Abstract
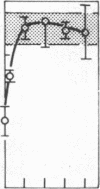
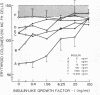
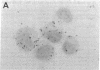
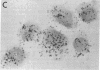
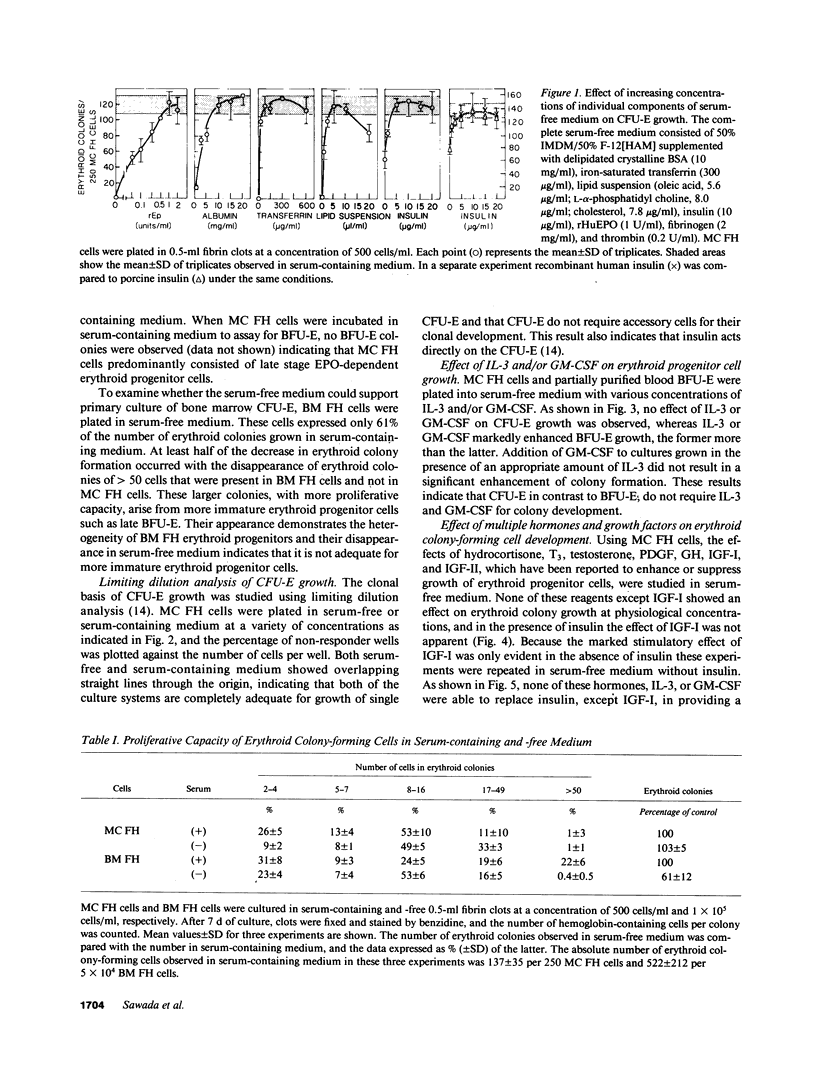
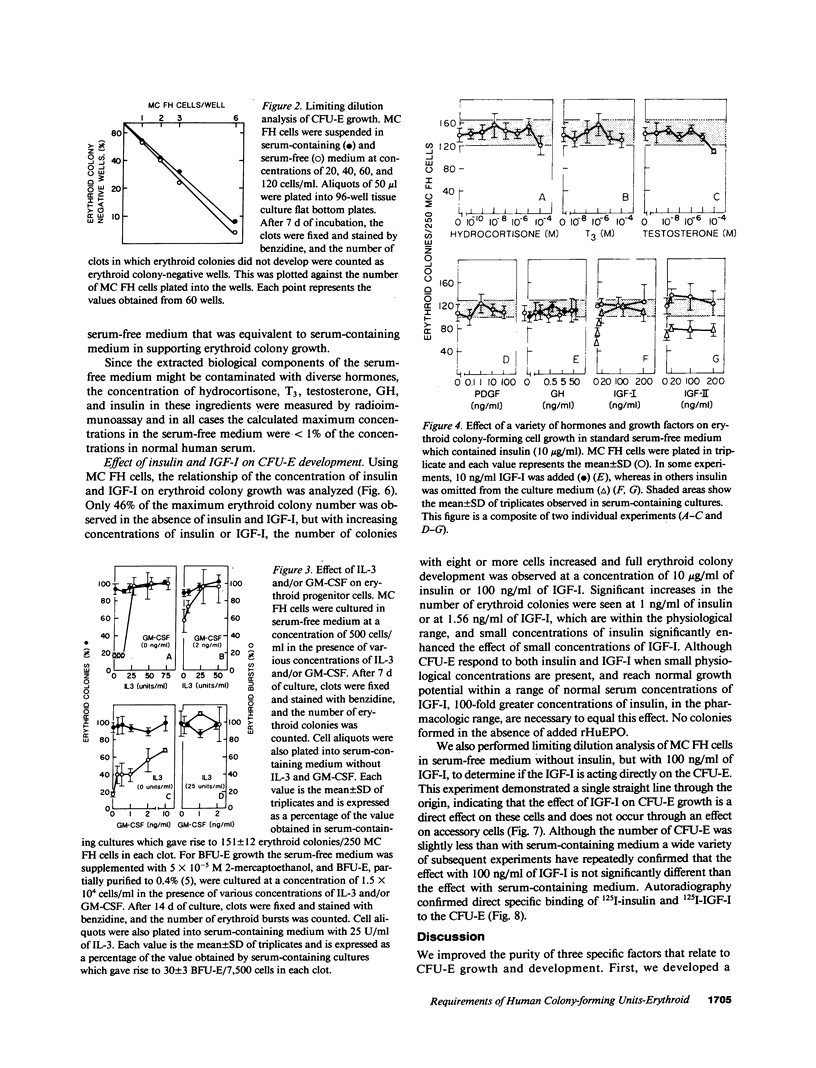
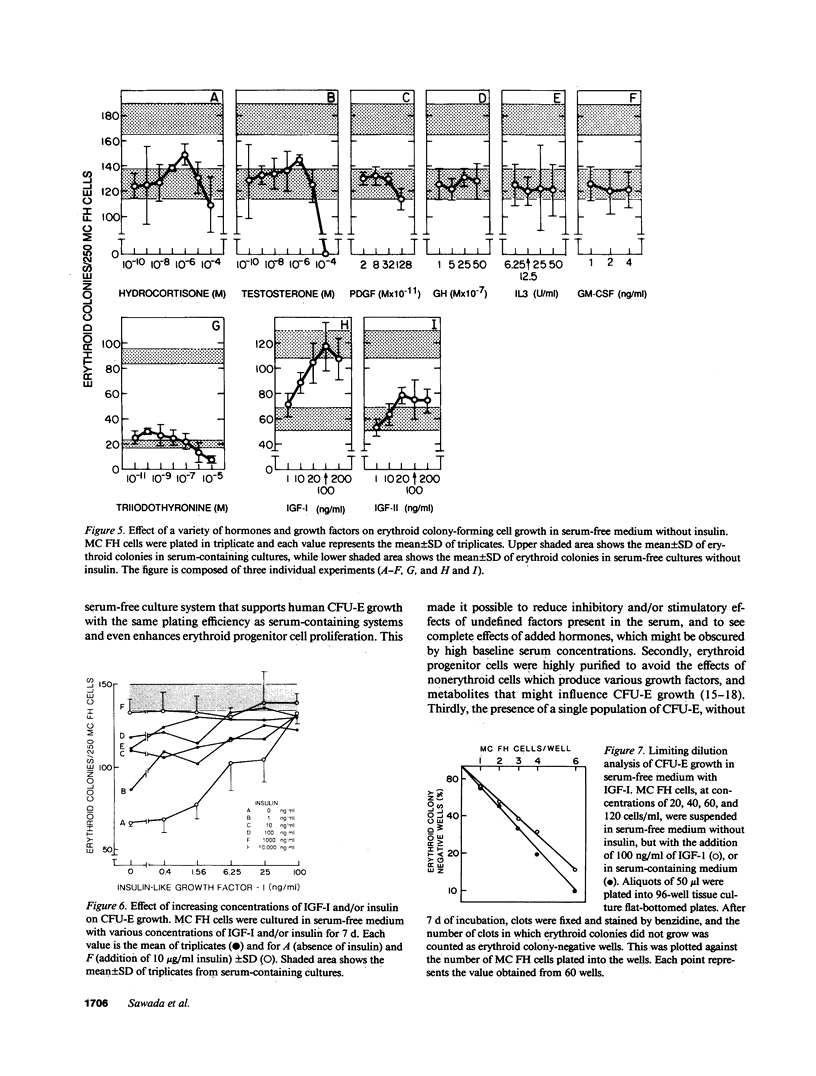
The presence of heterogeneous erythroid progenitor cells, contaminant cells, or serum may alter erythroid colony development in vitro. To obtain highly purified colony-forming units-erythroid (CFU-E), we cultured partially purified human blood burst-forming units-erythroid (BFU-E) in methylcellulose with recombinant human erythropoietin (rHuEPO) for 7 d and generated cells that consisted of 30-60% CFU-E, but no BFU-E. A serum-free medium was used that allowed development of the same number of erythroid colonies as serum containing medium, but with a greater percentage of larger colonies. This medium consisted of delipidated crystalline bovine serum albumin, iron saturated transferrin, lipid suspension, fibrinogen, thrombin, Iscove's modified Dulbecco's medium/F-12[HAM], and insulin plus rHuEPO. When CFU-E were cultured in a limiting dilution assay and the percentage of nonresponder wells was plotted against cell concentration, both serum-free cultures and serum-containing cultures yielded overlapping straight lines through the origin indicating that CFU-E development did not depend on accessory cells and that insulin acted directly on the CFU-E. Human recombinant interleukin 3 (IL-3) and/or granulocyte-macrophage colony-stimulating factor had no effect on CFU-E growth, while they markedly enhanced BFU-E growth. Physiological concentrations of recombinant human insulin-like growth factor I (IGF-I) enhanced CFU-E growth in the absence of insulin and, together with rHuEPO in serum-free medium, provided a plating efficiency equal to that of serum-containing medium. Limiting dilution analysis in serum-free medium with IGF-I showed a straight line through the origin indicating that IGF-I also acted directly on the CFU-E and not through an effect on accessory cells. These data demonstrate that CFU-E do not require accessory cells, but do require IGF-I and/or insulin which act directly on the CFU-E.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane K., Tojo A., Urabe A., Takaku F. Pure erythropoietic colony and burst formations in serum-free culture and their enhancement by insulin-like growth factor I. Exp Hematol. 1987 Aug;15(7):797–802. [PubMed] [Google Scholar]
