Abstract
Objective
Higher body mass index (BMI) has been associated with more sleep disturbance and depressive symptoms, but the combined effects of depression and BMI on sleep have not been studied in children. This study evaluated the relationship between BMI and polysomnography in children with major depressive disorder (MDD), compared to healthy controls (HCs).
Method
The sample of 104 subjects included 72 children, 8–17 years old, with MDD and 32 similarly aged HCs with no personal or family history of psychopathology. BMI was adjusted using the CDC formula for percentiles by age. Subjects were categorized as 1) normal weight (5th to 84th percentile) or 2) high weight, which included at risk of overweight and overweight (≥ 85th percentile). All analyses were adjusted for sex and Tanner maturational stage scores.
Results
In the MDD group only, higher BMI was significantly correlated with decreased sleep efficiency, decreased percentage of rapid eye movement sleep (REM%), and higher percentage of time spent awake-and-movement moving (TSPAM). In the HC group only, higher BMI correlated with higher total sleep time. Multivariate analyses revealed significant interactions between the BMI and diagnostic groups for several REM sleep parameters, such that high-weight children from the HC and MDD groups had increases and decreases in REM sleep, respectively. TSPAM increased in the high-weight MDD group, but decreased in the high-weight HC group.
Conclusions
Although limited by small sample size, these findings suggest that children and adolescents with MDD and a high BMI have more fragmented sleep than other children. The increased REM sleep patterns observed with MDD in this and other studies normalized in high-weight children with MDD. Prevention and treatment strategies should target both sleep and weight as factors that can potentially influence the development and course of MDD.
Keywords: Body mass index, sleep, polysomnography, depression, overweight, children
1. Introduction
Major depressive disorder (MDD) and being overweight are significant health problems. The prevalence of overweight among school-aged children in the United States is more than 15% [1]. The prevalence for MDD in children and adolescents ranges from 8% to 15% with higher rates among adolescents [2, 3]. Some studies have suggested that obese people are at elevated risk for depression [4–6], although these studies are not conclusive. Longitudinal studies in Finland revealed that obesity in adolescence may be associated with later depression in young adulthood [7]. On the other hand, clinical studies suggest that children and adolescents with MDD may be at increased risk for becoming overweight [8–11]. In the study by Pine et al., juvenile onset of MDD was associated with an increased body mass index (BMI) in adulthood, even when participants who were overweight during childhood were excluded at baseline [10]. Other studies in adolescents also have revealed an association between concomitant obesity and depressive symptoms [12, 13]. Overall, the relationship between obesity and depression is bidirectional.
The mechanism by which MDD and obesity are related to each other is not clear, but one common factor is sleep disturbance. Both MDD and obesity are associated with sleep disturbances. Over 90% of children and adolescents with MDD [14], for example, report subjective sleep complaints. Sleep disturbances also increase the risk for developing depression across the life cycle [15–17]. Moreover, short sleep duration has been linked to obesity in children [18, 19] and adolescents [20]. Recent studies that used sleep questionnaires and wrist actigraphy suggest that higher BMI is associated with greater sleep disturbances in adolescents [21–23]. Polysomnography (PSG) measures of sleep in adolescents have shown that BMI was associated with lighter and less deep sleep [23].
Because sleep disturbances are associated with both MDD and obesity, one might expect that individuals with both conditions would have worse sleep than individuals with only one or neither condition. We reasoned that such a finding would be clinically significant because sleep disturbances may both increase the risk to develop MDD [15–17] and worsen the course of MDD after it has developed [24, 25]. Moreover, both sleep and weight are factors that treatment interventions can target in order to interrupt their complex, bidirectional relationships with MDD and each other.
The primary aim of the present study was to investigate objective measures of sleep as a function of BMI and MDD in children and adolescents. In particular, we hypothesized that depressed overweight children would have the worst sleep.
2. Methods
2.1. Subjects
The sample included 104 children, 8–17 years of age, including 72 outpatients diagnosed with unipolar, nonpsychotic major depressive disorder (MDD), single or recurrent, and 32 healthy controls (HC) with no personal or family history of psychopathology. Subjects with MDD were symptomatic and unmedicated for a minimum of four weeks prior to the study. There were 40 females and 32 males in the sample of outpatients with MDD, and 11 females and 21 males in the sample of HCs. A number of children and adolescents in the depressed group also met criteria for other psychiatric illness (Table 1). None of the participants had an independent sleep disorder (e.g., sleep apnea, periodic limb movement disorder, bruxism, or restless legs syndrome) based on history and confirmed on the first night in this study. No subject met diagnostic criteria for anorexia, bulimia, or substance use disorders in the past 6 months.
Table 1.
