commonly used transfusion triggers.1–4 Transfusion guidelines became common in neonatal intensive care units (NICUs) after several clinical erythropoietin (EPO) trials showed that the institution of guidelines alone helped to decrease the frequency of transfusions in preterm infants.5 These transfusion guidelines are based on expert opinion, rather than evidence-based, and therefore vary from hospital to hospital, with some units favoring restrictive guidelines and others more liberal guidelines.2,3
Many symptoms have been attributed to chronic anemia in preterm infants including tachycardia, poor weight gain, apnea, and lactic acidosis, but there is no definitive evidence that when using any current guidelines, transfusion of PRBCs results in clinical benefit. Earlier studies show contradictory results about whether transfusions improve cardiorespiratory status6–11 or weight gain12,13 in preterm infants, but none focus specifically on extremely low birth weight (ELBW) infants.
There is increasing awareness that transfusion of blood products is not benign. In adults, anemia is an independent risk factor for increased cardiac and surgical morbidity and mortality, but correction of anemia with transfusion does not ameliorate this risk. Transfusion is an independent predictor of death, with an associated increased incidence of multiple-organ system failure, length of hospital stay, infection risk, and long-term immune modulation.14,15 In addition to the transmission of infection (viral or bacterial), transfusions have been associated with other morbidities, including transfusion-related acute lung injury,16 transfusion-related immune modulation,17 and transfusion-related cardiac overload.16 The incidence of these problems in neonates has not been well studied. Preterm infants in particular are at risk for problems not encountered in the adult population: PRBC transfusions have been implicated in the development of bronchopulmonary dysplasia (BPD),18–21 necrotizing enterocolitis (NEC),22,23 and retinopathy of prematurity (ROP).24,25
Our goal was to determine whether, when using the transfusion guidelines in place in the University of Washington Medical Center (UWMC) NICU, the transfusion of PRBCs was associated with any discernible benefit or harm in ELBW infants. We hypothesized that no clinical benefit would be detected and that transfusions would be associated with neonatal morbidities.
Methods
We conducted a retrospective chart review. All analyses were approved by the Human Subjects Division of the University of Washington and met all applicable Health Information Portability and Accountability Act standards. All patients admitted to the UWMC NICU during the 2006 calendar year were screened for study inclusion criteria: birth weight (BW) between 500 and 1000 g, with gestational age (GA) estimated at ≤28 completed weeks. Infants underwent transfusion on the basis of the criteria listed in Table I.
Table I.
Transfusion guidelines.
| 2006 guidelines* | Revised guidelines |
|---|---|
Transfuse for:
|
Transfuse for:
|
These guidelines were adapted from Shannon et al26 (with permission).
Instability is defined as an increased risk for poor oxygen delivery, for example
PRBC transfusions are standardized in our NICU: infants initially undergo transfusion of 15 mL/kg of blood in a 4-hour interval. If the HCT 4 hours after transfusion is <35%, an additional 10 mL/kg of blood is transfused; if the HCT is between 35% and 40%, 5 mL/kg of blood is transfused. If no change in HCT is noted, the patient receives an additional 15 mL/kg of blood. One adult unit of Optisol AS-5-preserved PRBC (HCT-57%) is aliquoted into 8 assigned aliquots and stored for as long as 42 days. For later transfusions, citrate-phosphate-dextrose-preserved PRBC (HCT-72%) is used. All PRBC units are cytomegalovirus safe (antibody negative or leukoreduced), Hg S negative, and irradiated.