- Bersch N., Groopman J. E., Golde D. W. Natural and biosynthetic insulin stimulates the growth of human erythroid progenitors in vitro. J Clin Endocrinol Metab. 1982 Dec;55(6):1209–1211. doi: 10.1210/jcem-55-6-1209. [DOI] [PubMed] [Google Scholar]
- Casadevall N., Vainchenker W., Lacombe C., Vinci G., Chapman J., Breton-Gorius J., Varet B. Erythroid progenitors in polycythemia vera: demonstration of their hypersensitivity to erythropoietin using serum free cultures. Blood. 1982 Feb;59(2):447–451. [PubMed] [Google Scholar]
- Clarke B. J., Housman D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1105–1109. doi: 10.1073/pnas.74.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claustres M., Chatelain P., Sultan C. Insulin-like growth factor I stimulates human erythroid colony formation in vitro. J Clin Endocrinol Metab. 1987 Jul;65(1):78–82. doi: 10.1210/jcem-65-1-78. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Hoffman R., Ritchey A. K., Floyd V., Callahan M. In vitro steroid sensitivity testing: a possible means to predict response to therapy in primary hypoproliferative anemia. Am J Hematol. 1980;9(4):401–412. doi: 10.1002/ajh.2830090407. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Kreczko S. Interactions of insulin, insulinlike growth factor II, and platelet-derived growth factor in erythropoietic culture. J Clin Invest. 1985 Sep;76(3):1237–1242. doi: 10.1172/JCI112079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Sutter D., Kreczko S. L-triiodothyronine augments erythropoietic growth factor release from peripheral blood and bone marrow leukocytes. Blood. 1986 Dec;68(6):1289–1297. [PubMed] [Google Scholar]
- Eaves A. C., Eaves C. J. Erythropoiesis in culture. Clin Haematol. 1984 Jun;13(2):371–391. [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. IMMUNOASSAY OF HUMAN GROWTH HORMONE IN PLASMA. Nature. 1963 Aug 24;199:784–787. doi: 10.1038/199784a0. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Chopra I. J., Cline M. J. Thyroid hormones stimulate erythropoiesis in vitro. Br J Haematol. 