Demographic and clinical characteristics of the sample by diagnostic group.
| Variable | MDD N = 72 |
NC N = 32 |
All N = 105 |
p |
|---|---|---|---|---|
| Females | 40 (55.6) | 11 (34.4) | 51 (49.0) | 0.075a |
| Age (years) | 12.19 (3.10) | 12.41 (2.72) | 12.26 (2.98) | NS |
| Tanner stage scores (1–5) | 2.95 (1.54)b | 3.16 (1.48) | 3.01 (1.51) | NS |
| BMI raw | 22.64 (5.99) | 22.27 (5.85) | 22.53 (5.92) | NS |
| BMI percentile | 70.79 (25. 37) | 72.06 (24.70) | 71.18 (25.05) | NS |
| BMI Z-score | 0.88 (1.00) | 0.85 (0.93) | 0.87 (0.97) | NS |
| FGAS | 63.36 (11.40) | 90.17 (4.27) | 68.72 (14.94) | < 0.001 |
| CGAS | 52.08 (7.02) | 87.83 (11.32) | 59.23 (16.45) | < 0.001 |
| CDRS-R | 57.89 (9.37) | 20.53 (9.03) | 46.39 (19.63) | < 0.001 |
| Age of onset (years) | 10.99 (3.07) | - | - | < 0.001 |
| Length of current episode (mo) | 14.55 (19.32) | - | - | |
| Suicidal ideation (1–5 scale) | 2.25 (0.80) | 1.17 (0.71) | 2.03 (0.89) | < 0.001 |
| Suicide attempts | 0 (0) | - | - | |
| Family history of MDD | 19 (26.4) | - | - | |
| Comorbid illness | 43 (59.7) | - | - | |
| ADHD | 18 | - | - | |
| Enuresis | 1 | - | - | |
| Seasonal affective disorder | 1 | - | - | |
| Dysthymia | 15 | - | - | |
| Generalized anxiety | 4 | - | - | |
| Oppositional defiant disorder | 1 | - | - | |
| Adjustment disorder | 1 | - | - | |
| Phobia | 1 | - | - | |
| OCD | 1 | - | - |
Continuous variables are represented by their means (standard deviations) and dichotomous variables by their frequency, n (%). MDD = Major Depressive Disorder; HC = Healthy Controls; BMI = Body Mass Index; FGAS = Family Global Assessment Scale; CGAS = Children’s Global Assessment Scale; CDRS-R = Children’s Depression Rating Scale-Revised; ADHD = Attention Deficit Hyperactivity Disorder; OCD = Obsessive Compulsive Disorder
p value for chi square with continuity correction for 2X2 table.
This sample was drawn from a larger study (n=180) of sleep macro- and micro-architecture and rest-activity cycles in depressed and healthy children and adolescents [26–28]. Inclusion in the present study required complete data for height, weight, and Tanner stage score at the time of sleep study, resulting in the sample size of 104. Subjects were recruited from 1999 to 2003 via advertisements and posted flyers at community centers, hospitals, and outpatient clinics. Referrals from community clinicians and self-referrals were also permitted.
2.2. Diagnostic procedures
The study was approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center and the University of Michigan Medical School. Subjects were scheduled for a full evaluation after a telephone screen for inclusion and exclusion criteria. Before the initial interview, the study was explained and written informed consent was obtained from the parent(s) and assent from the subjects.
All children and their parents were interviewed separately using the Schedules for Affective Disorders and Schizophrenia for School-Aged Children: Present and Lifetime (K-SADS-PL), a revision to the K-SADS16 [29]. The K-SADS-PL is a semi-structured diagnostic interview to evaluate Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for MDD and other concurrent and lifetime psychiatric disorders. Final diagnoses were determined using information from interviews of the parents and child. To measure depression symptom severity, the Children’s Depression Rating Scale-Revised (CDRS-R) was used [30]. Children with MDD had to score a minimum of 40 on the CDRS-R for entry into the study. Family history, based on the Family History Diagnostic Interview, was obtained from parents by a separate interviewer. The Children’s Global Assessment Scale (CGAS) determined overall functioning [31]. Family function and adjustment were evaluated using the Family Global Assessment Scale (FGAS) [32]. Other clinical characteristics of depression included age of onset, length of current episode, and suicidal thoughts. Just before the sleep study an interview was conducted to repeat psychiatric assessment and inventories. In addition to a structured psychiatric interview, the initial evaluation for subjects included a medical review of systems and self-assessment of the Tanner staging sexual maturity score on a scale of 1–5 [28] as well as physical and neurological examinations. Further detail on the evaluation procedures, including Tanner self-assessment, is reported elsewhere [28, 33].
2.3. BMI measures
Height and weight were measured during the initial evaluation by trained research assistants using a medical balance beam scale with height bar. Body mass index (BMI) was calculated as weight/height2 expressed in kg/m2 units and then transformed into a z-score and percentiles for age and sex using the Centers for Disease Control (CDC) reference values [34]. Based on these, childhood overweight was defined as a BMI of ≥ 95th percentile for age and sex, risk of overweight as a BMI ≥ 85th but less than 95th and normal weight as BMI less than the 85th percentile but > 4th percentile. For purposes of this study, MDD and HC subjects were categorized into two groups: 1) normal weight (fifth to 84th percentile) and 2) high weight, which included subjects either at risk of overweight or overweight (≥ 85th percentile). The at-risk of overweight and overweight groups were combined because of small sample sizes.