Detailed patient information was extracted from patient medical records. Weight, respiratory support, heart rate, and apnea number and severity were collected for a 6-day period: 2 days before, day of, and 3 days after a transfusion. The severity of apneic episodes were determined as follows: 1) mild—requiring a light touch (ie, stroke on the back or a tap on the foot) to resolve; 2) moderate— requiring moving the infant (ie, rolling over, rubbing back/sternum, repositioning) to resolve; or 3) severe— episode requiring prolonged vigorous stimulation or positive pressure breaths to resolve. Weight was also recorded for the 14th and 28th days of life (DOL) to calculate average daily weight gain. Infants who either died or were transferred to other hospitals before the 14th and 28th DOL were excluded from the calculations. Phlebotomized blood volume, volume and type of blood products transfused, presence and severity of intraventricular hemorrhage (IVH), NEC, ROP, BPD, sepsis, and use of diuretics and recombinant erythropoietin (rEpo) were recorded. We defined BPD as patients requiring any type of oxygen support (nasal canula, continuous positive airway pressure [CPAP], mechanical ventilation) at both DOL 28 and at 36 weeks corrected GA. The target oxygen saturation rate in our NICU is 88% to 93% for this patient population. The incidence and severity (grade) of IVH were determined by neuroradiologists evaluating routine cranial ultrasound scans and were defined by the presence of intracranial bleeding in any region of the brain. All grades of IVH were used for statistical analysis. NEC diagnosis was based on abdominal radiography and physical examinations for patients with symptoms (feeding intolerance, abdominal distention, heme-positive stools, absent bowel sounds). The severity of NEC was categorized by using Bell’s Staging criteria. ROP screening followed the criteria published in 2006.27 All infants were examined by the same ophthalmologist. The severity of the disease was based on the International Classification of Retinopathy of Prematurity. The volumes of blood withdrawn and transfused were tallied from nursing notes charted in the medical record from the day of admission until the day of the last transfusion. HCT levels at birth and pre- and post-transfusion HCT levels were recorded.
The data was analyzed with Stata software version 9 (StataCorp, College Station, Texas). For heart rate, percent inspired oxygen, weight gain, and apnea episodes per day, we computed the difference between the individual patient’s average in the 2 days before transfusion and the average in the 3 days after transfusion. A Generalized Estimating Equation model was used to calculate the standard error estimates for the mean pre- to post-transfusion difference and to derive the 95% CI, while accounting for within-person correlation. This model was also used to evaluate whether there were any relationships between the pre- to post-transfusion changes in heart rate, or inspired oxygen and the volume of the transfusion (mL/kg of patient weight). For these models, the volume of a transfusion per kg of patient weight was included as a linear term.
Non-parametric Wilcoxon rank-sum tests were used to determine associations among BPD, ROP, and rEpo and diuretic use with PRBC transfusions. Pearson and Fisher Exact tests were used to examine the association between rEpo administration and ROP. All infants who died, were transferred or discharged before DOL 42 (earliest ROP diagnosis day in this population) were excluded from analysis for ROP. Two definitions of BPD were used: oxygen requirement on DOL 28 and at 36 weeks corrected gestational age. All infants who died, were transferred or discharged before DOL 28 and 36 weeks corrected gestational age, respectively, were excluded from analysis for BPD.
The relative risk of sepsis after a PRBC transfusion was evaluated with a Cox regression model. For each patient, the total time at risk was partitioned into intervals without a recent transfusion and intervals that were ≤2 weeks after a transfusion. Subjects were excluded for sepsis outcome for death or transfer to another hospital at DOL 85. Subjects healthy enough to be discharged to home and without subsequent re-admission were assumed to be free of sepsis until day 85. The relative risk of NEC was analyzed similarly; however, a narrower time window of 7 days after transfusion was used.
Results
During the 12-month period from Jan 1, 2006, to Dec 31, 2006, 60 ELBW infants were admitted to the UWMC NICU; 6 died shortly after birth, and 2 were transferred to other hospitals within 7 days of admission and were excluded from the study. Of the remaining 52 infants, 47 (90%) underwent transfusion. These infants received a total of 179 PRBC transfusions and 31 other transfusions, including platelets (n = 26), fresh frozen plasma (n = 3), and cryoprecipitate (n = 2). The average number of PRBC transfusions per patient was 3.8 ± 3.0. Pre-transfusion HCT level averaged 28.4%, with a post-transfusion HCT rate average of 36.9%. Five ELBW infants remained in the UWMC NICU and received no transfusions. Patient demographics are shown in Table II.
Table II.
Population description.
| Transfused | Non-transfused | |
|---|---|---|
| Number of patients, n | 447 | 45 |
| BW, g* | 766 ± 106 | 793 ± 161 |
| GA, weeks* | 26.1 ± 1.3 | 27.3 ± 0.7 |
| Male sex, n (%) | 27 (57) | 0 (0) |
| Apgar, 1 minute* | 4 ± 2 | 4 ± 2 |
| Apgar, 5 minute* | 6 ± 2 | 6 ± 1 |
| Birth HCT* | 42% ± 6% | 42% ± 4% |
| Pre-transfusion HCT | 28.4% | |
| Post-transfusion HCT | 36.9% | |
| Mortality, n (%) | 8 (17) | 0 (0) |
| Cesarean section, n (%) | 29 (62) | 2 (40) |
| Twins, n (%) | 4 (8.5) | 0 |
Mean ± SD.
