Abstract
Objective
To test the hypothesis that disturbances in plasma melatonin distinguish pregnant and postpartum patients with major depression (DP) from matched healthy comparison (HC) women.
Method
In 25 pregnant (15 HC, 10 DP) and 24 postpartum (11 HC, 13 DP) women, we measured plasma melatonin every 30 minutes from 18:00 -11:00 hours (h) in dim (< 30 lux) light. The values were log-transformed and calculations made for baseline and synthesis onset and offset times, duration, peak concentration and area under the curve (AUC). Groups were compared by analyses of covariance using age, weeks pregnant or postpartum, breastfeeding status and body mass index (BMI) as covariates.
Results
Morning melatonin levels were significantly lower in pregnant DP from 02:00 – 11:00 h, but were significantly higher in postpartum DP across time intervals, relative to matched HC women. Pregnant (but not postpartum) women with a personal or family history of depression, regardless of current diagnosis, had significantly earlier melatonin synthesis and baseline offsets than those without such a history. In pregnant HC, but not in DP, melatonin levels increased during the course of pregnancy. No such relationship existed for postpartum HC or DP.
Conclusions
Plasma nocturnal melatonin concentrations, especially in the morning hours, were lower in depressed pregnant, but elevated in depressed postpartum women, compared with HC women. Melatonin timing measures were advanced in pregnant women with a personal or family history of depression. These findings implicate disturbances in the regulation of the melatonin generating system in pregnancy and postpartum depression.
INTRODUCTION
Major depression during pregnancy or postpartum can be a devastating illness to the mother, impair the neurocognitive and socio-emotional development of the child and increase the risks for mental and medical disorders in offspring later in life (1-5). The causes are unknown. Circadian rhythm dysregulation characterizes patients with mood disorders (6). Whether chronobiological disturbances also characterize patients with pregnancy and postpartum depressive disorders has not been investigated systematically. As estrogen and progesterone are among the few known endogenous modulators of circadian rhythm amplitude and phase (7), it is conceivable that the marked changes in gonadal steroids during pregnancy and postpartum may alter these biological rhythms, predisposing vulnerable women to develop mood disorders.
The best measure of circadian rhythmicity in humans is plasma melatonin (8). The hypothalamic circadian pacemaker regulates the secretion of melatonin, which is synthesized from serotonin in the pineal gland under the rate-limiting influence of norepinephrine. Melatonin generally is not secreted during the day, increases about two hours before sleep onset, and declines in the early morning hours. As even relatively dim light (200 lux) can suppress melatonin, it must be measured under controlled light conditions. Melatonin serves as an important regulator of other circadian rhythms, and in seasonally breeding animals, regulates cycles of reproduction and hibernation.
Melatonin circadian rhythms are of lower amplitude in some, but not all, depressed patients (9, 10). We observed blunted and phase-advanced (shifted earlier) melatonin rhythms in women with premenstrual dysphoric disorder (PMDD)(11, 12), but higher melatonin levels in women with menopausal depression (13). Melatonin studies of pregnant or postpartum women are few and do not include DP (cf. Parry et al. (14)).
The aim of the present study was to test the hypothesis that disturbances in melatonin circadian rhythms distinguish women with major depression from healthy women during pregnancy and postpartum. Based on our earlier work (13), we expected to find associations between altered melatonin rhythms and measures of depressed mood.
Subjects and Methods
Subjects
Details of subject recruitment procedures are described elsewhere (15). In brief, we telephone screened 20-45 year-old San Diego residents who were pregnant (up to 34 weeks, estimated) or postpartum (up to one year). To be eligible, women had to not smoke, not use hormonal contraceptives if postpartum and not use medications, herbs or over-the-counter preparations that would interfere with neuroendocrine measures. Participants agreed to multiple overnight hospital stays in the General Clinical Research Center (GCRC), where they were allowed to bring their child in with them if needed. Subjects had laboratory tests for a chemistry panel, thyroid indices, complete blood count, urinalysis and urine toxicology screens. They were without significant medical illness, and off medication that would interfere with the melatonin measures. DP were off antidepressant medication ≥ two weeks (four weeks for fluoxetine) before study. Patients with bipolar or primary anxiety disorders were excluded. DP and HC subjects were without alcohol abuse within the last year.
To establish DSM-IV (16) entrance and baseline criteria, trained clinicians gave each participant a structured psychiatric interview, a Structured Clinical Interview for DSM-IV (SCID) (17) and at least two baseline evaluation ratings scheduled one week apart using the 21-item Hamilton Depression Rating Scale (HDRS) (18) that included an atypical depressive symptom inventory (19), the Beck Depression Inventory (BDI) (20), and the Edinburgh Postnatal Depression Scale (EPDS) (21), also validated for use during pregnancy (22).