2.4. Recording procedures
Each participant maintained a regularized sleep/wake cycle for one week followed by 2 consecutive nights in the sleep lab. Night 1 served as an adaptation and allowed additional screening for independent sleep disorders. Night 1 recordings incorporated chest and abdomen respiration bands, nasal-oral thermistors, and leg electrodes. Electrode placement on the subsequent night included F1, F2, C3, C4, O3, O4, P3, P4, left and right EOG, recorded from the upper and lower canthi, and a bipolar, chin-cheek EMG. EEG electrodes were referenced to the ear lobes linked through a 10 KΩ resistor to minimize non-homogenous current flow and potential artifactual hemispheric asymmetries, as is standard in our laboratory. EEG was transduced by Grass™ P511 A/C amplifiers set at a sensitivity of 5 (50µV, 0.5 s calibration), corresponding to a gain of 50,000. The half-amp low- and high-bandpass filters were set at 0.3 and 30 Hz, respectively. A 60-Hz notch filter attenuated electrical noise.
The respiratory signals were also digitized to further assess potential reductions in airflow and effort. Any participant who showed 5 or more instances of >50% reduction in air flow of at least 5 seconds in duration per hour was excluded from further study. This together with visual assessment of respiratory signals, exclusion for parental report of snoring, or evidence of snoring in the lab, minimized the possibility of including those children/adolescents with sleep-disordered breathing.
According to standard sleep staging criteria [35], visual stage scoring of 30-second epochs was conducted by research personnel trained at better than 90% agreement on an epoch-by-epoch basis. Time in bed was defined as the time from lights out to lights on. Sleep onset latency (SOL) was defined as the first consecutive 10-minute block of any sleep stage (except rapid eye movement [REM] sleep) with no more than 2 minutes of awake time, reflecting persistent sleep onset. Total sleep period (TSP) was defined as the time from sleep onset to morning awakening including any awake-and-movement time. Total sleep time (TST) was defined as the total amount of REM and NREM sleep during the TSP. Sleep efficiency (SE) was calculated as the total amount of sleep time (TST) divided by the total time in bed. Minutes of each NREM sleep stage (Stages 1–4) as well as REM sleep were computed and expressed as a percentage of TSP. Stages 3 and 4 sleep were added together to calculate slow wave sleep (SWS). The REM sleep latency was defined as the duration in minutes from sleep onset to the first epoch of REM sleep with no minimum duration criterion. REM density (REM_D) was scored on a 5-point scale for each epoch, ranging from no eye movements (0) to more than 4 per epoch (4). The scores were then averaged per minute of REM.
Because of known REM-sleep differences between depressed patients and healthy controls, characteristics of REM sleep—divided by its first three cycles—were also considered. Normally, most REM sleep occurs in the last one-third of the night. In depressed patients, REM sleep latency is typically shortened on average, and a disproportionate amount of REM sleep occurs during the first third of the night. Thus, REM density, REM sleep duration, and net REM sleep duration (duration of true REM time during the duration of a REM sleep episode) were calculated for the first, second, and third REM sleep periods. Other sleep variables included awake-and-movement time both as amount in minutes and by percentage of TSP. The personnel who scored the records were blind to diagnostic group, age, and sex.
2.5. Statistical analyses
Continuous variables were checked for normal distribution. Non-normal variables were either transformed or analyzed with non-parametric statistics as indicated. The MDD and HC groups were compared in terms of demographics, BMI, and depression ratings using independent sample t tests (or Mann-Whitney U tests) for continuous variables and the chi square test for sex. All analyses were adjusted for sex and Tanner sexual maturation stage scores because both sex and Tanner stage have previously been shown to influence sleep in depressed children [28].
Pearson r correlations were performed to look for relationships between continuous BMI Z-scores and sleep parameters in each diagnostic group. Next, the MDD and HC groups were each further divided into normal-weight and high-weight groups. The high-weight group included all participants that were either overweight or at risk for becoming overweight. Multivariate analyses of covariance were performed to detect the interactive and main effects of diagnosis and BMI on sleep variables while controlling for sex and Tanner stage. The first set of ten sleep variables consisted of sleep continuity and architecture parameters: total sleep time, sleep latency, sleep efficiency, time spent awake and moving (TSPAM), S1%, S2%, SWS%, REM%, REM_D, and REM latency. After significant findings were observed for selected REM sleep parameters as well as for TSPAM, additional tests were performed to investigate time course of these variables over three time periods during the night while controlling for sex and Tanner stage. Effect sizes were calculated using the eta squared (η2) statistic. The guidelines proposed by Cohen [36] for interpreting these values are 0.01 = small effect, 0.06 = moderate effect, and 0.14 = large effect.
Finally, we performed a one-way MANCOVA for each set of sleep variables in the MDD group to investigate the covariate effects of clinical variables (age of onset of depression, depression severity score, ADHD comorbidity, family history of MDD) on the relationship between sleep and BMI.