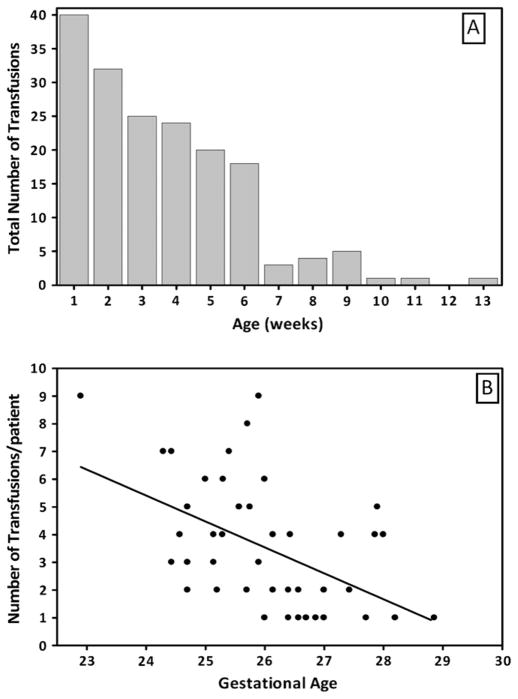
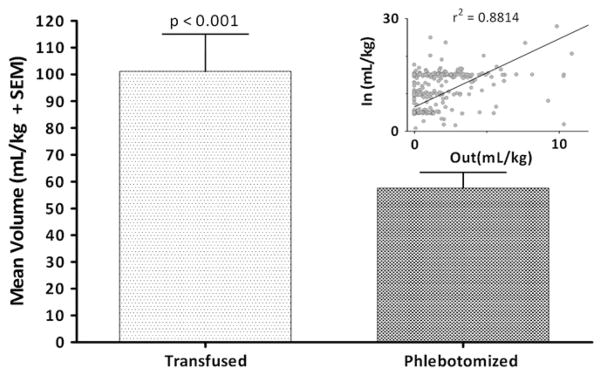
Figure 1 (available at www.jpeds.com) shows the timing of transfusions relative to birth in our population as a whole and the inverse relationship between gestational age and transfusion volume. The number and volume of transfusions increased as both BW and GA decreased (P < .05). The volume of PRBCs transfused was almost double the phlebotomized volume (P < .0001). The significance in the difference of volumes remained true when analysis was limited to the first 4 weeks of life (P < .001; Figure 2)
Figure 1.
Distribution of PRBC transfusions by weeks of life and corrected GA. The histogram shows that PRBC transfusions are inversely correlated with GA and occur most frequently in the first 6 weeks of life. A, The Y axis shows the total number of transfusions; the X axis shows the week of life. B, transfusions per patient (Y axis) are shown as a function of gestational age (X axis).
Figure 2.
Volume of PRBCs transfused and phlebotomized. The bar graph shows the mean (+ SEM) values for transfused and phlebotomized blood volumes per kg of birth weight. The scatter-plot compares the volumes and the Goodness of Fit value and regression line. Volume of blood transfused per kg of infant weight is 2 times higher than volume of blood phlebotomized (Non-parametric sign test, P < .0001).
Short-term Effects of Transfusions
The mean (within-person) changes in weight per day and percent weight gain for the post-transfusion period versus the pre-transfusion period were −8.8 g and −0.7%, respectively, and were not statistically significant from 0. For the average weight gain per day analysis, 15 of the 60 ELBW patients either did not survive or were transferred from the NICU before the 14th DOL, and 17 ELBW patients did not survive or were transferred from the NICU before the 28th DOL. The mean weight gain per day for the first 14 days of life was 7.3 and 8.0 g/kg for the transfused and non-transfused groups respectively (P = not significant [NS]). At the 28th DOL, weight gain increased to 11.5 and 12.5 g/kg per day in each group (P = NS). Thus, weight gain was not affected by transfusion.
PRBC transfusion was not associated with a change in heart rate (160 ± 1 beats per minute pre-transfusion, and 159 ± 1 post-transfusion; P = NS). The heart rate also did not change with the volume transfused. Excluding transfusion events for which the pre- and post-transfusion periods overlapped did not change the significance of our findings.