For study inclusion, DP had mean scores on the HDRS ≥ 14; BDI and EPDS ≥ 10, for two weeks. Postpartum DP had onset of a major depressive episode (MDE) within 3 months postpartum. HC subjects had mean HDRS ratings ≤ 8, and BDI ratings ≤ 5.
Methods
Subjects meeting entrance criteria were admitted to the UCSD GCRC at 16:00 h. After a night of adaptation to the sleep room, licensed nurses inserted an intravenous catheter at 17:00 h and drew blood (3cc) every 30 minutes from 18:00-11:00 h for measurement of plasma melatonin. Serum for estradiol and progesterone was obtained at 18:00 and 06:00 h. Subjects remained at bed rest in a single room with double doors and heavy drapery over the windows to block extraneous light from 16:00-11:00 h. Light panels kept daytime light exposure relatively dim (< 30 lux). We considered this light intensity too dim to substantially suppress melatonin in undilated pupils, disrupt sleep or shift circadian rhythms, yet not so dim that it might serve as a dark pulse (23). Subjects slept in the dark with an eye mask. Nurses or sleep technicians entered the room only when necessary (recorded by infrared camera), using a pen-size dim red flashlight. During sleep times, GCRC nurses threaded the intravenous catheter through a porthole in the wall and drew samples from an adjoining room to minimize sleep disturbances.
The University of California, San Diego Institutional Review Board, approved the protocol. All subjects gave written informed consent after the procedures had been explained fully.
Assays
Blood samples for melatonin were placed in ethylenediaminetetracetic acid-containing plastic tubes, centrifuged, frozen immediately, and stored at −70° C until assayed. All samples for the same subject were run in the same assay. Initial assays for melatonin, estradiol and progesterone were described in previously published manuscripts (24, 25). We assayed plasma melatonin concentrations of the first 44 subjects by radioimmunoassay (RIA) with kits manufactured by IBL Immuno-Biological Laboratories, Hamburg, Germany. As the manufacturer changed this kit, plasma for the last five subjects (3 pregnant, 2 postpartum) was assayed with Direct Melatonin RIA kits manufactured by Bühlmann Laboratories (ALPCO Diagnostics, Windham NH). This widely used RIA kit uses calibrators ranging from 1 - 81 pg/ml and reports intra- and inter-assay CVs of 6.7 % and 10.4 %, respectively. The standard range is from 1.0 - 81 pg/ml, with an analytical sensitivity of 0.8 pg/ml. For melatonin statistical analyses, assay type (IBL vs. Bühlmann) was included as a covariate to correct for differences between assays.
Analyses
From the melatonin secretory profile, we estimated measures of timing (onset time, synthesis and baseline offset time, duration) and quantity (peak concentration and area under the curve, AUC) by previously described methods (13).
Along with attempting to match DP with HC subjects on weeks pregnant and weeks postpartum, we included Weeks Pregnant/Postpartum as a covariate in analyses, to control statistically for differences in that parameter.
For the initial repeated measures (e.g., Time x Diagnosis x Pregnant/Postpartum) analyses, a three-factor “mixed” analysis of variance (ANOVA) was used with the Geisser-Greenhouse correction for sphericity of repeated measures. Follow-up analyses were done on pregnant and postpartum women, separately, with two-factor (Time x Diagnosis) analyses. Plasma melatonin timing and quantitative measures were analyzed with multivariate analyses of covariance (MANCOVA), followed by univariate ANCOVA on individual variables of interest. For pregnant subjects, weeks pregnant and assay method (IBL vs. Bühlmann) were applied as covariates; for postpartum subjects, weeks postpartum, Log BMI and breastfeeding status were applied as covariates.