3. Results
There were no significant differences in age, Tanner stage scores, or BMI scores between the MDD and control groups (Table 1). As expected, depressive symptom severity was significantly higher in the MDD group compared to controls (p < 0.001). The MDD group was also significantly more impaired than controls on the Family Global Assessment Scale and the Children’s Global Assessment Scale, where values below 60 indicate mild-to-moderate dysfunction on both scales (p < 0.001). The depressed group, however, had more females (56%) than the healthy control group (34%) which was marginally significant (p=0.075). Even though healthy control subjects were recruited with an aim to match depressed subjects for sex and age, the exclusion of subjects because of missing data for BMI led to this sex discrepancy. Therefore, multivariate analyses of sleep variables were controlled for sex; and interactions between sex, weight, and diagnosis on sleep variables were explored.
In the MDD group, significant correlations were found between BMI Z-score and selected sleep parameters while controlling for sex and Tanner stage (Table 2). Specifically, higher BMI was associated with decreased SE (p = 0.025) and a higher percentage of awake-and-movement time (p = 0.001). When looking at the distribution of awake-and-movement time across the night in the MDD group, higher BMI was associated with a longer TSPAM during the middle (p = 0.048) and last thirds (p = 0.005) of the sleep period. By contrast, higher BMI in the HC group was associated only with more minutes of TST (p = 0.026; Table 2). Because of the smaller size of the HC group, however, the correlation coefficients were examined further without reference to p values. The results, shown in Table 2, revealed opposite correlations, although similar in absolute value, between sleep and BMI in the HC vs. MDD groups with regard to four selected variables. As examples, the correlation coefficients between net REM time in the first REM sleep period and BMI for the control vs. MDD groups were r = 0.332 vs. −0.277, respectively. Similarly, for duration of the first REM sleep period, the respective correlations for the control vs. MDD groups were r = 0.330 vs. −0.266. Similar patterns were observed for REM% and TSPAM in the middle third of the night. The opposite direction of correlations between diagnostic groups suggested the likelihood of group interactions.
Table 2.
Significant correlations between body mass index Z-score (BMI Z-score) with sleep parameters in children with major depressive disorder (MDD) as compared to healthy control (HC) children, adjusting for sex and Tanner stage scores.
| MDD (N=72) | HC (N=32) | |||
|---|---|---|---|---|
| BMI Z-score | p | BMI Z-score | p | |
| TST | 0.105 | 0.389 | 0.407 | 0.026 |
| SE% | −0.268 | 0.025 | 0.138 | 0.466 |
| REM% | −0.243 | 0.043 | 0.235 | 0.212 |
| 1st REM period duration (min) | −0.266 | 0.026 | 0.330 | 0.075 |
| 1st REM period net REM time (min) | −0.277 | 0.020 | 0.332 | 0.073 |
| 1st REM period REM density | −0.250 | 0.037 | 0.153 | 0.419 |
| % Awake-&-movement time | 0.373 | 0.001 | −0.164 | 0.385 |
| Time spent awake and moving in middle third of night (min) | 0.237 | 0.048 | −0.285 | 0.127 |
| Time spent awake and moving in last third of night (min) | 0.329 | 0.005 | 0.019 | 0.920 |
Significant p values are bolded
Statistics for PSG variables are presented for the MDD and control groups in Table 3 by BMI group. The MANCOVA for the first set of 10 sleep parameters, entering BMI group and diagnostic group as main factors while controlling for sex and Tanner stage scores, revealed an overall multivariate main effect for the covariate variable, Tanner stage scores [F(10,89)=4.77, p<0.0005; η2 =0.35]. When the dependent variables were separately examined, TST, SWS%, and REM latency all decreased significantly as subjects became more sexually mature. No other variables were significant, but a nearly significant overall multivariate interaction between BMI group and diagnostic group was observed with a large effect size [F(10,89)=1.85, p=0.063; η2 =0.17]. Three sleep parameters contributed to this overall marginally significant interaction with effect sizes (partial η2 values) ranging from small to medium: REM% (F=6.63, df=1, p=0.012; η2 = 0.06), REM density (F=4.52, df=1, p=0.036; η2 = 0.04), and percentage of TSPAM (F=6.32, df=1, p=0.014; η2 = 0.06). The high-weight depressed children had the highest percentage of TSPAM. In addition, the normal-weight depressed children had increased REM% and REM density compared to normal-weight controls, whereas the high-weight depressed children had decreased REM% and REM density compared to high-weight controls. When sex was entered as an independent factor, rather than a covariate, the overall multivariate interaction effect between BMI group and diagnostic group maintained its non-significant, but large effect size (p=0.08, η2 =0.13). Neither sex nor its interactions with diagnosis or BMI group, however, was significantly related to any of the sleep variables.
Table 3.