1977 Oct;37(2):173–177. doi: 10.1111/j.1365-2141.1977.tb06833.x. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Li C. H. Growth hormone: species-specific stimulation of erythropoiesis in vitro. Science. 1977 Jun 3;196(4294):1112–1113. doi: 10.1126/science.870971. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Identification of the colony-stimulating cell in human peripheral blood. J Clin Invest. 1972 Nov;51(11):2981–2983. doi: 10.1172/JCI107124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., Lin N., Adamson J. W. Interleukin 1 stimulates fibroblasts to synthesize granulocyte-macrophage and granulocyte colony-stimulating factors. Mechanism for the hematopoietic response to inflammation. J Clin Invest. 1988 Jan;81(1):92–97. doi: 10.1172/JCI113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. J., Koekebakker M., Barr R. D. Modulation of human erythropoiesis by hydrocortisone in vitro. Eur J Haematol. 1987 Feb;38(2):137–140. doi: 10.1111/j.1600-0609.1987.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Bauer C. A new candidate for the regulation of erythropoiesis. Insulin-like growth factor I. FEBS Lett. 1982 Nov 22;149(1):105–108. doi: 10.1016/0014-5793(82)81081-8. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Bauer C. Insulin stimulates erythroid colony formation independently of erythropoietin. Br J Haematol. 1983 Feb;53(2):311–316. doi: 10.1111/j.1365-2141.1983.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Meuer S. C., Blohm D., Mertelsmann R. H., Herrmann F. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988 Feb 1;140(3):837–839. [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Morell B., Froesch E. R. Fibroblasts as an experimental tool in metabolic and hormone studies. II. Effects of insulin and nonsuppressible insulin-like activity (NSILA-S) on fibroblasts in culture. Eur J Clin Invest. 1973 Mar;3(2):119–123. doi: 10.1111/j.1365-2362.1973.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., MacEachern M. D., Avila L. Human marrow erythropoiesis in culture: II. Heterogeneity in the morphology, time course of colony formation, and sedimentation velocities of the colony-forming cells. Am J Hematol. 1977;3:29–36. doi: 10.1002/ajh.2830030104. [DOI] [PubMed] [Google Scholar]