RESULTS
Subject Characteristics
Of women meeting screening criteria, one pregnant subject was dropped as an outlier because her delayed melatonin onset (24:30 h) and advanced baseline offset (05:30 h) differed from the group mean by more than three standard deviations. We studied the remaining 25 pregnant women (15 HC and 10 DP): 17 Caucasian, 3 Hispanic, 3 African American, 1 Asian and 1 Multiethnic. Two pregnant HC reported a family history of depression; two others had no family history, but reported one previous MDE, each; five of the 10 DP reported a previous history of MDE, from 1- 6 previous episodes (mean = 1.1 ± 1.9). Based on retrospective reports, depression onset occurred during pregnancy (1-36 weeks) for all 10 subjects (mean = 12.1 ± 3.8 weeks). The current episode ranged in duration from 3 to 28 weeks (mean = 13.6 ± 3.4). We dropped two postpartum women from study -- one whose sleep onset time was later (01:58 h vs. 11:34 h), and a second whose melatonin offset time was earlier than the group mean (06:00 h vs. 09:56 h) by more than three standard deviations. We analyzed the data of the remaining 24 postpartum women (11 HC and 13 DP) who met screening criteria: 11 Caucasian, 8 Hispanic, 3 Asian and 2 Multiethnic. Two HC women reported one prior MDE, each, as well as family histories of depression; a third subject reported only a family history of depression. Ten of 13 DP reported a prior history of MDE, from 1 - 8 previous episodes (mean = 2.0 ± 2.2). Based on retrospective reports, MDE onset occurred during pregnancy (4 - 32 weeks) for six women (mean = 18.2 ± 4.7 weeks); and postpartum (1 – 22, mean = 5.7± 3.0 weeks) for seven DP. The current episode ranged in duration from 3 to 40 weeks (mean = 17.0 ± 3.9 weeks). See Table 1 for data on age, parity, breast feeding status, personal and family history of depression(s), and mood ratings.
Table 1.
Demographics, Mood and Depression Histories in Pregnant and Postpartum Women: Healthy Comparison (HC) women vs. Depressed Patients (DP)
| DEMOGRAPHICS | MOOD | HISTORY OF DEPRESSION | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GROUP | AGE | PARITY | BREAST- FEEDING |
HAMILTON | BECK | EDINBURGH | FAMILY | PERSONAL | POSTPARTUM | NON- POSTPARTUM |
| (N) | MEAN (±SD) |
MEAN (±SD) |
(N/total) | MEAN (±SD) |
MEAN (±SD) |
MEAN (±SD) |
(N/total) | (N/total) | (N/total) | (N/total) |
| PREGNANT | ||||||||||
| HC | 24.9 | 0.8 | N/A | 5.58 | 3.03 | 6.45 | 13.30% | 13.30% | 6.70% | 6.70% |
| (N=15) | (± 4.8) | (± 0.7) | N/A | (± 3.47 ) | (± 1.99) | (± 2.20) | (2/15)a | (2/15)a | (1/15) | (1/15) |
| DP | 27.1 | 1.7 | N/A | 17.04 | 8.61 | 14.01 | 30.00% | 50.00% | 20.00% | 30.00% |
| (N=10) | (± 5.2) | (± 1.1) | N/A | (± 4.40 ) | (± 3.49) | (±9.61 ) | (3/10) | (5/10) | (2/10) | (3/10) |
| P* | 0.282 | 0.016 | - | 0.000 | 0.000 | 0.000 | 0.307 | 0.045 | 0.315 | 0.119 |
| POSTPARTUM | ||||||||||
| HC | 28.5 | 1.7 | 81.80% | 3.71 | 3.11 | 3.18 | 27.30% | 18.20% | 9.10% | 9.10% |
| (N=11) | (± 6.5) | (± 0.9) | (9/11) | (±2.04) | (±2.26) | (±2.49) | (3/11)b | (2/11)b | (1/11) | (1/11) |
| DP | 28.3 | 2 | 38.50% | 18.72 | 20.21 | 11.25 | 53.80% | 76.90% | 53.80% | 53.80% |
| (N=13) | (± 6.7) | (± 1.1) | (5/13) | (±7.65) | (±8.53) | (±3.37) | (7/13) | (10/13) | (7/13) | (7/13) |
| P* | 0.957 | 0.508 | 0.032 | 0.000 | 0.000 | 0.000 | 0.188 | 0.004 | 0.020 | 0.020 |
P-values refer to significance of differences between HC and DP groups.
Two pregnant HC subjects with a Family History of depression had no Personal History; two with a Personal History had no Family History.
Two of 3 postpartum HC subjects had both Personal and Family Histories of depression; one had a Family History but no Personal History.