Sleep parameters in groups of children with Major Depressive Disorder (MDD) and Healthy Controls (HC) in relation to Body Mass Index (Mean ± SD)1
| MDD (N=73) | HC (N=32) | MANCOVA | ||||
|---|---|---|---|---|---|---|
| Normal weight | ≥ 85 percentile | Normal weight | ≥ 85 percentile | F | p | |
| N | 43 | 29 | 20 | 12 | ||
| 1) Total Sleep Time (min) | 471.38 ± 51.22 | 471.79 ± 52.58 | 454.58 ± 41.58 | 490.13 ± 50.18 | NS | |
| 2) Sleep Efficiency, % | 93.32 ± 3.24 | 91.61 ± 3.98 | 93.34 ± 3.60 | 93.58 ± 2.09 | NS | |
| 3) Sleep Latency (min) | 16.15 ±13.10 | 16.45 ± 14.60 | 10.52 ± 10.02 | 13.92 ± 10.95 | NS | |
| 4) % Awake-&-Movement time | 3.61 ± 1.71 | 5.29 ± 2.57 | 4.69 ± 3.20 | 3.92 ± 1.40 | 6.32 | 0.014* |
| 5) Sleep Stage 1 % | 7.74 ± 4.57 | 11.27 ± 6.90 | 9.19 ± 4.69 | 9.63 ± 7.41 | NS | |
| 6) Sleep Stage 2% | 50.10 ± 7.99 | 49.21 ± 6.95 | 49.80 ± 6.79 | 45.78 ± 9.24 | NS | |
| 7) Slow Wave Sleep % | 18.92 ± 8.06 | 16.48 ± 6.43 | 18.45± 5.74 | 19.89 ± 5.86 | NS | |
| 8) REM Latency (min) | 115.09 ± 50.42 | 106.28 ± 64.84 | 97.22 ± 36.59 | 108.12 ± 51.38 | NS | |
| 9) REM Sleep % | 19.62 ± 4.76 | 17.75 ± 4.97 | 17.87 ± 4.01 | 20.77 ± 3.79 | 6.63 | 0.012* |
| 10) REM Density | 2.28 ± 0.60 | 2.10 ± 0.54 | 1.95 ± 0.57 | 2.37 ± 0.82 | 4.52 | 0.036* |
| Time spent awake & moving in middle 3rd of night (min) | 7.15 ± 5.18 | 10.03 ± 8.50 | 10.08 ± 10.42 | 6.83 ± 3.98 | 3.98 | 0.049** |
| Time spent awake & moving in last 3rd of night | 6.67 ± 4.62 | 11.74 ± 9.22 | 7.42 ± 4.41 | 7.08 ± 2.91 | 4.42 | 0.038** |
| 1st REM period duration (min) | 15.88 ± 12.79 | 9.62 ± 6.29 | 12.23 ± 8.35 | 17.29 ± 9.28 | 7.42 | 0.008** |
| 1st REM period net REM time (min) | 14.02 ± 11.77 | 8.03 ± 5.41 | 9.03 ± 6.46 | 14.63 ± 9.45 | 9.44 | 0.003** |
| REM Density 1st period | 1.61 ± 0.75 | 1.29 ± 0.69 | 1.29 ± 0.80 | 1.68 ± 1.07 | 4.20 | 0.043** |
Means ± SD are unadjusted
p-values for 1st MANCOVA with ten dependent sleep variables showing that the interaction between diagnostic group and BMI group was significant while controlling for sex and Tanner stage score.
p-values for 2nd MANCOVA with five of 12 dependent sleep variables by time period, showing that the interaction between diagnostic group and BMI group was significant while controlling for sex and Tanner stage score
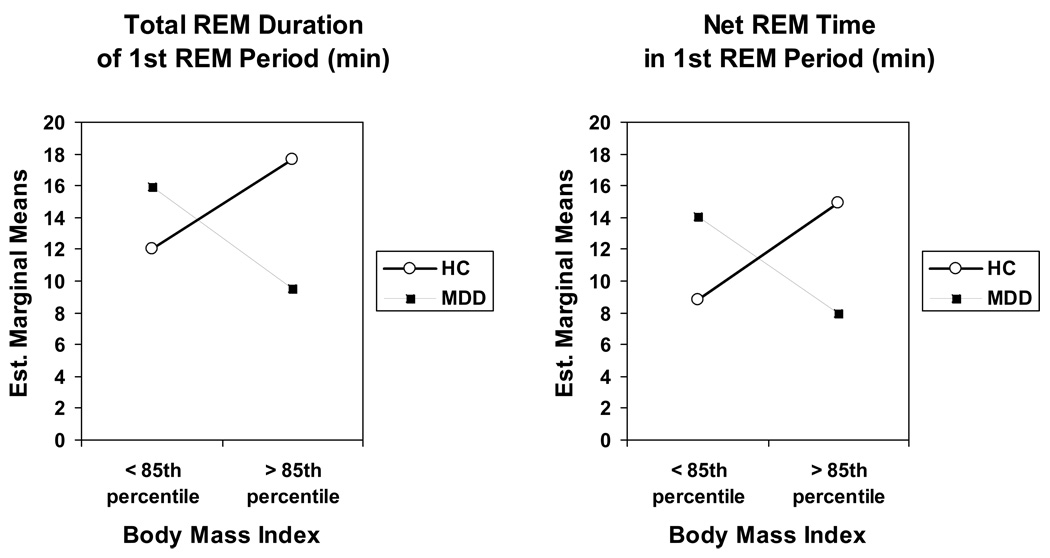
Focusing on the significant sleep parameters from the first MANCOVA, a second MANCOVA was performed to discover when the four groups most differed from one another over the course of the night. For TSPAM, the night was divided into three time periods or thirds. For REM sleep variables, the first three REM sleep periods were analyzed. Altogether, then, a total of 12 dependent sleep variables were entered: minutes of TSPAM for each third of the night and REM density, minutes of net REM time, and REM duration for each of the first three REM sleep periods of the night. Results are shown in Table 3. In this analysis, Tanner stage score was not significant, but the overall multivariate interaction between diagnostic group and BMI group was significant with a large effect size: F (12, 87) =1.97, p=0.037, η2 =0.21. Five variables contributed to the overall significance: TSPAM during both the middle and last thirds of the night and each of the three REM sleep variables during the first REM sleep period. Specifically, depressed children in the high-weight group had a longer duration of TSPAM during the last two-thirds of the night than both the normal-weight depressed children and the high-weight HCs (Table 3), although the effect sizes were small (η2 =0.04 and 0.05). Likewise, the effect size for REM density during the first REM period was also small (η2 =0.04), but paralleled the nature of the interactions observed for duration of, and net REM time during, the first REM period (Figure 1), which had medium effect sizes (η2 =0.07 and 0.09, respectively). The high-weight MDD group when compared to the normal-weight MDD group showed a shorter first REM sleep period with less net REM sleep time (Fig. 1). On the other hand, the high-weight HC group had the longest total duration of the first REM period with the most net REM time.