PRBC transfusion was associated with a decrease of 0.9 episodes of apnea per day (P < .05). Moderate and severe episodes were decreased by 0.3 events per day, also showing a significant decline in the post-transfusion period (P < .05). The change in apneic episodes per day from pre- to post- transfusion for every 10 mL/kg of transfusion volume was not significant. The results were not different when the data were filtered to exclude all infants on mechanical ventilation.
Before PRBC transfusion (n = 179 events total), 7 infants were in room air, 21 infants required nasal cannula oxygen, 33 infants required CPAP, 107 infants required conventional mechanical ventilation, and 6 infants required high frequency oscillation. On average, FiO2 increased 0.025 from baseline in the post-transfusion period (P < .05). Of the infants in room air, nasal cannula, or CPAP before transfusion, 30 required increasing support after transfusion (eg, nasal cannula advances to CPAP), 22 stayed the same, and 7 required less support. For infants already receiving mechanical ventilation, 2 were transitioned from conventional to high frequency oscillation, 89 remained on the same mode of support, and 18 had a decrease in their level of support. Excluding any transfusion events for which the pre- and post-periods overlapped did not change any of the conclusions. Thus, ventilatory support modes did not change significantly after transfusion, although the percent of patients requiring mechanical ventilation and oxygen supplementation increased after PRBC transfusions.
Long-term Effects of Transfusions
Forty-three infants were examined for BPD at 28 days of age, and 39 infants were examined at 36 weeks corrected GA (Table III). The incidence of BPD at 28 days of age correlated with the number of transfusions received (P < .01). When reassessed at 36 weeks corrected GA, no association remained between the number or volume of transfusions and BPD. Of the 47 infants who underwent transfusion, 25 (53%) received diuretics during the course of their stay. Diuretic use was correlated with both the number of PRBC transfusions alone and combined transfusion of all blood products (P < .01). Twenty-three of the 25 patients receiving diuretics met the criteria for BPD at DOL 28; for the patients reassessed for BPD at 36 weeks corrected GA, 12 of the 13 received diuretics.
Table III.
Morbidities and their association with transfusions.
| Non-transfused incidence | Transfused incidence | P value | |
|---|---|---|---|
| BPD (DOL 28) | 3/5 | 36/43 | <.01 |
| BPD (36 week corrected GA) | 3/3 | 17/39 | NS |
| Sepsis | 1/5 | 14/47 | NS |
| NEC | 0 | 8/47 | <.05 |
| ROP | 0 | 26/38 | NS |
| ROP grade and transfusion | 0 | 26/38 | <.01 |
There was no association between HCT level at birth and severity of IVH or between IVH severity and HCT level immediately before the first transfusion (Figure 3; available at www.jpeds.com). This analysis excluded infants who were known to have IVH before receiving their first transfusion.
Figure 3.
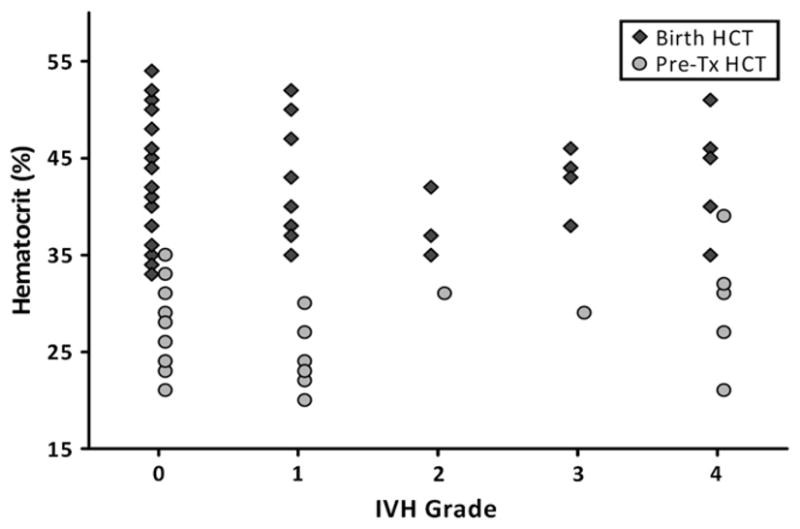
Relationship of HCT to IVH in the first week of life. The graph shows both the birth HCT level (filled diamonds) and pre-transfusion HCT level (filled circles) as a function of IVH. For pre-transfusion HCT level, only the first transfusion was considered. No relationship existed between intracranial bleeding and HCT.