Plasma Melatonin
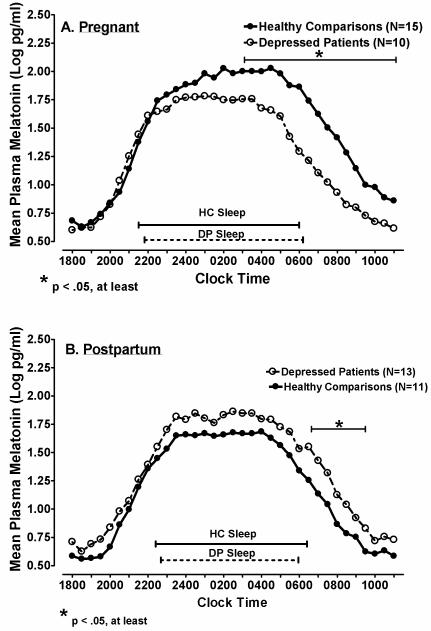
A repeated measures ANOVA on log-transformed melatonin concentrations across 35 successive half-hour intervals yielded a highly significant Time x Diagnosis X Pregnant/Postpartum Status interaction (F=11.8, df=1, 44, p = .001). Subsequent ANCOVA performed in pregnant subjects, with weeks pregnant and assay type (IBL vs. Bühlmann) as covariates, showed a significant Diagnosis effect (F=8.02, df=1, 21, p = .010) as well as a Time x Diagnosis interaction (F=3.19, df=34, 714, p = .028). Analysis of the interaction showed DP melatonin concentrations were lower than HC from 02:00 – 11:00 h (all p < .05, see Fig. 1 A). In contrast, in postpartum women, melatonin levels were significantly higher in DP than HC women, across time points (F=6.13, df=1, 20, p = .022; see Fig. 1 B), when weeks postpartum and breastfeeding status were applied as covariates. Although the Time x Diagnosis interaction was not significant (p = .727), because pregnant DP vs. HC showed morning melatonin differences, we calculated individual paired comparisons in postpartum women, which showed the most reliable differences in melatonin secretion between DP and HC women occurred from 06:30-09:30 h (all p < .05).
Figure 1.
Mean plasma melatonin concentrations at successive time points in Healthy Comparison (HC; closed circles) women and Depressed Patients (DP; open circles) in (A) Pregnant and (B) Postpartum women.
Baseline and Synthesis Melatonin Measures
In pregnant women, the omnibus MANCOVA was significant for Diagnosis (p = .037), with weeks pregnant and melatonin assay type as covariates. Univariate ANCOVA showed AUC (F=7.66, df=1, 21, p = .012) and Synthesis AUC (F=6.27, df=1, 21, p = .021) were lower in DP vs. HC (see Table 2). Melatonin onset, synthesis offset and baseline offset did not differ between groups (all p > .05). In postpartum women, the omnibus MANCOVA was marginally significant for diagnosis (p = .055) for the quantitative melatonin measures, when breastfeeding status and log BMI were included as covariates. In contrast to pregnant subjects, univariate ANCOVA showed AUC (F=9.02, df=1, 20, p = .007), Synthesis AUC (F=8.17, df=1, 20, p = .010) and log Peak melatonin concentration (F=7.89, df=1, 20, p = .011) were greater in postpartum DP vs. HC (see Table 2). Melatonin onset, synthesis offset and baseline offset did not differ between groups (all p > .05).
Table 2.
Melatonin Timing and Quantitative Measures in Healthy Comparison (HC) women and Depressed Patients (DP)
| GROUP | Onset | Baseline Offset | Duration | AUC | Log Peak | SYN Offset | SYN Duration | SYN AUC |
|---|---|---|---|---|---|---|---|---|
| (N) | hh:mm/(± SD) | hh:mm/(± SD) | h/(± SD) | mean/(± SD) | mean/(± SD) | hh:mm/(± SD) | h/(± SD) | mean/(± SD) |
| PREGNANT | ||||||||
| HC (N=15 ) | 20:36 | 10:53 | 14.28 | 2223 | 2.12 | 5:42 | 9.10 | 1847 |
| (±1:01) | (±1:35) | (±1.5) | (±1267) | (±0.3) | (±0:54) | (±1.5) | (±1129) | |
| DP (N=10) | 19:54 | 10:09 | 14.27 | 1114* | 1.95 | 5:12 | 14.30 | 946* |
| (±1:04) | (±1:45) | (±2.1) | (±311) | (±2.1) | (±1:32) | (±1.5) | (±264) | |
| POSTPARTUM | ||||||||
| HC (N= 11) | 20:17 | 10:56 | 14.63 | 1004 | 1.81 | 5:33 | 9.25 | 879 |
| (±0:50) | (±0:57) | (±1.3) | (±491) | (±0.27) | (±0:33) | (±1.5) | (±469) | |
| DP (N= 13) | 20:41 | 10:16 | 13.57 | 1337** | 1.98* | 6:09 | 9.46 | 1169** |
| (±1:07) | (±1:45) | (±2.2) | (±469) | (±0.19) | (±1:01) | (±1.1) | (±402) | |
Asterisks denote significance of differences between HC and DP
p < .05
p < .01
We compared melatonin concentrations in pregnant HC and DP with their postpartum counterparts. ANCOVA showed pregnant HC were higher than postpartum HC in melatonin concentrations across time intervals (F=7.84, df=1,20, p = .011). In contrast, pregnant DP did not differ significantly from postpartum DP (F=.050, df=1,17, p = .826).