Figure 1.
Significant interactions between diagnostic group and body mass index for REM sleep variables during the first REM sleep period: Total Duration (p=0.008) and Net REM Time (p=0.003). Est. = Estimated. REM = rapid eye movement.
The last analysis was performed to characterize relationships between sleep and BMI group in the MDD group after adjusting for ADHD, family history of MDD, age of onset of MDD, and depression severity. Two MANCOVAs were performed with BMI group as the independent variable and each set of 10–12 sleep parameters as dependent variables while also adjusting for sex and Tanner stage score. Neither BMI group nor any covariates were significant in these multivariate tests.
4. Discussion
The a priori hypothesis of this study was that high-weight depressed children would have worse sleep than both normal-weight depressed children and healthy control children of all weights. Our correlation analyses supported this hypothesis inasmuch as lower SE and a higher percentage of awake-and-movement time were correlated with higher BMI in depressed children, but not in healthy controls. Similarly, the multivariate analyses also supported this hypothesis both in terms of percentage of TSPAM over the entire night and duration of TSPAM during the last third of the night.
Although we were not able to confirm a significant association between higher BMI and shorter total sleep time [21, 22, 37–40], our finding of disrupted sleep in the high-weight subjects are consistent with those of Resta et al. [41], who found that obese patients had more frequent awakenings during sleep than normal-weight subjects, and Vgontzas et al. [42], who reported significantly more fragmented sleep in obese people compared to non-obese subjects, excluding those with sleep apnea.
A second major finding of the study was that several REM sleep parameters differed between children with and without major depressive disorder as a function of BMI. Significant group interactions (diagnosis X BMI group) were found for REM% and REM density across the night and for several parameters during the first REM period of the night. Thus, the high-weight control group had more net REM sleep time and REM density in the first REM period and a longer total duration of the first REM period than normal-weight healthy controls. In contrast, the high-weight depressed children had less net REM sleep time and REM density that occurred during a shorter first REM period than depressed subjects of normal weight. Interestingly, these REM sleep parameters in high-weight depressed children were quite similar to those observed in normal-weight healthy control children.
The reasons for the REM-sleep interactions are unknown. Two known abnormalities in the REM sleep of depressed patients are prolonged duration of the first REM sleep period and increased density of rapid eye movements [43], which are consistent with the findings for normal-weight depressed children in this study but inconsistent with the findings for high-weight depressed children. In a multivariate analysis of sleep variables and depressive symptoms, Perlis et al. [16] demonstrated that a constellation of 15 depressive symptoms, including weight loss, was correlated with nine sleep variables, in particular increased REM activity. Similarly, we observed increased REM sleep parameters only in the normal-weight depressed children. Therefore, it is possible that depressed subjects who either gain weight or are overweight do not share these characteristics of increased REM sleep, which distinguishes them from normal-weight depressed children.
Liu et al. [44] reported that overweight children with or without internalizing disorders had decreased REM sleep parameters. Their results are consistent with our finding of decreased REM sleep parameters in high-weight depressed children, but at odds with our finding of increased REM sleep parameters in high-weight healthy control children. Several differences in methodology, however, may have contributed to these results. First, their analyses combined children with either anxiety or mood disorders into one group of children with internalizing disorders, and then compared them to children without internalizing disorders. Second, they excluded children with extreme obesity such that the mean BMI Z-score for their sample was 0.45 (0.98) compared to 0.82 (0.99) in this study. Third, they did not analyze REM sleep parameters by thirds of the night.