Fifteen infants had sepsis during their stay; of those, 14 underwent transfusion. Ten of the 14 infections occurred within 2 weeks after a PRBC transfusion. There was no significant association between receiving a transfusion and risk of sepsis.
In the transfused population, NEC of stage 2A or greater developed in 8 patients; all cases occurred within 7 days of a PRBC transfusion. NEC did not develop in any of the patients who didn’t undergo transfusion. The relative risk of NEC developing after a transfusion was statistically significant (P < .05). One case (stage 3) occurred on DOL 3, 48 hours after a transfusion. Five cases occurred between DOL 21 and 29, within 7 days of a PRBC transfusion, and 2 cases occurred at DOL 36 and 40. Symptoms of NEC in these last 2 infants began 0 to 1 day after the transfusion.
Of the 38 ELBW infants examined, ROP developed in 26; 9 of those had grade II ROP or higher. There was no association between the number or volume of transfusions and the incidence of ROP, but a significant association was seen with the severity of the disease (P < .05). There was no ROP in the non-transfused population.
Early rEpo was given to 22 infants on DOL 1, 2, and 3 as part of an unrelated study,28 and it was dosed at either 500, 1000, or 2500 U/kg for 3 daily doses. Of these infants, 10 also received rEpo later for the treatment of anemia (either 200 U/kg IV of rEpo daily, or 400 U/kg/dose given Monday, Wednesday, and Friday for 2 weeks minimum). ROP developed in 7 of 10 patients who received combined early and late rEpo (3 had stage 2 or higher ROP). RPO developed in 6 of 12 infants who received early rEpo alone or early rEpo plus <2 weeks of late rEpo (2 had stage 2 or higher ROP). Eight infants were treated with late rEpo for >2 weeks, and ROP developed in 6 of these infants (1 had stage 2 or higher ROP). Two infants were treated with a 1-week regimen of rEpo at Epo dosing; ROP developed in 1(stage 2). ROP developed in 6 of the 15 infants who did not receive any rEpo . There was no correlation between rEpo treatment (either time frame) and ROP, nor with the number or volume of PRBC transfusions.
Discussion
We evaluated possible benefits and adverse effects of transfusions in ELBW infants with somewhat liberal guidelines modified from Shannon et al.26 Theoretically, the primary goal of transfusion is to correct anemia and thereby improve oxygen transport and cardiorespiratory status (oxygen dependency, apnea), with the hope of improving long-term neurodevelopmental outcome. There is, however, scant evidence that transfusions in ELBW infants with current guidelines actually leads to clinical improvement. To answer this question, we analyzed the effects of transfusion on weight gain, apnea, respiratory support, and heart rate. We examined possible associations between transfusions and common problems of prematurity, including BPD, IVH, ROP, NEC, and mortality. The effects of rEpo on transfusion needs and ROP were also analyzed.
Few studies have examined the relationship between transfusions and weight gain, and these showed conflicting results.12,13 In our study, the average daily weight gain was not significantly different between the pre- and post-transfusion periods. There was also no difference in the mean weight gain per day between the transfused and non-transfused groups.
Cardiorespiratory signs such as tachycardia, bradycardia, and apnea can be caused by anemia and may therefore improve after transfusion of PRBCs.6–10,29 However, this relationship may only be true at very low circulating hemoglobin levels.11 Reports of the effects of transfusion on cardiorespiratory status in preterm infants have not been consistent, with some studies demonstrating improvement after transfusion,7 and others showing no such benefit.10 Our study did not demonstrate any clinical improvement in cardiorespiratory status after PRBC transfusions, with the exception of a small decrease in moderate and severe apneic episodes. These results are unlikely to be clinically significant, because the decrease in episodes was 0.3 events per day. We noted that acute oxygen supplementation increased after a transfusion in 29 of 47 patients (66 transfusions total). No respiratory deteriorations were noted after transfusions of fresh frozen plasma.
Transfusions have been implicated in the development or worsening of BPD. Possible mechanisms include increased oxidative injury (caused by an increase in non-transferrin bound iron) or inflammatory mediators present in stored blood products.18–20 We found the incidence of BPD to be significantly associated with the number of transfusions at DOL 28, but this relationship disappeared by 36 weeks corrected GA.