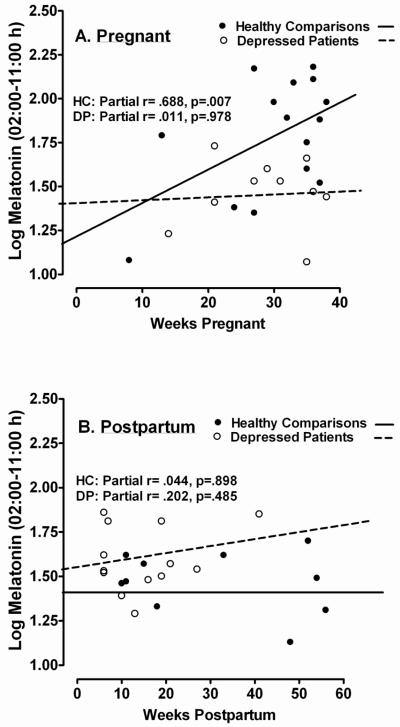
As Weeks Pregnant was a significant covariate (p < .05, at least) in some melatonin analyses, we determined the partial correlation between Weeks Pregnant and log-transformed melatonin concentrations from 02:00-11:00 h, controlling for age. For pregnant HC, the correlation was highly significant (r = .688, p = .007), but for pregnant DP, it was not (r = .011, p = .879; see Fig. 2A). Thus, melatonin increased significantly across weeks of pregnancy in HC, but not in DP. Similar postpartum analyses showed melatonin concentrations were unrelated to weeks postpartum in both HC (r = .044, p = .898) and DP groups (r = .202, p = .485; see Fig. 2B).
Figure 2.
Relationship of morning plasma melatonin concentrations (2:00 – 11:00 h) to (A) Weeks Pregnant and (B) Weeks Postpartum in Healthy Comparison (HC; closed circles) women and Depressed Patients (DP; open circles).
Correlations of Melatonin Measures with Mood and Depressive History
Overall, partial correlations between mood measures and melatonin measures (controlling for age and weeks pregnant or postpartum) were small and not significant in both pregnant (N = 25) and postpartum (N = 24) subjects. In contrast, a personal or family history of depression was positively correlated with HDRS score in both pregnant (r = .466, p = .025) and postpartum (r = .500, p = .018) subjects, as was number of prior episodes of depression in pregnant women (r = .543, p = .007). In pregnant, but not postpartum women, partial correlation (controlling for age and weeks pregnant) showed that number of prior episodes of depression was negatively correlated with melatonin synthesis offset time (r = −.677, p = .0004) and baseline offset time (r = −.629, p = .001) in DP + HC combined (N = 25). Thus, earlier synthesis and baseline offsets were associated with greater number of previous depressive episodes in pregnant subjects, in whom melatonin quantity measures were reduced in DP vs. HC.
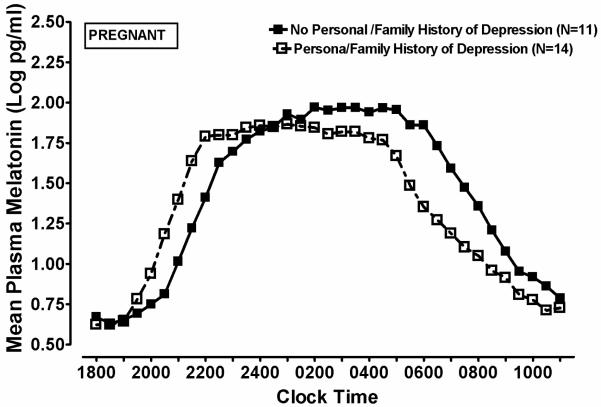
On melatonin timing measures, omnibus MANCOVA revealed a significant main effect of Personal or Family History of Depression (p = .023), but no significant main effects or interactions for Diagnosis, in pregnant subjects (HC + DP combined, n = 25), when weeks pregnant and melatonin assay type were included as covariates in the analysis. The univariate ANCOVA suggested that melatonin timing parameters were temporally advanced in history-positive, relative to history-negative subjects (See Figure 3). Pregnant women with a positive personal or family history of depression had, on average, marginally earlier melatonin onset times (19.8 vs. 20.7 h, F = 3.62, df=1, 19, p = .073), significantly earlier synthesis offset (4.8 vs. 6.4 h, F = 12.33, df=1, 19, p = .002) and baseline offset times (9.6 vs. 11.4 h, F = 9.17, df=1, 19, p = .007). In contrast, melatonin timing measures in postpartum women were not significantly affected (all p > .05) by personal or family history of depression. Thus, depression history affected melatonin timing measures in pregnant, but not in postpartum women.