Hormones such as cortisol, ghrelin, leptin, and orexin provide another link between depression, sleep, and weight. Leptin, for example, is an adipose-derived hormone which functions in the hypothalamus to decrease appetite and increase energy expenditure [20]. It has been demonstrated that plasma leptin levels are decreased in some depressed patients [45–47]. Low leptin levels might be expected to facilitate weight gain inasmuch as high leptin levels signal satiety. Leptin has also been shown to suppress REM sleep in rats [48], and genetic studies of mice with leptin deficiency showed higher levels of sleep fragmentation than wild-type mice [49]. Therefore, low leptin levels, as observed in some depressed individuals [45–47], might be associated with weight gain, relatively increased REM sleep, and increased sleep fragmentation – consistent with the sleep findings for high-weight depressed children in this study. The neurobiology of feeding behavior, depression and sleep, however, are obviously complex. Moreover, leptin levels were not measured in the present study; thus we can only speculate on their possible relationship to REM sleep parameters in these groups.
4.1. Limitations
The present findings should be interpreted carefully and the analyses should be considered exploratory. Because of unequal sample sizes across groups, the small sample size of the control group, and lack of power in multivariate analyses, we might have missed significance in some important relationships. Indeed, after applying Bonferroni corrections to the two major multivariate analyses of dependent sleep variables, the resulting p values of 0.005 (0.05/10 variables) and 0.004 (0.05/12 variables) would have precluded significance in most case cases. Nevertheless, the power to detect multivariate interaction effects was sufficient for the MANCOA involving sleep across thirds of the night. Moreover, the use of effect sizes, revealed medium to large effect sizes for all significant comparisons except REM density in the first REM sleep period and across the night and TSPAM in the last two-thirds of the night.
Another limitation was a lack of any biochemical or endocrinological markers which could be associated with overweight and sleep problems like leptin, ghrelin or cortisol. Further research is needed to examine the role of biochemical markers as mediators in the relationship between sleep parameters and BMI in depressed children. Nor did we categorize children on the basis of family history of obesity. We also did not subtype children for depression-related weight loss vs. weight gain. In addition, the majority of depressed children in this study had co-occurring psychiatric disorders. Therefore, our results may not generalize to all depressed children, but may be representative of those seen in clinical practice. It should also be noted that we did not compare sleep results with daily activity using self reports or actigraphs. Finally, this was a cross-sectional study, so no conclusions about causal relationships can be made.
4.2. Conclusions
Overall, our findings indicate a stronger relationship between high weight and more fragmented sleep in children with MDD than in high-weight HC children as measured by PSG. If replicated, then these findings may have strong clinical significance. Since poor sleep already poses a greater risk of relapse and recurrence of depression, then increasing BMI may further increase that risk. Depressed children with poor sleep and high BMI may be particularly appropriate for additional screening and intervention efforts targeted at improving sleep and reducing weight.
Acknowledgments
Financial Support: This study was supported by: NIMH R01 MH56953; NIAAA K24 AA00304; Fogarty International Center/NIDA International Substance Abuse Research Program grant D43 TW05818, Fogarty International Center/NIAAA International Collaborative Alcohol & Injury Research Training Program grant D43 TW007569.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
Dr. Brower was a member of Pfizer’s Varenicline Advisory Board in 2007. Dr. Armitage was an Advisory Board member for Takeda Pharmaceuticals in Dec 2007. Dr. Emslie receives research support from Eli Lilly, Organon, and Forest Laboratories; is a consultant to Eli Lilly, GlaxoSmithKline, Wyeth-Ayerst, Shire, and BioBehavioral Diagnostics, Inc.; and is on the speakers’ bureau of McNeil. The other authors report no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Fleming JE, Offord DR. Epidemiology of childhood depressive disorders: a critical review. J Am Acad Child Adolesc Psychiatry. 1990;29:571–580. doi: 10.1097/00004583-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Wight RG, Sepulveda JE, Aneshensel CS. Depressive symptoms: how do adolescents compare with adults? J Adolesc Health. 2004;34:314–323. doi: 10.1016/j.jadohealth.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Anderson SE, Cohen P, Naumova EN, Jacques PF, Must A. Adolescent obesity and risk for subsequent major depressive disorder and anxiety disorder: prospective evidence. Psychosom Med. 2007;69:740–747. doi: 10.1097/PSY.0b013e31815580b4. [DOI] [PubMed] [Google Scholar]
- 5.Dong C, Sanchez LE, Price RA. Relationship of obesity to depression: a family-based study. Int J Obes Relat Metab Disord. 2004;28:790–795. doi: 10.1038/sj.ijo.0802626. [DOI] [PubMed] [Google Scholar]
- 6.Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J Epidemiol. 2000;152:163–170. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- 7.Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K, Joukamaa M. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 2006;30:520–527. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- 8.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- 9.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65:634–651. doi: 10.4088/jcp.v65n0507. quiz 730. [DOI] [PubMed] [Google Scholar]