Some studies suggest that maintaining a higher HCT level in the first week of life might protect preterminfants from intraventricular hemorrhage (IVH).30,31 Our study showed no association between either HCT at birth and IVH or HCT level before first transfusion and IVH (Figure 3). Recently, 2 prospective randomized trials were published in which the impact of transfusions on IVH was discussed, although neither had this as a primary outcome variable. In a single center study of 103 infants randomized to liberal versus restrictive transfusion guidelines, Bell et al found no difference in IVH rate or IVH grades III and IV combined in the restrictive transfusion group, but did note a trend for more infants with grade IV IVH in this group (4 versus 0, P = .054). When infants with grade IV IVH or pericentricular leukomalacia were combined, this severe brain injury was more likely to occur in the restrictive group (6 versus 0, P = .012).2 Limitations of this study’s ability to evaluate the association between transfusion guidelines and brain injury include that patients were not enrolled within the first DOL, and cranial ultrasound scanning was not conducted before study entry, so it is not known whether this finding was incidental or related to transfusion. The larger (n = 451), multicenter study titled “Premature Infants in Need of Transfusion” showed no association between IVH and transfusion guidelines used.3 Because this remains an unresolved issue, and intracranial bleeding is most common in the first week of life, our revised transfusion guidelines (Table I) have taken into account the possibility that a higher HCT level in the first week of life may decrease the risk of IVH.
We found no association between transfusion and incidence of sepsis. Although most sepsis events (71%) occurred within 2 weeks of a transfusion, the relationship was not significant. Studies in adult populations have shown a relationship between transfusions and immune function32; however, this is another area in which few studies have been done in preterm infants.
NEC was associated with PRBC transfusion in our population. Although NEC was diagnosed in only 8 patients in our study, all cases occurred within 7 days after a transfusion. Only a few studies have been conducted to evaluate this relationship. McGrady et al found an association between PRBC transfusions and NEC; however, there was no analysis of temporal association.23 Mally et al evaluated the association of late-onset NEC and blood transfusions in stable premature infants and found a significant relationship.22
We found no significant association between volume or number of transfusions and the incidence of ROP, but there was a correlation with the severity of the disease. The possible association with rEpo has been controversial and may depend on the timing of treatment. Suk et al have shown that late rEpo may be associated with increased risk of ROP,33 and Ohlsson et al have published the opposite.34 In our population, neither early nor late rEpo was associated with incidence or severity of ROP, although this study was not adequately powered to determine an association. Fortes Filho et al reported that ROP was associated with blood transfusions, although the study only concluded that transfusions were a risk factor with lower BW, GA, and rEpo treatment.25 However, transfusions were identified as independent risk factors for ROP by several other studies, even when the data was adjusted for the aforementioned factors.21,24
In adult medicine, there is an increasing awareness of the acute and long-term harmful effects of blood transfusions. 14,16,17 There is also increasing appreciation giving transfusions to patients who are anemic does not necessarily achieve the goal of improving outcomes. In our patient population, the most common causes of anemia are phlebotomy loss and anemia of prematurity.35 Reducing phlebotomy losses can be achieved by using bedside or in-line analyzers, implementing discriminating blood drawing schedules, and decreasing the use of umbilical catheters.35–37 Use of supplemental iron, with or without rEpo, and the implementation of more stringent transfusion guidelines also decrease transfusion needs.38–41
Because this was a retrospective study, certain limitations exist. For instance, transfusion guidelines may not always have been followed. Apneic episodes were recorded by the nursing staff and may not be completely accurate. Furthermore, oxygen dependence at 28 days and 36 weeks corrected GA was not verified with physiologic testing. Another limitation is patients were not followed long-term, so other complications of transfusion or prematurity may have been missed. Because of the number of limitations a retrospective study poses, we believe that a randomized prospective study would be needed to further evaluate the effects of transfusions in the ELBW infant population.
In our study of all ELBW infants treated at UWNICU during 2006, we did not identify any clinical benefits of transfusing PRBCs with the guidelines in place at the time. Contrary to clinical expectations, a significant subset of babies required increased respiratory support after transfusions. Of some concern, we also identified associations between transfusions and common problems associated with preterm birth, such as NEC and BPD. Diuretic use was also more common in infants who underwent transfusion. As a result of this study, the transfusion guidelines at the UWMC NICU were made more restrictive.