Figure 3.
Mean plasma melatonin concentrations at successive time points in Pregnant women, with (closed squares) and without (open squares) a Personal or Family History of Depression.
Menstruation, breastfeeding status and presence of child in room on testing nights
None of the pregnant women brought children to the GCRC on test nights. Two of 11 postpartum HC and 2 of 13 postpartum DP reported resumption of menstruation prior to testing. One HC was studied in the follicular, and one in the luteal phase; likewise, one DP was studied in the follicular, and one in the luteal phase. Seven of 11 postpartum HC women brought and nursed children in the GCRC; two others pumped their breasts but did not nurse children on study nights. Five of 13 DP women brought and breast-fed children. Total sleep time, sleep latency, sleep efficiency, and wake after sleep onset, ascertained by polysomnography (PSG) recording, were available for all 25 of the pregnant and 23 of 24 postpartum women. Analyses of the PSG data showed no significant differences in sleep quality variables as a function of Breastfeeding Status, Child Presence in Room, or their interaction (all p > .05). Furthermore, Breastfeeding Status, Child Presence in Room, and their interaction, were without significant effect on morning melatonin secretion (all p > .05).
Reproductive Hormones
MANCOVA with weeks pregnant or postpartum (and breastfeeding status, postpartum) showed no significant main effect of Diagnosis, Personal or Family History of Depression, or their interaction, on log-transformed estradiol or progesterone in pregnant and postpartum subjects (all p > .05). Partial correlation, controlling for age, weeks pregnant or postpartum, and breastfeeding status, where appropriate, revealed no significant correlations between mood measures (HDRS, atypical ratings, BDI, EPDS) and reproductive hormones (estradiol, progesterone) in pregnant (N = 25) or postpartum (N = 24) women, when DP and HC were combined (all p > .05). Similar tests revealed no significant partial correlations between melatonin measures and reproductive hormones (all p > .05) in pregnant and postpartum women.
DISCUSSION
The main findings of this study were that, compared with matched HC women, pregnant DP had decreased, whereas postpartum DP had increased levels of nocturnal plasma melatonin, particularly in the early morning hours. We also found that pregnant women who had a depressive history had earlier melatonin offset times, and that melatonin secretion increased during pregnancy in HC, but not in DP.
The findings of decreased levels of plasma melatonin in pregnant DP parallel findings in women with Premenstrual Dysphoric Disorder (PMDD) (11, 12). In contrast, the increased levels of plasma melatonin in postpartum DP parallel the findings in menopausal DP (13). Taken together, the findings from these studies suggest that depressive disorders related to the reproductive cycle in women (premenstrual, pregnancy, postpartum and menopause) may each be a specific disorder with its own characteristic pathophysiological mechanism(s), rather than sharing a common pathophysiology. As O’Hara observed (26), social support and life events may contribute differently to prepartum and postpartum depression.
One explanation for decreased melatonin secretion in pregnant DP and increased secretion in postpartum DP compared with HC is that DP may be less sensitive to the effects of estradiol or progesterone on melatonin receptors. As a result, the increase in gonadal hormones during pregnancy would increase melatonin secretion in pregnant HC, but not in DP, as observed. In postpartum women, the declining levels of estradiol and progesterone would decrease melatonin levels in HC women, but not in DP, resulting in higher melatonin levels in postpartum DP vs. HC, also as observed. The effect of estradiol and progesterone on melatonin production may occur through the rate-limiting catecholamine pathways (27-30).
An altered sensitivity to gonadal steroids in DP may explain other depressive disorders related to the reproductive cycle. Schmidt et al. (31) found differential behavioral effects of gonadal steroids in women with and without depressive symptoms related to the menstrual cycle, and Bloch et al. (32) found that simulated hormonal excursions of pregnancy and postpartum induced depressive relapses in women with a history of perinatal mood disorder, but not in women without such a history. Although we did not find significantly different levels of estradiol or progesterone in the present study, individual variability in sensitivity to these hormones may have affected results. For example, as a function of individual variability, estradiol treatment in postmenopausal women produced either an increase or a decrease in melatonin in different patients (33).