- 10.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 11.Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, Connell F. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med. 2003;157:739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- 12.Richardson LP, Garrison MM, Drangsholt M, Mancl L, LeResche L. Associations between depressive symptoms and obesity during puberty. Gen Hosp Psychiatry. 2006;28:313–320. doi: 10.1016/j.genhosppsych.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Sjoberg RL, Nilsson KW, Leppert J. Obesity, shame, and depression in school-aged children: a population-based study. Pediatrics. 2005;116:e389–e392. doi: 10.1542/peds.2005-0170. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RE, Lewinsohn PM, Seeley JR. Symptoms of DSM-III-R major depression in adolescence: evidence from an epidemiological survey. J Am Acad Child Adolesc Psychiatry. 1995;34:1608–1617. doi: 10.1097/00004583-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 16.Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, Tu X. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 17.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 18.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91:881–884. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutson KL, Lauderdale DS. Sleep duration and overweight in adolescents: self-reported sleep hours versus time diaries. Pediatrics. 2007;119:e1056–e1062. doi: 10.1542/peds.2006-2597. [DOI] [PubMed] [Google Scholar]
- 21.Beebe DW, Lewin D, Zeller M, McCabe M, MacLeod K, Daniels SR, Amin R. Sleep in overweight adolescents: shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing. J Pediatr Psychol. 2007;32:69–79. doi: 10.1093/jpepsy/jsj104. [DOI] [PubMed] [Google Scholar]
- 22.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14:762–768. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- 23.Landis AM, Parker KP. A retrospective examination of the relationship between body mass index and polysomnographic measures of sleep in adolescents. J Adolesc Health. 2007;40:89–91. doi: 10.1016/j.jadohealth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dombrovski AY, Cyranowski JM, Mulsant BH, Houck PR, Buysse DJ, Andreescu C, Thase ME, Mallinger AG, Frank E. Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress Anxiety. 2008 doi: 10.1002/da.20467. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Buysse DJ. Sleep and youth suicidal behavior: a neglected field. Curr Opin Psychiatry. 2006;19:288–293. doi: 10.1097/01.yco.0000218600.40593.18. [DOI] [PubMed] [Google Scholar]
- 26.Armitage R, Hoffmann R, Emslie G, Rintelman J, Moore J, Lewis K. Rest-activity cycles in childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry. 2004;43:761–769. doi: 10.1097/01.chi.0000122731.72597.4e. [DOI] [PubMed] [Google Scholar]
- 27.Armitage R, Hoffmann R, Emslie G, Rintelmann J, Robert J. Sleep microarchitecture in childhood and adolescent depression: temporal coherence. Clin EEG Neurosci. 2006;37:1–9. doi: 10.1177/155005940603700103. [DOI] [PubMed] [Google Scholar]
- 28.Robert JJ, Hoffmann RF, Emslie GJ, Hughes C, Rintelmann J, Moore J, Armitage R. Sex and age differences in sleep macroarchitecture in childhood and adolescent depression. Sleep. 2006;29:351–358. doi: 10.1093/sleep/29.3.351. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Poznanski EO, Freeman LN, Mokros HB. Children’s Depression Rating Scale - Revised (September 1984) Psychopharmacol Bulletin. 1985;21:979–989. [Google Scholar]
- 31.Shaffer D, Gould MS, Brasic J. A Children’s Global Assessment Scale (CGAS) (for children 4 to 16 years of age) J Psychopharmacol Bulletin. 1985;21:747–748. [Google Scholar]
- 32.Mrazek DA, Masterton J. The family global assessment scale (FGAS): initial reliability and validity. 32nd Annual Meeting of American Academy of Child Psychiatry; San Antonio, TX. 1985. [Google Scholar]
- 33.Emslie GJ, Armitage R, Weinberg WA, Rush AJ, Mayes TL, Hoffmann RF. Sleep polysomnography as a predictor of recurrence in children and adolescents with major depressive disorder. Int J Neuropsychopharmacol. 2001;4:159–168. doi: 10.1017/S1461145701002383. [DOI] [PubMed] [Google Scholar]
- 34.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 35.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System For Sleep Stages of Human Subjects. Washington: U.S. Government Printing Office: National Institutes of Health; 1968. [Google Scholar]
- 36.Cohen JW. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 37.Biggs SN, Dollman J. Association between sleep, BMI and waist girth in children and adolescents: a retrospective analysis. Acta Paediatr. 2007;96:1839–1840. doi: 10.1111/j.1651-2227.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 38.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the 'Quebec en Forme' Project. Int J Obes (Lond) 2006;30:1080–1085. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 39.Lumeng JC, Somashekar D, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–1029. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- 40.Sekine M, Yamagami T, Handa K, Saito T, Nanri S, Kawaminami K, Tokui N, Yoshida K, Kagamimori S. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 41.Resta O, Foschino Barbaro MP, Bonfitto P, Giliberti T, Depalo A, Pannacciulli N, De Pergola G. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med. 2003;253:536–543. doi: 10.1046/j.1365-2796.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–1337. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 43.Benca RM. Mood Disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier Saunders; 2005. pp. 1311–1326. [Google Scholar]
- 44.Liu X, Forbes EE, Ryan ND, Rofey D, Hannon TS, Dahl RE. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry. 2008;65:924–932. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 47.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7:648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8:197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 49.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]