- Perrine S. P., Greene M. F., Lee P. D., Cohen R. A., Faller D. V. Insulin stimulates cord blood erythroid progenitor growth: evidence for an aetiological role in neonatal polycythaemia. Br J Haematol. 1986 Nov;64(3):503–511. doi: 10.1111/j.1365-2141.1986.tb02206.x. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Zapf J., Nissley S. P., Froesch E. R., Moses A. C., Podskalny J. M., Schilling E. E., Humbel R. E. Interactions of insulin-like growth factors I and II and multiplication-stimulating activity with receptors and serum carrier proteins. Endocrinology. 1980 Nov;107(5):1451–1459. doi: 10.1210/endo-107-5-1451. [DOI] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Kans J. S., Dessypris E. N., Sawyer S., Glick A. D., Civin C. I. Purification of human erythroid colony-forming units and demonstration of specific binding of erythropoietin. J Clin Invest. 1987 Aug;80(2):357–366. doi: 10.1172/JCI113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Insulin-like growth factors stimulate synthesis of nucleic acids and glycogen in cultured calvaria cells. Calcif Tissue Int. 1983 Jul;35(4-5):578–585. doi: 10.1007/BF02405097. [DOI] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett. 1983 Sep 5;161(1):117–121. doi: 10.1016/0014-5793(83)80742-x. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Stewart S., Zhu B., Axelrad A. A "serum-free" medium for the production of erythropoietic bursts by murine bone marrow cells. Exp Hematol. 1984 Jun;12(5):309–318. [PubMed] [Google Scholar]
- Zapf J., Froesch E. R., Humbel R. E. The insulin-like growth factors (IGF) of human serum: chemical and biological characterization and aspects of their possible physiological role. Curr Top Cell Regul. 1981;19:257–309. doi: 10.1016/b978-0-12-152819-5.50024-5. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. Insulin-like growth factors I and II: some biological actions and receptor binding characteristics of two purified constituents of nonsuppressible insulin-like activity of human serum. Eur J Biochem. 1978 Jun 15;87(2):285–296. doi: 10.1111/j.1432-1033.1978.tb12377.x. [DOI] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981 Nov;68(5):1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]