Acknowledgments
This study was made possible by a grant from the University of Washington Department of Pediatrics Academic Enrichment Fund and a grant (number 1 UL1 RR025014-01) from the National Center for Research Resources, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health.
- BPD
Bronchopulmonary dysplasia
- BW
Birth weight
- CPAP
Continuous positive airway pressure
- DOL
Day of life
- ELBW
Extremely low birth weight
- Epo/rEpo
Erythropoietin/recombinant erythropoietin
- GA
Gestational age
- HCT
Hematocrit
- IVH
Intraventricular hemorrhage
- NEC
Necrotizing enterocolitis
- NICU
Neonatal intensive care unit
- NS
Not significant
- PRBC
Packed red blood cell
- ROP
Retinopathy of prematurity
- UWMC
University of Washington Medical Center
Footnotes
The authors declare no conflicts of interest.
References
- 1.Brown MS, Berman ER, Luckey D. Prediction of the need for transfusion during anemia of prematurity. J Pediatr. 1990;116:773–8. doi: 10.1016/s0022-3476(05)82670-8. [DOI] [PubMed] [Google Scholar]
- 2.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Ross MP, Christensen RD, Rothstein G, Koenig JM, Simmons MA, Noble NA, et al. A randomized trial to develop criteria for administering erythrocyte transfusions to anemic preterm infants 1 to 3 months of age. J Perinatol. 1989;9:246–53. [PubMed] [Google Scholar]
- 5.Bifano EM, Curran TR. Minimizing donor blood exposure in the neonatal intensive care unit. Current trends and future prospects. Clin Perinatol. 1995;22:657–69. [PubMed] [Google Scholar]
- 6.DeMaio JG, Harris MC, Deuber C, Spitzer AR. Effect of blood transfusion on apnea frequency in growing premature infants. J Pediatr. 1989;114:1039–41. doi: 10.1016/s0022-3476(89)80459-7. [DOI] [PubMed] [Google Scholar]
- 7.Joshi A, Gerhardt T, Shandloff P, Bancalari E. Blood transfusion effect on the respiratory pattern of preterm infants. Pediatrics. 1987;80:79–84. [PubMed] [Google Scholar]
- 8.Stute H, Greiner B, Linderkamp O. Effect of blood transfusion on cardiorespiratory abnormalities in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:F194–6. doi: 10.1136/fn.72.3.f194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westkamp E, Soditt V, Adrian S, Bohnhorst B, Groneck P, Poets CF. Blood transfusion in anemic infants with apnea of prematurity. Biol Neonate. 2002;82:228–32. doi: 10.1159/000065891. [DOI] [PubMed] [Google Scholar]
- 10.Keyes WG, Donohue PK, Spivak JL, Jones MD, Jr, Oski FA. Assessing the need for transfusion of premature infants and role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 1989;84:412–7. [PubMed] [Google Scholar]
- 11.Alkalay AL, Galvis S, Ferry DA, Simmons CF, Krueger RC., Jr Hemodynamic changes in anemic premature infants: are we allowing the hematocrits to fall too low? Pediatrics. 2003;112:838–45. doi: 10.1542/peds.112.4.838. [DOI] [PubMed] [Google Scholar]
- 12.Keller A, Hermanussen M, Vogtmann C, Kiess W, Keller E. Effect of erythrocyte transfusion on longitudinal bone growth of premature infants assessed by mini-knemometry. Eur J Pediatr. 1999;158:871–2. doi: 10.1007/s004310051230. [DOI] [PubMed] [Google Scholar]
- 13.Stockman JA, III, Clark DA. Weight gain: a response to transfusion in selected preterm infants. Am J Dis Child. 1984;138:828–30. doi: 10.1001/archpedi.1984.02140470028009. [DOI] [PubMed] [Google Scholar]
- 14.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–10. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 15.Hebert PC, Wells G, Tweeddale M, Martin C, Marshall J, Pham B, et al. Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. Am J Respiratory Crit Care Med. 1997;155:1618–23. doi: 10.1164/ajrccm.155.5.9154866. [DOI] [PubMed] [Google Scholar]
- 16.Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34:243–4. doi: 10.1016/j.transci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. 2006;66:355–64. doi: 10.1016/j.mehy.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr. 1997;156:47–50. doi: 10.1007/s004310050551. [DOI] [PubMed] [Google Scholar]