The finding that depressive history and number of prior episodes were associated with earlier melatonin offset times in pregnant women suggests that abnormalities in melatonin timing parameters may be markers of vulnerability to depressive illness during pregnancy. The mechanism for this vulnerability may be that women with depressive histories have a lower strength or output of the hypothalamic circadian pacemaker that regulates melatonin secretion. Alternatively, as early morning light suppresses melatonin secretion, causing an earlier offset, these women may have an increased sensitivity to early morning light as do some other patients at risk for depression (34), or they may experience more light in the morning due to early morning awakening that occurs in DP.
Prepartum psychiatric illness is a risk factor for development of postpartum mood disorders. Andersson et al. (35) reported depression and/or anxiety were prevalent in 29.2% of pregnant, versus 16.5% of postpartum, women. The variables most predictive of postpartum depression include antenatal depression and a previous history of postpartum depression (36). Mothers with a previous psychiatric history are four times more likely to develop depression postpartum, six times more likely to have recurrent depressive symptoms and more likely to experience physical and mental illness compared to women without depressive symptoms (37). Mood symptoms may manifest particularly in pregnancy in women with a previous psychiatric disorder. Effects on newborn physiology depend more on prepartum than on postpartum maternal depression and on duration of depressive symptoms (38). Thus, melatonin timing parameters, as manifested especially in measures of melatonin offset, appear to be sensitive physiological markers for vulnerable women whose history of depressive illness may predispose them to future episodes.
These findings have important treatment implications. The low melatonin levels in depressed pregnant women may compromise their ability to use melatonin as a regulator of other circadian rhythms. As a result, desynchronization of circadian rhythms may predispose them to further depressive mood changes (6). As melatonin treatment can alter reproductive function (39), light therapy would be a better strategy to synchronize circadian rhythms and thereby mitigate depression. Bright light at critical times of the day is one of the most potent synchronizers of circadian rhythms and has been used in the treatment of mood disorders, including those related to the reproductive cycle (see review, Parry and Maurer (40)). As light has the ability not only to entrain circadian rhythms, but also to suppress melatonin, the elevated melatonin levels in postpartum depressed women also might be amenable to critically-timed light administration. Further work is needed to define more precisely the abnormalities of melatonin secretion in pregnant and postpartum DP as a basis for designing light, sleep or pharmacological therapies that affect melatonin regulation.
ACKNOWLEDGEMENTS
This work was supported by NIH Grant No. R01 MH070788 and by NIH Clinical Research Center (CRC) Grant No. M01-RR-00827.
Footnotes
DISCLOSURES: The authors have no interests to disclose.
REFERENCES
- 1.Murray L, Woolgar M, Cooper P, Hipwell A. Cognitive vulnerability to depression in 5-year-old children of depressed mothers. J Child Psychol Psychiatry. 2001;42(7):891–9. doi: 10.1111/1469-7610.00785. [DOI] [PubMed] [Google Scholar]
- 2.Murray L, Halligan SL, Adams G, Patterson P, Goodyer IM. Socioemotional development in adolescents at risk for depression: the role of maternal depression and attachment style. Dev Psychopathol. 2006;18(2):489–516. doi: 10.1017/S0954579406060263. [DOI] [PubMed] [Google Scholar]
- 3.Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002;159(11):1889–95. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163(6):1001–8. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- 5.Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, Hughes CW, Garber J, Malloy E, King CA, Cerda G, Sood AB, Alpert JE, Trivedi MH, Rush AJ. Remissions in maternal depression and child psychopathology: a STAR*D-child report. Jama. 2006;295(12):1389–98. doi: 10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- 6.Wehr TA, Wirz-Justice A. Internal coincidence model for sleep deprivation and depression. In: Koella WP, editor. Sleep 1980. Karger; Basel: 1981. pp. 26–33. [Google Scholar]
- 7.Leibenluft E. Do gonadal steroids regulate circadian rhythms in humans? J Affect Disord. 1993;29(2-3):175–81. doi: 10.1016/0165-0327(93)90031-e. [DOI] [PubMed] [Google Scholar]
- 8.Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23(1-2):21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, Parry B, Cardinali DP. Melatonin in mood disorders. World J Biol Psychiatry. 2006;7(3):138–51. doi: 10.1080/15622970600571822. [DOI] [PubMed] [Google Scholar]
- 10.Parry BL, Meliska CJ, Martinez LF, Lopez AM, Sorenson DL, Nowakowski S, Loving RT. Melatonin circadian rhythms in women with depression: relationship to the reproductive cycle. In: Pandi-Perumal SR, Cardinali DP, editors. Melatonin: From Molecules to Therapy. Nova Publishers; New York: 2007. pp. 571–580. [Google Scholar]