- 20.Hirano K, Morinobu T, Kim H, Hiroi M, Ban R, Ogawa S, et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:F188–93. doi: 10.1136/fn.84.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooke RW, Clark D, Hickey-Dwyer M, Weindling AM. The apparent role of blood transfusions in the development of retinopathy of prematurity. Eur J Pediatr. 1993;152:833–6. doi: 10.1007/BF02073381. [DOI] [PubMed] [Google Scholar]
- 22.Mally P, Golombek SG, Mishra R, Nigam S, Mohandas K, Depalhma H, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. 2006;23:451–8. doi: 10.1055/s-2006-951300. [DOI] [PubMed] [Google Scholar]
- 23.McGrady GA, Rettig PJ, Istre GR, Jason JM, Holman RC, Evatt BL. An outbreak of necrotizing enterocolitis. Association with transfusions of packed red blood cells. Am J Epidemiol. 1987;126:1165–72. doi: 10.1093/oxfordjournals.aje.a114754. [DOI] [PubMed] [Google Scholar]
- 24.Hesse L, Eberl W, Schlaud M, Poets CF. Blood transfusion. Iron load and retinopathy of prematurity. Eur J Pediatr. 1997;156:465–70. doi: 10.1007/s004310050641. [DOI] [PubMed] [Google Scholar]
- 25.Fortes Filho JB, Eckert GU, Procianoy L, Barros CK, Procianoy RS. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye. 2009;23:2530. doi: 10.1038/sj.eye.6702924. [DOI] [PubMed] [Google Scholar]
- 26.Shannon KM, Keith JF, III, Mentzer WC, Ehrenkranz RA, Brown MS, Widness JA, et al. Recombinant human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very low birth weight preterm infants. Pediatrics. 1995;95:1–8. [PubMed] [Google Scholar]
- 27.Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 28.Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122:383–91. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- 29.Bifano EM, Smith F, Borer J. Relationship between determinants of oxygen delivery and respiratory abnormalities in preterm infants with anemia. J Pediatr. 1992;120:292–6. doi: 10.1016/s0022-3476(05)80447-0. [DOI] [PubMed] [Google Scholar]
- 30.Linder N, Haskin O, Levit O, Klinger G, Prince T, Naor N, et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics. 2003;111:e590–5. doi: 10.1542/peds.111.5.e590. [DOI] [PubMed] [Google Scholar]
- 31.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117:1235–42. doi: 10.1542/peds.2005-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reading FC, Brecher ME. Transfusion-related bacterial sepsis. Curr Opin Hematol. 2001;8:380–6. doi: 10.1097/00062752-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Suk KK, Dunbar JA, Liu A, Daher NS, Leng CK, Leng JK, et al. Human recombinant erythropoietin and the incidence of retinopathy of prematurity: a multiple regression model. J Aapos. 2008;12:233–8. doi: 10.1016/j.jaapos.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299–306. doi: 10.1542/peds.2004-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol. 2005;25:21–5. doi: 10.1038/sj.jp.7211201. [DOI] [PubMed] [Google Scholar]
- 37.Lin JC, Strauss RG, Kulhavy JC, Johnson KJ, Zimmerman MB, Cress GA, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 2000;106:E19. doi: 10.1542/peds.106.2.e19. [DOI] [PubMed] [Google Scholar]
- 38.Badiee Z, Pourmirzaiee MA, Kelishadi R, Naseri F. Recombinant human erythropoietin and blood transfusion in low-birth weight preterm infants under restrictive transfusion guidelines. Saudi Med J. 2006;27:817–20. [PubMed] [Google Scholar]
- 39.Ohls RK, Harcum J, Schibler KR, Christensen RD. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double-blind, placebo-controlled study. J Pediatr. 1997;131:661–5. doi: 10.1016/s0022-3476(97)70089-1. [DOI] [PubMed] [Google Scholar]
- 40.Franz AR, Pohlandt F. Red blood cell transfusions in very and extremely low birthweight infants under restrictive transfusion guidelines: is exogenous erythropoietin necessary? Arch Dis Child Fetal Neonatal Ed. 2001;84:F96–100. doi: 10.1136/fn.84.2.F96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier RF, Obladen M, Muller-Hansen I, Kattner E, Merz U, Arlettaz R, et al. Early treatment with erythropoietin beta ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr. 2002;141:8–15. doi: 10.1067/mpd.2002.124309. [DOI] [PubMed] [Google Scholar]