- 11.Parry BL, Berga SL, Kripke DF, Klauber MR, Laughlin GA, Yen SS, Gillin JC. Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry. 1990;47(12):1139–46. doi: 10.1001/archpsyc.1990.01810240059010. [DOI] [PubMed] [Google Scholar]
- 12.Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12(1):47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- 13.Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Hauger RL, Elliott JA. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab. 2008;93(1):54–60. doi: 10.1210/jc.2006-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson DL, Meliska CJ. Sleep rhythms and women’s mood. Part I: Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10(2):129–144. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Gamst A, Hauger R. Hormonal basis of mood and postpartum disorders. Curr Womens Health Rep. 2003;3(3):230–5. [PubMed] [Google Scholar]
- 16.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D. C.: 1994. [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/Pm, version 2.0) Biometerics Research Dept., New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 18.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal NE, Heffernan MM. Bulimia, carbohydrate craving and depression: a central connection? In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. Vol. 7. Raven Press; New York: 1986. [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 22.Green J, Murray D. The use of the Edinburgh Postnatal Depression Scale in research to explore the relationship between antenatal and postnatal dysphoria. In: Cox J, editor. Perinatal Psychiatry: Use and Misuse of the Edinburgh Postnatal Depression Scale. Gaskell; London: 1994. pp. 180–198. [Google Scholar]
- 23.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4(1):66–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Brzezinski A, Lynch HJ, Seibel MM, Deng MH, Nader TM, Wurtman RJ. The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab. 1988;66(5):891–5. doi: 10.1210/jcem-66-5-891. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28(2):179–96. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 26.O’Hara MW. Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry. 1986;43(6):569–73. doi: 10.1001/archpsyc.1986.01800060063008. [DOI] [PubMed] [Google Scholar]
- 27.Masana MI, Soares JM, Jr., Dubocovich ML. 17Beta-estradiol modulates hMT1 melatonin receptor function. Neuroendocrinology. 2005;81(2):87–95. doi: 10.1159/000084897. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson M, Arendt J. Effects of oestrogen and progesterone on rat pineal N-acetyl transferase activity and melatonin production. Experientia. 1978;34(5):667–9. doi: 10.1007/BF01937023. [DOI] [PubMed] [Google Scholar]
- 29.Cagnacci A, Zanni AL, Veneri MG, Menozzi R, Volpe A, Rio GD. Influence of exogenous melatonin on catecholamine levels in postmenopausal women prior and during oestradiol replacement. Clin Endocrinol (Oxf) 2000;53(3):367–72. doi: 10.1046/j.1365-2265.2000.01099.x. [DOI] [PubMed] [Google Scholar]
- 30.Kostoglou-Athanassiou I, Athanassiou P, Treacher DF, Wheeler MJ, Forsling ML. Neurohypophysial hormone and melatonin secretion over the natural and suppressed menstrual cycle in premenopausal women. Clin Endocrinol (Oxf) 1998;49(2):209–16. doi: 10.1046/j.1365-2265.1998.00504.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–16. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 32.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–30. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 33.Bartsch C, Seeger H, Muck AO, Lippert TH. The effect of estradiol on the production of melatonin in postmenopausal women. Int J Clin Pharmacol Ther. 1995;33(7):401–3. [PubMed] [Google Scholar]
- 34.Nurnberger JI, Jr., Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E. Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A preliminary report. Neuropsychopharmacology. 1988;1(3):217–23. doi: 10.1016/0893-133x(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 35.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet Gynecol Scand. 2006;85(8):937–44. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- 36.Dennis CL, Ross LE. The clinical utility of maternal self-reported personal and familial psychiatric history in identifying women at risk for postpartum depression. Acta Obstet Gynecol Scand. 2006;85(10):1179–85. doi: 10.1080/00016340600697595. [DOI] [PubMed] [Google Scholar]
- 37.Josefsson A, Sydsjo G. A follow-up study of postpartum depressed women: recurrent maternal depressive symptoms and child behavior after four years. Arch Womens Ment Health. 2007;10(4):141–5. doi: 10.1007/s00737-007-0185-9. [DOI] [PubMed] [Google Scholar]
- 38.Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67(1):63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- 39.Diaz BL, Llaneza PC. Endocrine regulation of the course of menopause by oral melatonin: first case report. Menopause. 2008;15(2):388–92. doi: 10.1097/gme.0b013e31812503f2. [DOI] [PubMed] [Google Scholar]
- 40.Parry BL, Maurer EL. Light treatment of mood disorders. Dialogues in Clinical Neuroscience. 2003;5(4):353–365. doi: 10.31887/DCNS.2003.5.4/bparry. [DOI] [PMC free article] [PubMed] [Google Scholar]